Abstract
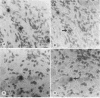
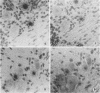
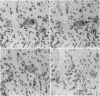
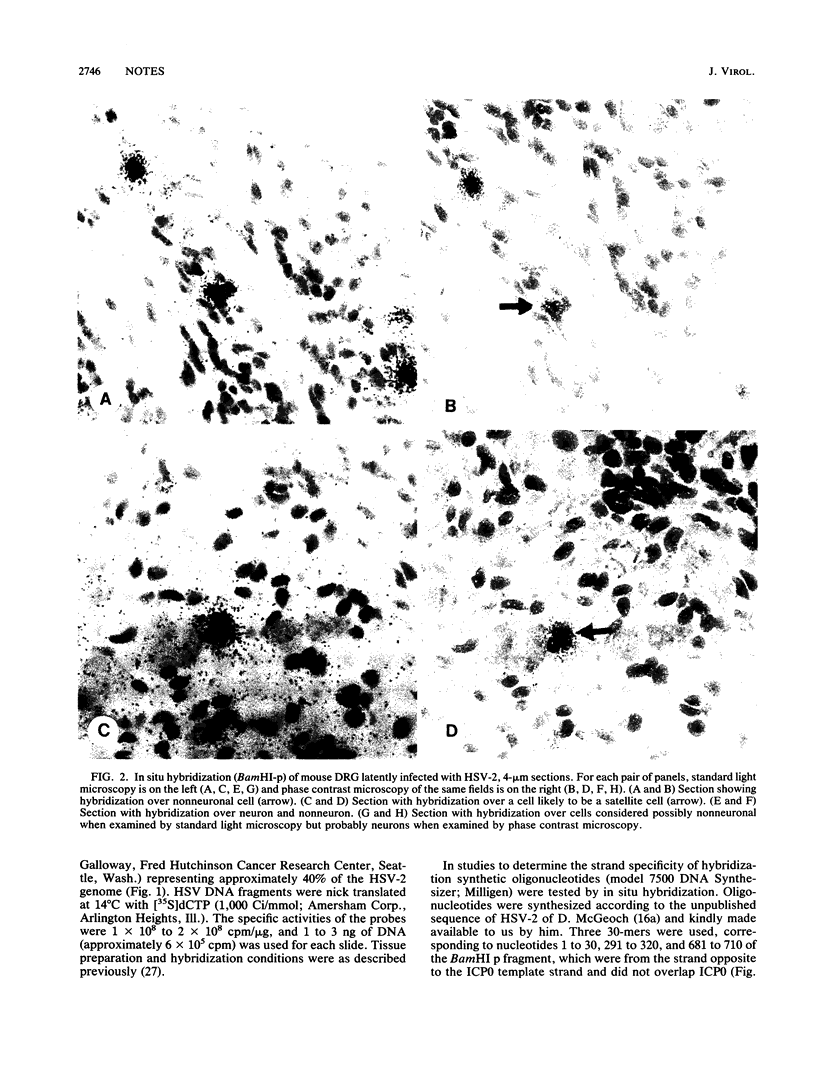
The presence of herpes simplex virus type 2 (HSV-2) transcription during in vivo latent infection was investigated by in situ hybridization. Latent infection of mouse dorsal root ganglion was investigated with the BamHI p fragment of HSV-2, which resulted in evidence of ganglion hybridization, and other fragments representing approximately 40% of the genome, which did not result in hybridization. Strand specificity of hybridization was investigated in studies with synthetic oligonucleotides, which supported the conclusion that a latency-associated transcript(s) had been detected. Hybridization was detected with oligonucleotides complementary to the infected-cell polypeptide 0 (ICP0) template strand but not with oligonucleotides synthesized from the ICP0 template strand. Although most hybridization occurred over neurons, in some instances hybridization appeared to occur over nonneuronal ganglion cells, and this was more evident when tissue sections were examined by phase contrast microscopy. Although these results supported the usual neuronal site of HSV-2 latency, latency in nonneuronal cells may be important in considering the pathobiology of HSV-2 infections.
