Abstract
Two gene-targeted immunoglobulin heavy chain transgenic mouse strains, TgH(KL25) and TgH(VI10), expressing neutralizing specificities for lymphocytic choriomeningitis virus and vesicular stomatitis virus, respectively, have been generated. Three days after lymphocytic choriomeningitis virus infection, TgH(KL25) mice showed a thymus-independent neutralizing IgM response followed by thymus-dependent (TD) IgG. In contrast, WT mice mounted only a TD IgG response around day 80. These observations indicated that not only structural properties of the virus but also immunological parameters such as the frequency of B cells were indicative for the induction of thymus-independent versus TD Ig responses. Naïve vesicular stomatitis virusspecific Ig heavy chain transgenic mice displayed greatly elevated natural antibody titers. However, despite these high naïve titers, de novo activation of naïve CD4+ T and B cells was not blocked. Therefore, B cells giving rise to natural antibodies do not participate in virus-induced antibody responses.
Lymphocytic choriomeningitis virus (LCMV) and vesicular stomatitis virus (VSV) are widely used viral models for studying antiviral immune responses in mice. Whereas LCMV is mainly cleared by the action of cytotoxic T lymphocytes (CTL) in a perforin-dependent manner (1–3), control of VSV strongly depends on natural or early induced antibodies (4, 5). CTL are dispensable for survival of a VSV infection (6).
The humoral responses elicited by LCMV and VSV in C57BL/6 mice display fundamental differences. VSV is a potent thymus-independent (TI) inducer of neutralizing IgM antibodies. These early neutralizing antibodies are decisive for the survival of the infected animals. Between days 6 and 8, isotype class-switched and affinity-matured antibodies become detectable and remain elevated lifelong. In contrast, neutralizing antibodies against LCMV develop late after infection, usually between days 30 and 100. Although antibodies are dispensable for early virus clearance, if adoptively transferred, they can reduce the initial viral load and thus prevent CTL exhaustion after high-dose infection with LCMV. Importantly, neutralizing antibodies are crucial for the long-term control of LCMV (7–10).
Previously, comprehensive analysis of humoral antiviral immunity against LCMV or VSV has been impaired by the limited differentiation capacities of IgM transgenic B cells (11, 12). Gene targeting approaches that introduced rearranged VHDJH regions 5′ of the intron enhancer at the physiological correct position resulted in the generation of Ig heavy chain (IgH) transgenic mice with normal B cell differentiation capacities, normal isotype switch, and normal hypermutation rates. Such transgenic mice with various specificities have been generated and successfully used to study allelic exclusion (13), receptor editing (14–18), B cell tolerance (19, 20), autoimmunity (21–23), allergy (24), and anergy (20).
Here, we present two gene-targeted mouse strains expressing the IgH variable regions of virus-neutralizing antibodies to analyze the role of specific B cell frequencies and protective humoral immunity against virus infections.
Materials and Methods
mAbs. KL25 is an LCMV-WE, and VI10 is a VSV serotype Indiana (IND) neutralizing mAb, described in refs. 25 and 26, respectively. mAbs were purified from culture supernatant by affinity chromatography (Protein G Sepharose 4 Fast Flow, Amersham Pharmacia Biosciences) and directly labeled with FITC (Fluka), tetramethylrhodamine isothiocyanate (Fluka), or NHS-LC-Biotin (Pierce).
Generation of Gene-Targeted Mice. A detailed description of the generation of the gene-targeted mice can be found in Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org.
Generation of Anti-Idiotypic Antibodies to KL25 and VI10. Two milligrams of an iso- and allotype-matched unrelated mAb was injected i.v. to tolerize Browns Norway rats against murine Ig. After initial immunization with 300 μg of KL25 or VI10 in complete Freund's adjuvant s.c, rats received two to three booster immunizations (200–300 μg in incomplete Freund's adjuvant s.c.) at 2- to 3-week intervals. Final immunization was performed 1 month after the last booster immunization on day –4 by 200 μg antibody in incomplete Freund's adjuvant i.p. and on day –3 by 200 μg antibody in PBS i.v. Fusion to the mouse myeloma cell line X63AG8.653 was performed according to standard procedures (27). Positive clones were identified by flow cytometry on HL25 splenocytes (11) or ELISA on VI10-coated plates.
