Abstract
The majority of organisms can be grouped into those relying solely on photosynthesis (phototrophy) or those relying solely on the assimilation of organic substances (heterotrophy) to meet their requirements for energy and carbon. However, a special life history trait exists in which organisms combine both phototrophy and heterotrophy. Such “mixotrophy” is a widespread phenomenon in aquatic habitats and is observed in many protozoan and metazoan organisms. The strategy requires investment in both photosynthetic and heterotrophic cellular apparatus, and the benefits must outweigh these costs. In accordance with mechanistic resource competition theory, laboratory experiments revealed that pigmented mixotrophs combined light, mineral nutrients, and prey as substitutable resources. Thereby, they reduced prey abundance below the critical food concentration of competing specialist grazers [Rothhaupt, K. O. (1996) Ecology 77, 716–724]. Here, we demonstrate the important consequences of this strategy for an aquatic community. In the illuminated surface strata of a lake, mixotrophs reduced prey abundance steeply. The data suggest that, as a consequence, grazers from higher trophic levels, consuming both the mixotrophs and their prey, could not persist. Thus, the mixotrophs escaped from competition with and losses to higher grazers. Furthermore, the mixotrophs structured prey abundance along the vertical light gradient, creating low densities near the surface and a pronounced maximum of their algal prey at depth. Such deep algal accumulations are typical features of nutrient-poor aquatic habitats, previously explained by resource availability. We hypothesize instead that the mixotrophic grazing strategy is responsible for deep algal accumulations in many aquatic environments.
The majority of organisms rely either on the use of light, inorganic carbon, and mineral nutrients for photosynthesis (phototrophy) or on the heterotrophic use of organic compounds in the form of prey items (phagotrophy) or dissolved organic carbon (saprotrophy). However, a special life history trait exists in which organisms combine both phototrophy and heterotrophy. This strategy is termed “mixotrophy.” Mixotrophy is not a new “invention”; it is regarded as an evolutionarily derived character (1). Mixotrophy occurs in many single-celled aquatic organisms (flagellates, ciliates, and radiolarians) as well as in sponges, corals, rotifers, and even in higher plants.
The mixotrophic life history has significant physiological implications. Mixotrophic organisms have to invest in the synthesis and maintenance of both a photosynthetic apparatus and in mechanisms for prey uptake and its subsequent digestion. These energetic costs may lower the mixotroph's resource use efficiency and/or lower photosynthetic performance, resulting in, for example, a reduced maximum growth rate compared with a phototrophic or heterotrophic specialist (2). A mixotroph is therefore expected to be inferior if it competes with specialist phototrophs for light or specialist phagotrophs for prey (3). However, there are also inherent advantages to being mixotrophic. For example, the capability of mixotrophs to use particulate food as a source of mineral nutrients and growth factors gives them a distinct advantage over specialist phototrophs if dissolved minerals limit phototrophic growth (4). Furthermore, laboratory experiments have demonstrated that the supplementary use of light and mineral nutrients may allow mixotrophs to outcompete specialist phagotrophs for prey (3). Here, we demonstrate the consequences of the mixotrophic strategy in an aquatic community, in particular in creating a pronounced deep chlorophyll maximum (DCM) of their algal prey at depth.
DCM represent accumulations of phototrophs (cyanobacteria or eukaryotic algae) in subsurface strata. They are a common feature and one of the most striking characteristics of nutrientpoor waters, such as central ocean gyres and clearwater lakes (5, 6), and occur to depths of ≈120 m. Although cell-specific chlorophyll contents have been observed to increase with depth, DCM typically represent biomass maxima and often constitute a substantial proportion of the phototrophic biomass (7–12). The formation of DCM has traditionally been interpreted from a bottom-up perspective by enhanced nutrient availability at depth (13–17). The biomass achievable at a given nutrient concentration, however, depends on cellular growth. As shown in several chemostat experiments, when growth balanced loss, the algae depleted the soluble limiting nutrients to a concentration just sufficient to allow them to realize this growth rate. Thus, the biomass of algae depends on total nutrient concentration and cellular growth rate. Higher growth rates to compensate for, e.g., grazing losses require higher external and internal nutrient concentrations and are therefore associated with lower achievable algal biomass. This means that even in situations when growth balances loss, nutrients alone cannot sufficiently explain biomass patterns.