Full text
PDF
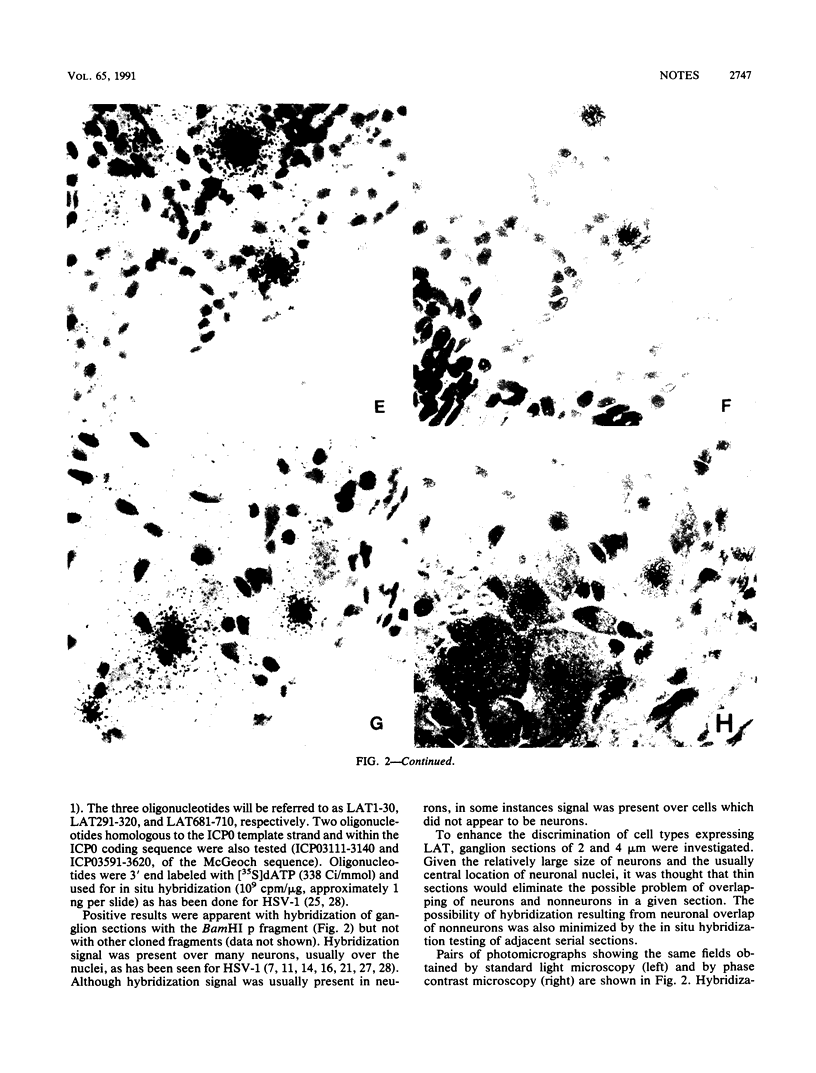
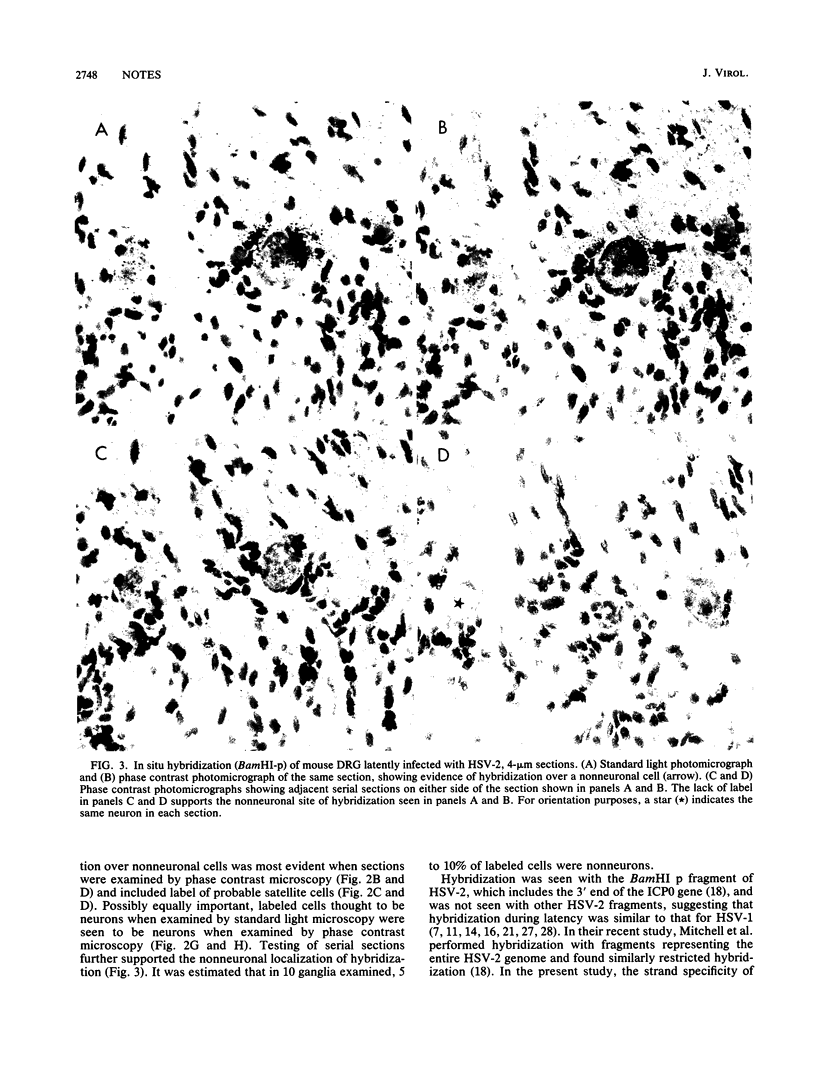


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Saadi S. A., Clements G. B., Subak-Sharpe J. H. Viral genes modify herpes simplex virus latency both in mouse footpad and sensory ganglia. J Gen Virol. 1983 May;64(Pt 5):1175–1179. doi: 10.1099/0022-1317-64-5-1175. [DOI] [PubMed] [Google Scholar]
- Clements G. B., Subak-Sharpe J. H. Herpes simplex virus type 2 establishes latency in the mouse footpad. J Gen Virol. 1988 Feb;69(Pt 2):375–383. doi: 10.1099/0022-1317-69-2-375. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Bastone V. B., Stevens J. G. Evidence that neurons harbor latent herpes simplex virus. Infect Immun. 1974 May;9(5):946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. D., Batra S. K., Brown S. M. Recovery of herpes simplex virus from the corneas of experimentally infected rabbits. J Gen Virol. 1987 Jul;68(Pt 7):2013–2017. doi: 10.1099/0022-1317-68-7-2013. [DOI] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Straus S. E. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard S. H., Cheatham W. J., Moses H. L. Electron microscopy of zosteriform herpes simplex infection in the mouse. Lab Invest. 1972 Apr;26(4):391–402. [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C., Shevchuk M., McDougall J. K. Detection of herpes simplex RNA in human sensory ganglia. Virology. 1979 May;95(1):265–268. doi: 10.1016/0042-6822(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Rozenman Y., Murray R., Devlin M., Vafai A. Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann Neurol. 1987 Sep;22(3):377–380. doi: 10.1002/ana.410220315. [DOI] [PubMed] [Google Scholar]