Mice and Viruses. C57BL/6, HL25 (11), and YEN mice were obtained from the Institute of Laboratory Animal Science, University of Zurich. Once generated, heterozygous TgH(KL25) and TgH(VI10) mice were housed at the Institute of Laboratory Animal Science. All mice were kept under specific pathogen-free conditions. Depletion of CD4+ T cell was performed by two i.p. injections of 1–2.5 mg of the rat–anti-mouse antibody YTS191.1.2 (28) at days –3 and –1. Absence of CD4+ T cells was verified by flow cytometry on the day of infection.
LCMV-WE was originally provided by F. Lehmann-Grube (University of Hamburg, Hamburg, Germany) and propagated on L-929 cells, and VSV IND (Mudd-Summers isolate) was originally obtained from D. Kolakofsky (University of Geneva, Geneva) and propagated on BHK-21 cells.
Immunohistology. Standard procedures (29) were used with the following modifications. For the detection of LCMV-specific B cells, cryosections of spleens of infected mice were blocked in PBS containing 10% FCS, 10% rat serum, and 10 μg/ml rat anti-FcγRI/II (2.4G2). Subsequently, anti-KL25 mAb (10 μg/ml IIIC4.8-FITC, 2 μg/ml B2.5-tetramethylrhodamine isothiocyanate) was added in PBS containing 10% FCS. After washing in PBS, sections were mounted by using DAKO fluorescent mounting medium (DakoCytomation, Glostrup, Denmark) containing anti-bleach and examined under a fluorescence microscope. For overlay, digital pictures were imported into photoshop (Adobe Systems, San Jose, CA), converted to false colors, and adjusted in brightness and contrast and overlaid.
Flow Cytometry. Flow cytometric analysis was performed on a FACScan, and all antibodies used were obtained from BD Biosciences (San Diego, CA) unless otherwise indicated.
Virus Neutralization Assay. Standard procedures (30–32) were used.
Results
Generation of Gene-Targeted Mouse Strains Expressing the IgH of Either KL25 or VI10. Homologous recombination was used to replace the endogenous DQ52 and all four JH elements of the IgH locus in C57BL/6-derived embryonic stem cells by rearranged VHDJH regions and their genuine promoters (Fig. 5 and ref. 33). For the targeting, the VHDJH regions of two different virus neutralizing antibodies were used, i.e., KL25 (25), an LCMV-neutralizing antibody, and VSV-IND-neutralizing antibody VI10 (26). The resulting gene-targeted mouse strains Igh-Jtm1(VDJ-KL25)zbz and Igh-Jtm1(VDJ-VI10)zbz are referred to as TgH(KL25) and TgH(VI10), respectively.
Generation of mAbs Against the Variable Regions of KL25 and VI10 and Enumeration of B Cells Expressing the Transgenic Heavy Chain. To identify B cells expressing the transgenic VHDJH regions in TgH(KL25) and TgH(VI10) mice, anti-KL25 and anti-VI10 mAbs were generated. The rat mAb IIIC4.8 specifically bound the VH of KL25, and flow cytometric analysis of TgH(KL25)-derived blood cells revealed that >95% of all B cells expressed the transgenic IgH (Fig. 1A). IIIC4.8 was able to inhibit LCMV-WE neutralization by KL25 (data not shown); however, IIIC4.8 binding was not indicative for neutralizing capacity because IIIC4.8 also bound various mAbs isolated from naïve TgH(KL25) mice that did not neutralize LCMV-WE (Fig. 1B).