Several studies demonstrated high cell division rates at the DCM (7, 8, 18). Such sustained growth at the DCM implies that phototroph abundance is the result of both production and loss. Grazing is known to be the most important loss process for these phototrophs, facilitating sustained growth via nutrient remineralization (9). If grazing, rather than resources, stimulates the formation of the DCM, vertical gradients in grazing pressure must balance growth over a period of many months, a situation that may arise via the following mechanism.
The Hypothesis
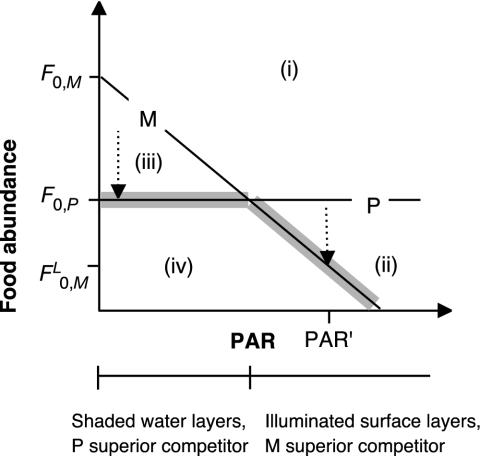
Using the mechanistic resource competition theory (19), Rothhaupt (3) demonstrated that in the dark specialist grazers (phagotrophs) reach zero net population growth at food concentrations (F0) lower than mixotrophic grazers (F0,P<F0,M). Pigmented mixotrophic grazers are able to use organic carbon (e.g., prey items) and light as substitutable energy sources. Thus, conversely, at sufficient light, mixotrophs require lower food densities than the nonpigmented phagotrophs 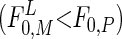 and are able to outcompete them (3) (Fig. 1). We hypothesize that high phototroph abundance in the DCM reflects higher phagotroph food thresholds (F0,P) compared with those of mixotrophs illuminated at the surface
and are able to outcompete them (3) (Fig. 1). We hypothesize that high phototroph abundance in the DCM reflects higher phagotroph food thresholds (F0,P) compared with those of mixotrophs illuminated at the surface 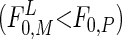 . This may not apply to some lakes where the DCM is formed by phototrophs less susceptible to grazing [e.g., colonial chrysophytes (20)] and to systems in which an obligate resource for the phototrophs is available only within a deep boundary layer (e.g., reduced sulfur for pigmented sulfur bacteria). Although mixotrophy includes many forms, in the model and in our discussion, we define our use of the term “mixotroph” to the combination of phagotrophy and phototrophy. This type of mixotrophy must have been evaluated functionally by their possession of pigments and their experimentally demonstrated ability to take up prey (21) (see The Mixotrophic Nature of Ochromonas in Lake 111 in Supporting Methods, which is published as supporting information on the PNAS web site, www.pnas.org).
. This may not apply to some lakes where the DCM is formed by phototrophs less susceptible to grazing [e.g., colonial chrysophytes (20)] and to systems in which an obligate resource for the phototrophs is available only within a deep boundary layer (e.g., reduced sulfur for pigmented sulfur bacteria). Although mixotrophy includes many forms, in the model and in our discussion, we define our use of the term “mixotroph” to the combination of phagotrophy and phototrophy. This type of mixotrophy must have been evaluated functionally by their possession of pigments and their experimentally demonstrated ability to take up prey (21) (see The Mixotrophic Nature of Ochromonas in Lake 111 in Supporting Methods, which is published as supporting information on the PNAS web site, www.pnas.org).
Fig. 1.
Application of mechanistic resource competition theory (3, 19) to explain the mechanisms of DCM formation. Lines indicate resource combinations at which the net population growth of a phagotrophic (P) and a mixotrophic (M) organism are zero, the zero net growth isoclines. Sections i–iv show resource combinations allowing the growth of M+P(i),M(ii),P(iii), and neither M nor P (iv, only theoretically). F0,P and F0,M, refer to the zero net growth food concentrations in the dark. In the context of Lake 111, food is represented by phototrophs (Chlamydomonas) plus bacteria. The consumption vectors (arrow) indicate resource changes by grazing assuming no variation of PAR attenuation due to varying predator or prey densities. Starting in i, grazing by M or P reduces the food abundance until the population growth of the superior competitor equals zero (thick gray line), indicated by the lower F0 derived from the zero net growth isocline and a given PAR. In shaded strata (DCM), F0,P determines the food abundance. FL0,M represents phototrophic abundance at the surface as a function of PAR at half epilimnion depth (PAR′), assuming vertical mixing.