- Gordon Y. J., Johnson B., Romanowski E., Araullo-Cruz T. RNA complementary to herpes simplex virus type 1 ICP0 gene demonstrated in neurons of human trigeminal ganglia. J Virol. 1988 May;62(5):1832–1835. doi: 10.1128/jvi.62.5.1832-1835.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R. W., Ecker J. R., Tenser R. B. Varicella-zoster virus RNA in human trigeminal ganglia. Lancet. 1983 Oct 8;2(8354):814–816. doi: 10.1016/s0140-6736(83)90736-5. [DOI] [PubMed] [Google Scholar]
- Kennedy P. G., Al-Saadi S. A., Clements G. B. Reactivation of latent herpes simplex virus from dissociated identified dorsal root ganglion cells in culture. J Gen Virol. 1983 Jul;64(Pt 7):1629–1635. doi: 10.1099/0022-1317-64-7-1629. [DOI] [PubMed] [Google Scholar]
- Krause P. R., Croen K. D., Straus S. E., Ostrove J. M. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J Virol. 1988 Dec;62(12):4819–4823. doi: 10.1128/jvi.62.12.4819-4823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K., Vahlne A., Persson L. A., Lycke E. Neural spread of herpes simplex virus types 1 and 2 in mice after corneal or subcutaneous (footpad) inoculation. J Neurol Sci. 1978 Feb;35(2-3):331–340. doi: 10.1016/0022-510x(78)90013-8. [DOI] [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan J. L., Darby G. Herpes simplex virus latency: the cellular location of virus in dorsal root ganglia and the fate of the infected cell following virus activation. J Gen Virol. 1980 Dec;51(Pt 2):233–243. doi: 10.1099/0022-1317-51-2-233. [DOI] [PubMed] [Google Scholar]
- Mitchell W. J., Deshmane S. L., Dolan A., McGeoch D. J., Fraser N. W. Characterization of herpes simplex virus type 2 transcription during latent infection of mouse trigeminal ganglia. J Virol. 1990 Nov;64(11):5342–5348. doi: 10.1128/jvi.64.11.5342-5348.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. J., Taylor J. L. The isolation of herpes simplex virus from rabbit corneas during latency. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):357–364. [PubMed] [Google Scholar]