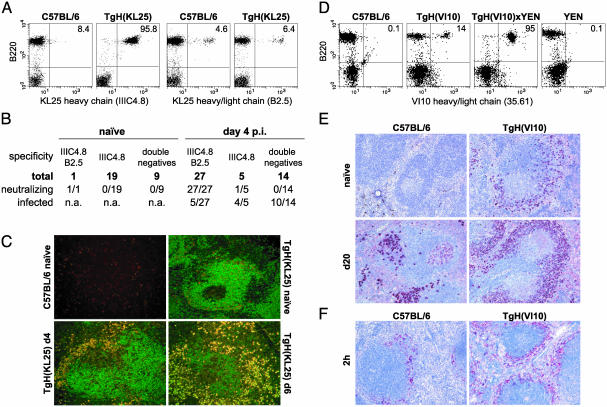
Fig. 1.
Idiotypic and histological analysis of TgH(KL25) and TgH(VI10) mice. (A) Expression of the KL25 heavy chain in TgH(KL25) mice. Peripheral blood lymphocytes from TgH(KL25) and C57BL/6 mice were stained with anti-B220 in combination with either IIIC4.8 (anti-KL25H) or B2.5 (anti-KL25HL). The percentage of B cells expressing the transgenic heavy chain or heavy/light chain combination is indicated in the corresponding quadrant. Data shown are representative of at least three experiments. (B) Analysis of hybridoma prepared from naïve and LCMV-infected TgH(KL25) mice. Spleen cells from naïve and day 4-infected mice (2 × 106 pfu of LCMV-WE i.v.) were fused to the myeloma X63Ag8.653 and subjected to hypoxanthine/aminopterin/thymidine selection. Surviving clones were expanded, and supernatants were analyzed by ELISA for the presence of the KL25 heavy chain (IIIC4.8) or the KL25 heavy/light chain combination (B2.5). In addition, supernatants were analyzed for LCMV-WE neutralizing activity and the presence of infectious virus. Supernatants referred to as double negative did not bind to either of the anti-KL25 mAbs and might also include Ig-nonproducing clones. (C) Localization of LCMV-specific B cells in spleen during infection with 2 × 106 pfu of LCMV-WE. Cryosections were stained with anti-KL25H-FITC (IIIC4.8, green) and anti-KL25HL-tetramethylrhodamine isothiocyanate (B2.5, red). Digital photos were taken and electronically overlaid. (D) Expression of the VI10 heavy chain in TgH(VI10) mice. Because no mAb was available directed against the heavy chain of VI10, the expression level of the VI10 heavy chain was assessed indirectly with mAb 35.61 specific for the VI10 heavy and light chain combination. This was achieved by breeding TgH(VI10) mice to the VI10-light chain expressing transgenic mouse strain YEN. Peripheral blood lymphocytes from double transgenic, the parental single transgenics, and WT mice were stained with mAb 35.61 in combination with anti-B220 and analyzed by flow cytometry. The percentage of B cells expressing heavy and light VI10-Ig chains are indicated. Data shown are representative of at least three experiments. (E) Splenic localization of VSV-specific B cells. C57BL/6 and TgH(VI10) mice were immunized with 2 × 106 pfu of VSV-IND. Spleen sections from infected (d20) and naïve mice were stained for VSV-specific B cells (red) as described in Materials and Methods. (F) Localization of VSV antigen 2 h after 109 pfu of VSV-IND i.v. infection. Spleen sections were stained for VSV (red) as described in Materials and Methods.
The second mAb B2.5 specifically bound to a determinant provided by the combination of the VH and Vκ regions of KL25. Flow cytometric (Fig. 1 A) and immunohistochemical analysis (Fig. 1C) of naïve TgH(KL25) mice revealed 5–10% B2.5-positive B cells in naïve TgH(KL25) mice. Interestingly, B2.5 also bound 2–7% of naïve WT B cells (Fig. 1 A), suggesting some cross-reactivity. Binding of B2.5 did not inhibit LCMV neutralization by KL25, indicating that the binding site of B2.5 is located distal from the antigen binding site of KL25. Twenty eight of 29 neutralizing antibodies prepared from naïve or day 4-infected TgH(KL25) mice were ELISA positive for both IIIC4.8 and B2.5 (Fig. 1B).