Methods
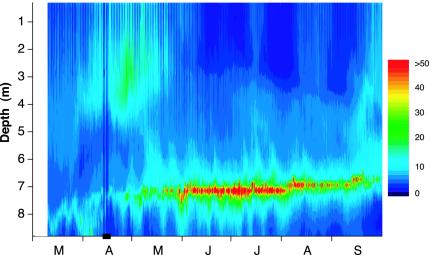
We tested our hypothesis on the low-diversity planktonic food web of an acidic mining lake [Lake 111, Germany, pH 2.6–3.3 (22, 23)] with a distinct DCM (Fig. 2). Physical data including in situ fluorescence were obtained by using an automatic probe (Idronaut, Brugherio, Italy). Photosynthetic available radiation (PAR) was measured by using spherical quantum sensors (SPQA, Li-Cor, Lincoln, NE). We took water samples by using tubing mounted near the probe's depth sensor. Samples were preacidified with H2SO4 to a final concentration of 0.3% and then fixed with Lugol's iodine to prevent iodine precipitation under lake water conditions.
Fig. 2.
The 1999 DCM in Lake 111. Relative units of in situ fluorescence (rough indicator of chlorophyll concentration) are shown. The highest summer fluorescence values corresponded to a chlorophyll a concentration of ≈60 μg/liter–1 (29 June, 7.0-m depth, linear scale). No data are shown from April 13 April 17 (horizontal bar).
Protozoan cell density was determined microscopically by counting in sedimentation chambers. Bacterial numbers were quantified by means of epifluorescence microscopy after staining with acridine-orange. Before staining, the color of the Lugol was removed by adding a few drops of 0.1 M sodium thiosulfate. Biovolumes were calculated by approximation to simple geometrical bodies.
Experiments were performed in the laboratory. We conducted grazing experiments to test the grazing impact of the mixotroph, Ochromonas, on the Chlamydomonas food algae and competition experiments of mixotrophs and phagotrophs, the ciliates Oxytricha, for prey. Bacteria (strain 99P5, University of Potsdam), the flagellates Chlamydomonas (11A2) and Ochromonas (1B3), and ciliates Oxytricha (99X4) were isolated from field samples. Each isolate was precultured at 20 ± 1°C in an incubator simulating the specific in situ light spectrum (24). A PAR of 150 or 60 μmol photons m–2·s–1 was supplied continuously or in a 16:8 h light/dark cycle in the competition and grazing experiments, respectively. We measured the PAR inside the culturing flasks with a quantum sensor (QSL-101, Biospherical Instruments, San Diego). We used an inorganic nutrientreplete medium, simulating the lake's chemical conditions (25). The experiments were run in duplicates. Abundances were monitored by microscopic cell enumeration.
Further laboratory experiments were conducted to test the growth of food algae and bacteria under in situ conditions and ambient dissolved organic carbon quality. We collected water from Lake 111 on July 24, 2000 (2.5-m depth), August 22, 2000 (2.5 m), August 30, 2000 (3.6 m), October 10, 2000 (6.0 m), and November 14, 2000 (8.5 m). The water was filtered with low pressure (10 kPa) by using capsules (0.22-μm pore size, MPGL 06 GH2, Millipore). We used bacterioplankton collected in situ that was adapted to the ambient dissolved organic carbon. Bacteria were taken from the sampled water and separated by gravity filtration through a 0.8-μm filter. The natural bacterioplankton and preincubated Chlamydomonas were inoculated in separate cultures at low densities (<2% of lake abundances) and maintained under a 16:8 h light/dark cycle. Due to the low pH, inorganic carbon is present only in the form of dissolved CO2. We therefore adjusted the medium's CO2 content to in situ conditions by pumping air containing 5% CO2 into the incubation chamber. The growth rates over the logarithmic growth phase (6–30 days) were calculated for pooled data from two replicates.