- Roizman B. The organization of the herpes simplex virus genomes. Annu Rev Genet. 1979;13:25–57. doi: 10.1146/annurev.ge.13.120179.000325. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Straus S. E. Clinical and biological differences between recurrent herpes simplex virus and varicella-zoster virus infections. JAMA. 1989 Dec 22;262(24):3455–3458. [PubMed] [Google Scholar]
- Stroop W. G., Rock D. L., Fraser N. W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984 Jul;51(1):27–38. [PubMed] [Google Scholar]
- Suzuki S., Martin J. R. Herpes simplex virus type 2 transcripts in trigeminal ganglia during acute and latent infection in mice. J Neurol Sci. 1989 Nov;93(2-3):239–251. doi: 10.1016/0022-510x(89)90194-9. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dawson M., Ressel S. J., Dunstan M. E. Detection of herpes simplex virus mRNA in latently infected trigeminal ganglion neurons by in situ hybridization. Ann Neurol. 1982 Mar;11(3):285–291. doi: 10.1002/ana.410110309. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Hay K. A., Edris W. A. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J Virol. 1989 Jun;63(6):2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B. Role of herpes simplex virus thymidine kinase expression in viral pathogenesis and latency. Intervirology. 1991;32(2):76–92. doi: 10.1159/000150188. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S. M., Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988 Nov;62(11):4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Davison A., Chartrand P., Stow N. D., Preston V. G., Timbury M. C. Recombination in herpes simplex virus: mapping of mutations and analysis of intertypic recombinants. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):827–840. doi: 10.1101/sqb.1979.043.01.089. [DOI] [PubMed] [Google Scholar]