Cryosections of spleens derived from C57BL/6 and TgH(KL25) mice were analyzed by fluorescence microscopy (Fig. 1C). Naïve C57BL/6 mice displayed only a few B2.5 single-positive cells. In contrast, most of the B cells in naïve TgH(KL25) mice were single positive for IIIC4.8 with only a minor fraction of naïve splenocytes staining double positive for IIIC4.8 and B2.5 on their surface (Fig. 1C). Upon infection, double-positive B cells expanded mostly in the border region between the red and white pulp of TgH(KL25) mice. At day 6, most B cells displayed a plasma cell phenotype and were positive for both intracellular IIIC4.8 and B2.5, whereas hardly any IIIC4.8 single-positive plasma cells were observed (Fig. 1C). Thus, in TgH(KL25) mice, KL25 heavy chain transgenic B cells can be identified by IIIC4.8. However, to identify LCMV-neutralizing B cells, both IIIC4.8 and B2.5 have to bind to the expressed Ig.
The mAb 35.61 raised against VI10 recognized a combinatorial determinant provided by VH and Vκ of VI10. Binding of 35.61 inhibited VSV neutralization by VI10 (data not shown). Analysis of B cells expressing the idiotypic determinant 35.61 revealed that 10–15% of TgH(VI10)-derived B cells expressed the VH and Vκ of VI10 (Fig. 1D). In contrast, (TgH(VI10)×YEN) double transgenic mice expressing both VH and Vκ of VI10 showed >90% 35.61-positive cells (Fig. 1D), whereas only negligible numbers of B cells from WT mice or single VI10 light chain transgenic YEN mice were positive (Fig. 1D).
Localization of VSV-specific B cells in WT and TgH(VI10) mice was assessed by immunohistochemistry using VSV particles in combination with anti-VSV reagents (29). As depicted in Fig. 1E, no VSV-specific B cells could be detected in the spleens of naive WT mice, which is in contrast to naïve TgH(VI10) mice where a considerable population of VSV-binding B cells was found in the marginal zone. After infection, VSV-specific B cells were distributed throughout the splenic red pulp and in junctional areas of WT mice. In contrast, VSV-specific B cells almost exclusively accumulated in the marginal zone of TgH(VI10) mice (Fig. 1E) where an increased amount of VSV antigen was also found (Fig. 1F). It is noteworthy to mention that an enlarged marginal zone B cell compartment was already detectable in naïve TgH(VI10) mice (Fig. 6, which is published as supporting information on the PNAS web site). However, this appears to be a common feature of Ig-transgenic mice and has been attributed to a bottleneck during B cell development (34).
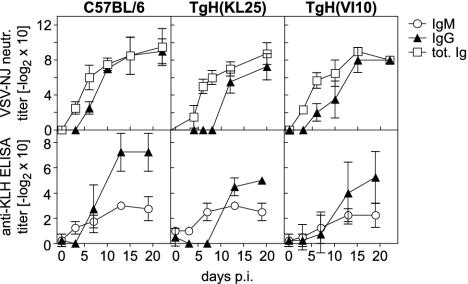
Although the vast majority of all B cells in our gene-targeted mice express the same heavy chain specificity, both TgH(KL25) and TgH(VI10) mice were found capable of mounting humoral responses 2–4 days delayed against other viruses and protein antigen (Fig. 2).
Fig. 2.
Antibody response against other antigens. The neutralizing antibody response after infection with 2 × 106 pfu of VSV serotype New Jersey was determined for TgH(KL25), TgH(VI10), and WT mice (Upper). The anti-keyhole limpet hemocyanin (KLH) antibody response was monitored after i.p. immunizations with 100 μgofKLH in alum on days 0 and 7 (Lower). The data shown represent the mean ± SD of at least two values.