Results
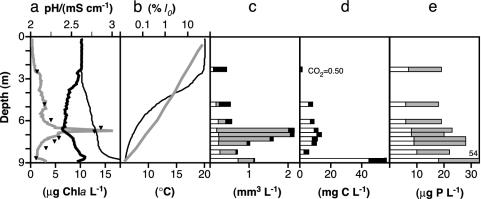
Important functional groups in acidic Lake 111 were represented by only one or a few species in the food web (22). These comprised the DCM-forming phototroph, Chlamydomonas sp.; the pigmented mixotrophic grazer, Ochromonas sp.; phagotrophic protozoans (heliozoans and ciliates); and nonpigmented bacteria. The mixotrophs were the dominant grazers throughout the water column, particularly in illuminated surface strata (Fig. 3). Due to their low specific chlorophyll a content (1.7 and 20.2 μg·mm–3 in light-saturated Ochromonas and Chlamydomonas cultures, respectively), the mixotrophs did not enhance surface chlorophyll concentrations significantly. Phagotroph biomass was very low. Mixo- and phagotroph prey biomass (phototrophs and nonfilamentous bacteria) was significantly lower in illuminated than in shaded strata. These observations agreed well with the mechanistic theory and support our hypothesis that grazing by mixotrophs steeply reduces phototrophic biomass at the surface. To exclude alternative hypotheses, we needed to show that (i) cellular growth of prey organisms was higher in surface strata than at DCM, (ii) the mixotrophs consumed the equally sized phototrophs, (iii) mixotrophs in the light had a lower F0 than mixotrophs and phagotrophs in the dark and, therefore, (iv) the mixotrophs outcompeted the phagotrophs in the light.
Fig. 3.
Vertical distribution of plankton taxa, physical parameters, and nutrients in Lake 111 (September 15, 1999). (a) Chlorophyll a (Chla, triangles) and in situ fluorescence (gray line), pH (thin line), and conductivity (κ25, thick line). Conductivity indicates the presence of a mixed epilimnion (0–3 m), an intermediate layer (down to 6.5 m), and a deeper water layer. (b) Temperature (black) and PAR (gray, I0–surface irradiation). (c) Biovolume of nonfilamentous bacteria (white), phototrophs (Chlamydomonas, gray), and mixotrophs (Ochromonas, black). Median biovolumes of nonfilamentous (edible) bacteria from several sampling occasions were lower in epilimnion than in layers down to the DCM (3.6–7.6 m depth) (11 vs. 17 samples, P = 0.0005, two-tailed U test). Phototrophs were also higher in the depth (P = 0.0024). Mixotrophs exhibited higher biovolumes in epilimnion (P = 0.0147). Phagotrophs (heliozoans plus ciliates) and filamentous bacteria (less edible) did not vary in the vertical range (not shown, P = 0.2561 and 0.0509, respectively). Phagotroph biovolumes were 1.6 orders of magnitude lower than those of mixotrophs. Nonprotozoan phagotrophs were of low importance (22). (d) CO2 (white) and dissolved organic carbon (black). (e) Total phosphorus (whole column) and soluble reactive phosphorus (white). Nitrogen was found in excess (≥3,000 μg liter–1
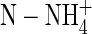 ). We did not detect H2S.
). We did not detect H2S.
The cellular growth of prey organisms in lake water from different depths is shown in Table 1. Growth rates of phototrophs and bacteria were highest in surface strata, but their abundances, in contrast, were much higher at depth (Fig. 3c). Although growth rates cannot be directly transferred to in situ conditions, this corroborates the hypothesis that loss processes controlled abundances.
Table 1. Growth of prey organisms at DCM and surface conditions.
| Depth, m | DOC, mg C liter-1 | PAR, μmol m-2·s-1 | Temperature, °C | μ phototrophs, d-1 | μ bacteria, d-1 |
|---|---|---|---|---|---|
| 2.5 | 1.3 | 60 | 17.0 | 0.60 ± 0.06 | 1.05 ± 0.05 |
| 2.5 | 0.8 | 60 | 22.1 | 0.60 ± 0.03 | 1.01 ± 0.06 |
| 3.6 | 0.9 | 20 | 16.4 | -0.02 ± 0.03 | 0.34 ± 0.06 |
| 6.0 | 3.7 | 7 | 10.3 | 0.05 ± 0.01 | 0.15 ± 0.01 |
| 8.5 | 2.9 | 2 | 7.8 | -0.01 ± 0.01 | 0.13 ± 0.02 |
Growth rates of separate cultures of natural bacterioplankton and phototrophs (Chlamydomonas) (μ ± SE). We used sterile filtered Lake 111 water from different depths. The PAR denotes that applied in the laboratory. In situ PAR values can be estimated on the basis of continuous solar radiation measurements at Lake 111 and light attenuation with depth (24). PAR was calculated as 58, 23, 3.1, and 0.4 μmol photons m-2·s-1 at 2.5, 2.5, 3.6, 6.0, and 8.5 m, respectively, over a mean 16 h, per day from May to August 1999. DOC, dissolved organic carbon.
