Abstract
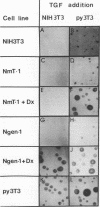
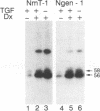
The middle tumor antigen (mT) of polyomavirus is unable to transform a clone of NIH 3T3 cells to anchorage independence (L. Raptis and J.B. Bolen, J. Virol. 63:753-758, 1989). However, this oncogene increased the responsiveness of these cells to the growth factors (alpha-like and beta-type transforming growth factors) produced by cells possessing the whole transforming region of polyomavirus. This resulted in the growth of NIH 3T3 cells, expressing mT under control of the dexamethasone-regulatable mouse mammary tumor virus promoter, in agar medium supplemented with these growth factors upon addition of the inducer. Therefore, mT, a transforming oncogene, is able to enhance the responsiveness of established cells to growth factors, a property previously attributed primarily to myc and other establishment type oncogenes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherington V., Morgan B., Spiegelman B. M., Roberts T. M. Recombinant retroviruses that transduce individual polyoma tumor antigens: effects on growth and differentiation. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4307–4311. doi: 10.1073/pnas.83.12.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Anderson C., Hunter T., Kaplan P. L. Transmission of the polyoma virus middle T gene as the oncogene of a murine retrovirus. Nature. 1984 Apr 19;308(5961):748–750. doi: 10.1038/308748a0. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Whitfield J. F. Evidence that the viral Ki-ras protein, but not the pp60v-src protein of ASV, stimulates proliferation through the PDGF receptor. Biochem Biophys Res Commun. 1987 Oct 14;148(1):376–383. doi: 10.1016/0006-291x(87)91121-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Kennedy B., Deuel T. F. Platelet-derived growth factor. Specific binding to target cells. J Biol Chem. 1982 Jul 25;257(14):8130–8136. [PubMed] [Google Scholar]
- Kaplan D. R., Pallas D. C., Morgan W., Schaffhausen B., Roberts T. M. Mechanisms of transformation by polyoma virus middle T antigen. Biochim Biophys Acta. 1989 Feb;948(3):345–364. doi: 10.1016/0304-419x(89)90006-1. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. L., Ozanne B. Polyoma virus-transformed cells produce transforming growth factor(s) and grow in serum-free medium. Virology. 1982 Dec;123(2):372–380. doi: 10.1016/0042-6822(82)90270-7. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., Cooper G. M. Transforming activity of human tumor DNAs. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1181–1184. doi: 10.1073/pnas.78.2.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Moses H. L. Modulation of transforming growth factor type beta action by activated ras and c-myc. Mol Cell Biol. 1987 Jul;7(7):2649–2652. doi: 10.1128/mcb.7.7.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W., Connell J. R. Induction of immortality is an early event in malignant transformation of mammalian cells by carcinogens. Nature. 1982 Oct 14;299(5884):633–635. doi: 10.1038/299633a0. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- Noda T., Satake M., Yamaguchi Y., Ito Y. Cooperation of middle and small T antigens of polyomavirus in transformation of established fibroblast and epithelial-like cell lines. J Virol. 1987 Jul;61(7):2253–2263. doi: 10.1128/jvi.61.7.2253-2263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis L., Bolen J. B. Polyomavirus transforms rat F111 and mouse NIH 3T3 cells by different mechanisms. J Virol. 1989 Feb;63(2):753–758. doi: 10.1128/jvi.63.2.753-758.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis L., Lamfrom H., Benjamin T. L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985 Sep;5(9):2476–2486. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzino A., Ruff E. Fibroblast growth factor induces the soft agar growth of two non-transformed cell lines. In Vitro Cell Dev Biol. 1986 Dec;22(12):749–755. doi: 10.1007/BF02621092. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Enhancement of polyoma virus middle T antigen tyrosine phosphorylation by epidermal growth factor. Nature. 1983 Aug 25;304(5928):742–744. doi: 10.1038/304742a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Drozdoff V., McKinney M. D., Zeitz L., Fleissner E. Potentiation of growth factor activity by exogenous c-myc expression. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8167–8171. doi: 10.1073/pnas.83.21.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Roberts A. B., Roche N. S., Sporn M. B., Weinberg R. A. Differential responsiveness of myc- and ras-transfected cells to growth factors: selective stimulation of myc-transfected cells by epidermal growth factor. Mol Cell Biol. 1986 Mar;6(3):870–877. doi: 10.1128/mcb.6.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Zullo J., Stiles C. D., Garcea R. L. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1210–1214. doi: 10.1073/pnas.84.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]