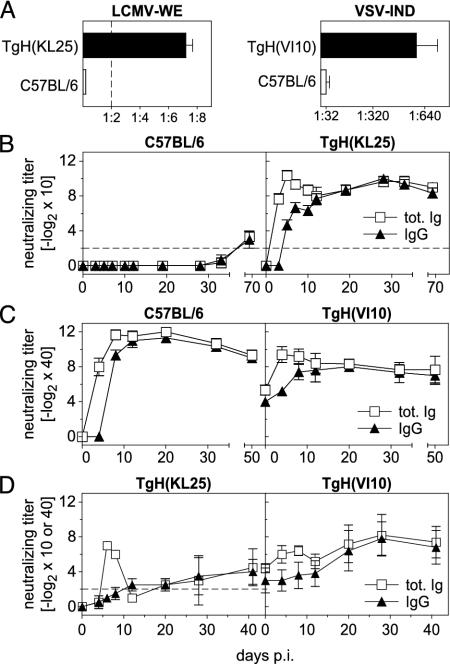
Elevated Preimmune Neutralizing Antibody Titers in TgH(KL25) and TgH(VI10) Mice. Unlike WT mice, naïve TgH(KL25) mice displayed low, but distinct, preimmune neutralizing antibody titers against LCMV-WE (Fig. 3A). After LCMV infection, a dramatic, but short-lived, neutralizing IgM response was induced in TgH(KL25) mice. Reduction-resistant, and thus isotype class-switched neutralizing antibodies (30), could be detected at day 4 postinfection with 2 × 106 plaque-forming units (pfu) of LCMV-WE. After day 12, serum-neutralizing activity was almost exclusively contributed by isotype class-switched antibodies. A temporary decrease in neutralizing activity of the sera was observed between days 8 and 20, which reflects the rapid decay of the IgM response before neutralizing IgG titers reached maximal levels (Fig. 3B and data not shown).
Fig. 3.
Analysis of the virus-neutralizing antibody response in TgH(KL25) and TgH(VI10) mice. (A) Total Ig-neutralizing titers were determined in serum from naïve C57BL/6, TgH(KL25), and TgH(VI10) mice. The detection limit of the assay is indicated by a dashed line. Note the different scales for LCMV and VSV. (B) C57BL/6 and TgH(KL25) mice were infected i.v. with 2 × 106 pfu of LCMV-WE, serum samples were collected at the time points indicated, and total Ig- and IgG-neutralizing titers were determined. The IgG detection limit of the assay is indicated by a dashed line. (C) C57BL/6 and TgH(VI10) mice were infected i.v. with 2 × 106 pfu of VSV-IND. Total Ig- and IgG-neutralizing titers were determined in the serum. (D)(Left) CD4-depleted TgH(KL25) mice were infected with 2 × 106 pfu of LCMV-WE, and virus-neutralizing antibodies were determined from 10-fold prediluted sera. (Right) Neutralizing antibody response elicited in CD4-depleted TgH(VI10) mice after infection with 2 × 106 pfu of VSV. Sera were 40-fold prediluted. In A, data represent the mean ± SD of five mice from one experiment. The data shown in B and C display the mean ± SD of three mice and represent one of three similar experiments. Data shown in D represent the mean ± SD of at least two animals with one of two experiments shown.