The ingestion of phototrophs by mixotrophs was microscopically and experimentally verified: Inoculated at typical epilimnion densities, phototroph net growth was lower when the phototrophs were grown together with the mixotrophs than when they were grown alone (0.49 ± 0.02 and 0.58 ± 0.02 d–1, respectively). Under laboratory conditions, phototrophic growth exceeded in situ growth, which might explain why Chlamydomonas production was not balanced by mixotroph grazing (see Control of Chlamydomonas in Lake 111 in Supporting Methods; see also Table 2 and Fig. 5, which are published as supporting information on the PNAS web site). It is known that Ochromonas feed on algae (26). The capability to ingest algae significantly larger than its own cell size has already been demonstrated for the closely related mixotrophic flagellate Poterioochromonas malhamensis (27).
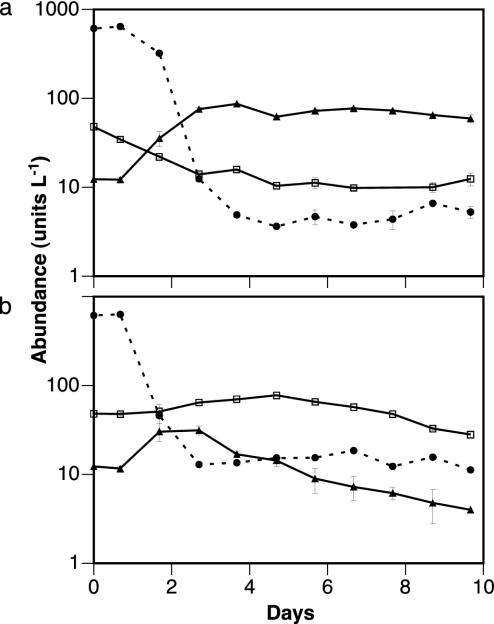
We determined the food thresholds (F0) of mixotrophs and phagotrophs experimentally by using bacterial prey (Fig. 4). We used bacteria rather than phototrophs as prey, because bacterial densities indicating F0 were assumed to be more stable in dark treatments. In the light, bacteria were grazed to lower densities than in the dark (minimum 0.36 108 and 1.10 108 liter–1, respectively). The final biomass ratio of mixotrophs to phagotrophs was 843:1 in the light and 6:1 in the dark. These results generally conform to the mechanistic competition theory and verify our hypothesis of a steeper prey reduction in the light than in the dark. The predicted competitive advantage of phagotrophs in the dark (Fig. 1) was less than expected. In the light, grazing by phagotrophs on mixotrophs may have prevented both further mixotrophic growth and the complete competitive removal of phagotrophs (Fig. 4a).
Fig. 4.
Competition between mixotrophs and phagotrophs for bacteria in batch experiments (a) in the light (150 μmol photons m–2·s–1, 20 ± 1°C) (a) and in the dark (b). Symbols represent the means and ranges of two replicates: •, 107 bacteria; ▴, 106 mixotrophs (Ochromonas sp.); □, 102 phagotrophs (the ciliate Oxytricha sp.).
Discussion
Our data indicate that loss processes caused the formation of the DCM in Lake 111. In illuminated surface strata, prey abundance was lowest, prey growth rates highest, and the mixotrophs were able to reduce prey abundance most steeply. Other grazers could not balance phototrophic and bacterial losses (see Control of Chlamydomonas in Lake 111 in Supporting Methods). These results support the predictions derived from the mechanistic resource competition theory (3, 19). Our interpretation is that the lower limit for the occurrence of phototrophs is set by their energetic requirements, which are met by light and potentially by the uptake of dissolved organic carbon. Although Chlamydomonas has the potential to use dissolved organic carbon (ref. 25; see Chlamydomonas Uses Organics for Growth at the DCM in Supporting Methods and Table 1, 3.6- and 6-m depth), we refer to it as a phototroph for simplicity. Within this energetically feasible depth range, phototroph abundance is determined by the critical food concentration (F0) of the dominant grazer. These are represented here by the mixotrophs throughout the water column (Fig. 3), because the phagotrophs turned out to be competitively inferior even at depth (Fig. 4). Although the latter observation derives from predictions (Fig. 1), consequences are expected to be of secondary importance. At depth, both competitors have a higher F0 than the mixotrophs at the surface. Therefore, the abundance of phototrophs should follow the zero growth isocline of the mixotrophs (Fig. 1), resulting in a pronounced DCM due to exponential light attenuation with depth (Fig. 3). We conclude that the observed formation of the DCM in Lake 111 was indeed the result of a steep reduction of phototroph abundance by the mixotrophs in illuminated strata.
The mixotrophic strategy potentially allows mixotrophs to do more than simply outcompete competitors. As indicated in our experiment (Fig. 4), phagotrophs at higher trophic levels may feed on the mixotroph's prey and on the mixotrophs themselves. If prey growth rates are low or moderate, low prey biomasses are associated with low prey production rates and therefore with low mixotroph biomasses to compensate for production. The deep reduction of prey biomass by mixotrophs in illuminated strata, therefore, means, that the overall available prey (mixotrophs plus mixotroph's prey) for a phagotroph at a higher trophic level is also low and will eventually be driven toward the phagotroph's critical food concentration. The mixotrophs would therefore escape from both competition for the same prey and from direct grazing losses. Although the vertical abundances of phagotrophs did not follow the predictions of the competition theory, the results from competition experiments (Fig. 4), combined with the absence of higher grazers, implies that the mixotrophs prevented phagotrophs from invading. In the illuminated surface strata of Lake 111, mixotrophic biomass exceeded that of phagotrophs 24- to 50-fold in different years.
If the cellular growth rate of the prey increases, the mixotrophs either keep prey abundance low by enhancing their own numbers or prey abundance runs out of grazing control. In both cases, the total food available for phagotrophs increases (prey biomass surpasses F0,P, Fig. 1), and the probability that the phagotrophs become dominant over the mixotrophs also increases [“intraguild predation” (28)]. This situation likely occurred in fertilized mesocosms in Lake 111. After fertilization (29), phototrophs and bacteria started to grow. Consequently, phagotrophs increased in abundance and ultimately equaled mixotrophic biomass. Conversely, in the control and in the lake, mixotrophs dominated (median mixotroph/phagotroph ratios 1.2 and 19, respectively, P < 0.05, n1 = 9, n2 = 27, two tailed U test). Overall, observations indicate that the mixotrophic strategy modifies processes at multitrophic levels. The mixotrophic life history trait has implications for both prey abundance and food web structure.
In biological oceanography literature, we found studies illustrating the ideas presented, although the authors themselves did not link their observations to the mechanistic theory (7, 30). For example, Havskum and Riemann (30) investigated the role of bacterivorous flagellates in the Bay of Aarhus, Denmark, and concluded “... that mixotrophic flagellates constituted half of the pigmented biomass in the nutrient-depleted top layer of the Bay of Aarhus. These mixotrophs were also responsible for almost the entire flagellate grazing on bacteria. The bacteria were grazed down to a low level, <1 × 106 ml–1, and our results strongly suggest that no flagellate group was able to sustain its growth solely on bacterivory. In the deeper, nutrient-rich environment, bacterivorous pigmented flagellates accounted, on average, for only 9% of the pigmented biomass. Here bacterial abundance was higher and colorless flagellates were mainly responsible for the grazing on bacteria.”
To demonstrate the effects of combining the use of two resources (i.e., light and organic carbon) in nature, we chose a system with a low-diversity food web that allowed us to use all of the constituent species in our experiments. We acknowledge that the extreme environmental conditions in our system provoke questions about the generality of the results presented here; other more common systems are larger, not acidic or more diverse. Nevertheless, the basic ecological mechanisms of species interaction, as illustrated in Fig. 1, have been proven to be independent of scale, habitat type, or the species involved [e.g., resource partitioning (31, 32)].