In contrast to TgH(KL25), TgH(VI10) mice displayed substantial preimmune VSV-neutralizing antibodies that consisted of equal quantities of IgM and IgG antibodies (Fig. 3 A and C). Despite these preexisting neutralizing titers, TgH(VI10) mice mounted a significant humoral response after infection with 2 × 106 pfu of VSV-IND. This response reached maximal levels ≈4 days earlier in TgH(VI10) mice compared with WT animals (Fig. 3C). However, the maximal titers measured in TgH(VI10) mice were typically two to four times lower compared with those observed in WT animals (Fig. 3C). This reduction might be explained by the possibility that preexisting antibodies neutralized the initial inoculum (see below), resulting in a lowered antigenic load. This notion is supported by the observation that TgH(VI10) mice infected with higher doses of virus also displayed higher maximal antibody titers (data not shown). With time, neutralizing titers of TgH(VI10) mice reached levels found in WT mice and remained elevated over preimmune level for at least 150 days (data not shown).
Theoretically, the increase in neutralizing titers observed in TgH(VI10) mice upon infection could also represent a secondary response of the same B and T cells that are responsible for production of the preimmune titers. In TgH(VI10) mice no augmentation of isotype class-switched neutralizing Abs was observed before 6 days after infection (Fig. 3C). However, in primed WT mice, IgM and IgG titers increased almost simultaneously (35, 36). Therefore, the observed delay in isotype class switching in TgH(VI10) mice provided evidence that the additional neutralizing Abs are produced by de novo-activated B cells that were helped by de novo-activated T cells. Conversely, T or B cells responsible for the production of preimmune IgG titers did not appear to be involved in newly induced antiviral responses.
Taken together, our results demonstrated that the introduction of the variable heavy chain derived from an LCMV-neutralizing antibody is sufficient to overcome the inability of WT mice to mount an early neutralizing antibody response against LCMV. Furthermore, experiments in TgH(VI10) mice indicate that high levels of preexisting antibodies are able to influence the extent of an immune response against VSV-IND, but they are not capable of preventing priming of naïve T helper and B cells. Moreover, our data provide evidence that B or T cells responsible for the production of preimmune antibodies of the same specificity are probably not participating in antiviral antibody responses in TgH(VI10) mice.
Induction of Neutralizing Antibodies in the Absence of CD4+ T Cells. Neutralizing antibodies against LCMV are considered to be strictly thymus dependent (37). To investigate to which extent early neutralizing IgM antibodies in TgH(KL25) mice require T help, CD4-depleted TgH(KL25) mice were infected with 2 × 106 pfu of LCMV-WE. A transient neutralizing IgM response was observed between days 4 and 12 (Fig. 3D), providing evidence that production of neutralizing IgM in TgH(KL25) mice is largely independent of T help. Isotype class-switched neutralizing antibodies appeared in CD4-depleted mice between days 12 and 30, which correlated with recovery of the CD4+ T cell compartment (Fig.7A, which is published as supporting information on the PNAS web site). Interestingly, these results suggest that structural features of LCMV-GP on the viral surface fulfill the requirements for TI B cell induction (i.e., repetitiveness, spacing, and rigidity of the antigen; ref. 38).
Although it is well established that in WT mice the IgM response against VSV is TI (39), only little is known about the influence of preexisting antibodies on TI production of neutralizing antibodies. Thus, TgH(VI10) mice were depleted of CD4+ T cells and infected with 2 × 106 pfu of VSV-IND. As depicted in Fig. 3D, TgH(VI10) mice increased their preexisting IgM titer above preimmune levels independently of CD4+ T cells between days 4 and 8. As in TgH(KL25) mice, these responses were transient and titers dropped to preimmune levels by day 12. When compared with CD4-depleted WT mice, the kinetics and the absolute magnitude of the IgM response in CD4-depleted TgH(VI10) mice were similar. The IgG antibody titer in VSV-infected TgH(VI10) mice remained constant during the time of CD4+ T cell depletion (Fig. 3D). With the reappearance of CD4+ T cells (days 12–28; Fig. 7A) IgG titers started to rise in both TgH(VI10) and WT mice. Thus, VSV was capable of eliciting a TI antibody response. Moreover, despite the drastically reduced amounts of VSV-GP antigen and viral replication (see below), sufficient antigen was apparently preserved in secondary lymphoid organs to allow for induction of T help and isotype class switch >20 days after infection.