Mechanistic theory predicts that mixotrophs reduce food abundances steeply in surface waters, if (i) significant losses to higher trophic levels do not occur, allowing the mixotrophs to take full advantage of their strategy (2, 3); (ii) organic carbon (prey items) is available to mixotrophs; and (iii) the mixotrophs are able to combine light and organic carbon resource use. The first prerequisite is rather given in oligotrophic areas, because predation generally increases with enrichment. In accordance with the second prerequisite, pigmented flagellates ingesting prey have been evaluated as important planktonic constituents, because they contribute to the consumption of the predominating small phototrophs (33, 34). In nutrient-poor areas, phototrophs generally consist of small cells that are efficiently consumed by protozoans within the microbial food web (35, 36). In accordance with the third prerequisite, the steep reduction of prey abundances by mixotrophs when light is supplied has been exemplified under laboratory and field conditions (2, 30, 37). Moreover, a number of studies have shown that mixotrophs are abundant and active in illuminated surface waters (4, 38–40). For example, in the oligotrophic Sargasso Sea, up to 50% of nanoplanktonic algae in surface waters ingested bacteria, and this proportion decreased with depth, not exceeding 0.5% at the DCM (21). Indeed, evidence for the significance of loss processes in DCM formation came from studies in marine and limnetic environments showing that cell division rates of phototrophs in surface layers were higher than or equal to those at DCM (7, 18, 41).
The emerging picture makes sense in the context of mechanistic theory and led us to hypothesize that light modifies grazing activity throughout the vertical water column in many aquatic environments. The use of many resources is connected with enhanced basic metabolic requirements and decreasing efficiency by using each single resource (2, 42). Although ecology paradigm predicts that specialization should be the most successful strategy for survival under stable conditions (43–45), our data indicate that the use of several resources with lower efficiency can be an equally successful strategy in nature.
Supplementary Material
Acknowledgments
We thank the staff of the UFZ Centre and Potsdam University for processing the plankton samples and chemical analyses. U. Riebesell, G. F. Fussmann, K. O. Rothhaupt, J. Spindler, A. Kremp, W. Geller, and two referees commented on the manuscript. V.B. was supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DCM, deep chlorophyll maximum (maxima); PAR, photosynthetic available radiation.
References
- 1.Raven, J. A. (1997) Limnol. Oceanogr. 42 198–205. [Google Scholar]
- 2.Rothhaupt, K. O. (1996) Ecology 77 706–715. [Google Scholar]
- 3.Rothhaupt, K. O. (1996) Ecology 77 716–724. [Google Scholar]
- 4.Nygaard, K. & Tobiesen, A. (1993) Limnol. Oceanogr. 38 273–279. [Google Scholar]
- 5.Cullen, J. J. (1982) Can. J. Fish. Aquat. Sci. 39 791–803. [Google Scholar]
- 6.Abbott, M. R., Denman, K. L., Powell, T. M., Richerson, P. J., Richards, R. C. & Goldman, C. R. (1984) Limnol. Oceanogr. 29 862–878. [Google Scholar]
- 7.Agawin, N. S. R. & Agusti, S. (1997) J. Plankton Res. 19 1599–1615. [Google Scholar]
- 8.Gross, H. P., Wurtsbaugh, W. A., Luecke, C. & Budy, P. (1997) Can. J. Fish. Aquat. Sci. 54 1177–1189. [Google Scholar]
- 9.Brock, J. C., Sathyendranath, S. & Platt, T. (1998) Mar. Ecol. Prog. Ser. 165 1–15. [Google Scholar]
- 10.Arin, L., Berdalet, E., Marrasé, C., Estrada, M., Guixa-Boixereu, N. & Dolan, J. (1999)) J. Plankton Res. 21 1299–1316. [Google Scholar]
- 11.Estrada, M., Marrasé, C., Latasa, M., Berdalet, E., Delgado, M. & Rierra, T. (1993) Mar. Ecol. Prog. Ser. 92 289–300. [Google Scholar]
- 12.Coon, T. G., Lopez, M., Richerson, P. J., Powell, T. M. & Goldman, C. R. (1987) J. Plankton Res. 9 327–344. [Google Scholar]
- 13.Letelier R. M., Bidigare, R. R., Hebel, D. V., Ondrusek, M., Winn, C. D. & Karl, D. M. (1993) Limnol. Oceanogr. 38 1420–1437. [Google Scholar]
- 14.Gin, K. Y. H., Guo, J. & Cheong, H.-F. (1998) Ecol. Model. 112 53–72. [Google Scholar]
- 15.Carney, H. J., Richerson, P. J., Goldman, C. R. & Richards, R. C. (1988) Ecology 69 664–678. [Google Scholar]
- 16.Reynolds, C. S. (1997) Vegetation Processes in the Pelagic: A Model for Ecosystem Theory (Ecology Institute, Oldendorf/Luhe, Germany), pp. 167–171.
- 17.Klausmeier, C. A. & Litchman, E. (2001) Limnol. Oceanogr. 46 1998–2007. [Google Scholar]
- 18.Partensky, F., Blanchot, J., Lantoine, F., Neveux, J. & Marie, D. (1996) Deep Sea Res. 43 1191–1213. [Google Scholar]
- 19.Tilman, D. (1982) Resource Competition and Community Structure (Princeton Univ. Press, Princeton). [PubMed]
- 20.Fee, E. J. (1976) Limnol. Oceanogr. 21 767–783. [Google Scholar]
- 21.Arenovski, A. L., Lim, E. L. & Caron, D. A. (1995) J. Plankton Res. 17 801–820. [Google Scholar]
- 22.Wollmann, K., Deneke, R., Nixdorf, B. & Packroff, G. (2000) Hydrobiologia 433 3–14. [Google Scholar]
- 23.Friese, K., Wendt-Potthoff, K., Zachmann, D. W., Fauville, A., Mayer, B. & Veizer, J. (1998) Water Air Soil Pollut. 108 231–247. [Google Scholar]
- 24.Koschorreck, M. & Tittel, J. (2002) Limnol. Oceanogr. 47 1197–1201. [Google Scholar]
- 25.Bissinger, V., Jander, J. & Tittel, J. (2000) Acta Hydrochim. Hydrobiol. 28 310–312. [Google Scholar]
- 26.Olrik, K. & Nauwerck, A. (1993) Hydrobiologia 249 15–24. [Google Scholar]
- 27.Zhang, X. & Watanabe, M. M. (2001) J. Phycol. 37 738–743. [Google Scholar]
- 28.Diehl, S. & Feissel, M. (2001) Ecology 82 2977–2983. [Google Scholar]
- 29.Koschorreck, M., Frömmichen, R., Herzsprung, P., Tittel., J. & Wendt-Potthoff, K. (2002) Water Air Soil Pollut. Focus 2 97–109. [Google Scholar]
- 30.Havskum, H. & Riemann, B. (1996) Mar. Ecol. Prog. Ser. 137 251–263. [Google Scholar]
- 31.Connell, J. H. (1961) Ecology 42 710–723. [Google Scholar]
- 32.Hylleberg, J. (1976) Oecologia 23 115–123. [DOI] [PubMed] [Google Scholar]
- 33.Sanders, R. W., Berninger, U.-G., Lim, E. L., Kemp, P. F. & Caron, D. A. (2000) Mar. Ecol. Prog. Ser. 192 103–118. [Google Scholar]
- 34.Havskum, H. & Hansen, A. S. (1997) Aquat. Microb. Ecol. 12 139–151. [Google Scholar]
- 35.Gieskes, W. W. & Kraay, G. W. (1986) Mar. Biol. 91 567–576. [Google Scholar]
- 36.Sherr, E. B., Sherr, B. F. & McDaniel, J. (1991) Mar. Ecol. Prog. Ser. 69 81–92. [Google Scholar]
- 37.Posch, T., Šimek, K., Vrba, J., Pernthaler, J., Nedoma, J. Sattler, B., Sonntag, B. & Psenner, R. (1999) Aquat. Microb. Ecol. 18 235–246. [Google Scholar]
- 38.Dolan, J. R. & Marrase, C. (1995) Deep Sea Res. 42 1965–1987. [Google Scholar]
- 39.Pitta, P., Giannakourou, A. & Christaki, U. (2001) Aquat. Microb. Ecol. 24 297–311. [Google Scholar]
- 40.Pitta, P. & Giannakourou, A. (2000) Mar. Ecol. Prog. Ser. 194 269–282. [Google Scholar]
- 41.Padisak, J., Krienitz, L., Koschel, R. & Nedoma, J. (1997) Eur. J. Phycol. 32 403–416. [Google Scholar]
- 42.Raven, J. A. (1995) in Chrysophyte Algae: Ecology, Phylogeny and Development, eds. Sandgren, C. D., Smol, J. & Kristiansen, J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 95–118.
- 43.MacArthur, R. H. & Connell, J. H. (1966) The Biology of Populations (Wiley, New York).
- 44.Dall, S. R. X. & Cuthill, I. C. (1997) Oikos 80 197–202. [Google Scholar]
- 45.Perlman, S. J. & Jaenike, J. (2001) Ecol. Lett. 4 577–584. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.