Accelerated Virus Clearance by Neutralizing Antibodies in IgH-Targeted Transgenic Mice. Because in both Ig gene-targeted mice substantial titers of neutralizing antibodies were detected before and/or after infection, an altered viral dissemination had to be expected. Because this finding also implied an altered antigen distribution, viral titers in different organs and blood of transgenic animals were analyzed and compared with WT animals.
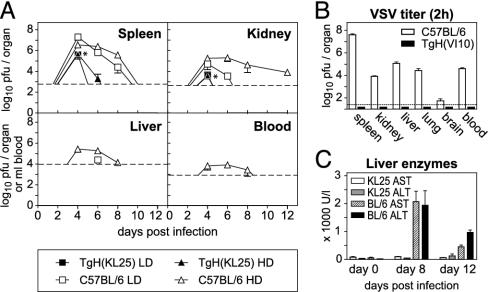
After LCMV-WE infection, TgH(KL25) mice displayed maximal virus titers that were between 10- and 1,000-fold lower than those of C57BL/6 control animals (Fig. 4A). After day 6, viral titers in TgH(KL25) mice were below the detection level in the blood and in all organs analyzed. In contrast, WT mice still exhibited virus up to day 12, depending on the size of the inoculum (Fig. 4A). Although in the experiment shown no blood viremia was observed, TgH(KL25) mice occasionally displayed detectable virus titers in blood (1,000–10,000 pfu/ml) between days 4 and 6 after high-dose infection.
Fig. 4.
Impact of preexisting or early inducible neutralizing antibodies on virus dissemination and liver pathology. (A) Clearance of LCMV-WE from spleen, kidney, liver, and blood of TgH(KL25) and C57BL/6 mice after i.v. infection with a high (2 × 106 pfu; HD) or a low (200 pfu; LD) dose of LCMV-WE. The detection limit for each organ is indicated by a dashed line, and * indicates data points where a filled triangle is covered by a filled box. Data represent mean ± SD of three animals with one of two experiments shown. (B) Viral titers in spleen, kidney, liver, lung, brain, and blood of TgH(VI10) and C57BL/6 mice determined 2 h after infection with 109 pfu of VSV-IND i.v. Data represent the mean ± SD of at least five animals with one of two experiments shown. (C) Comparison of the liver pathology in KL25 transgenic and WT mice. Activity of ALT and AST was determined in the serum of mice infected with 2 × 106 pfu of LCMV-WE. Data represent the mean ± SD of three animals with one of two experiments shown.
To analyze the influence of preexisting neutralizing antibody titers on VSV clearance, TgH(VI10) mice were infected i.v. with a very high virus dose (109 pfu) of VSV-IND (in comparison: LD50 of VSV-IND in C57BL/6 mice is 2–5 × 107 pfu; ref. 40). Virus titers in organs were determined 2 h later. Control mice displayed high virus titers in all organs analyzed, but TgH(VI10) mice were free of virus (Fig. 4B). Apparently, the VSV-specific preimmune titer in TgH(VI10) mice (Fig. 3 A and C) completely neutralized the initial inoculum in all organs tested. As mixing of spleen homogenates from infected TgH(VI10) mice with spleen homogenates from infected C57BL/6 mice did not decrease the viral titer in the C57BL/6 homogenates more than it would be expected from the dilution, in vitro neutralization by antibodies discharged from the tissue of VI10 mice can be excluded (Fig. 7B).
Interestingly, 2 h after infection with 109 pfu of VSV-IND more viral antigen was detectable histologically in splenic sections of TgH(VI10) compared with control mice (Fig. 1F). VSV antigens were located mainly in the marginal zone where the VSV-specific B cells were also detected (Fig. 1F). Thus, preexisting antibodies appear to completely neutralize infectivity of the inoculated VSV and trap the immune complexes in the marginal zone (4, 41).
Enhanced Disease or Protection from Immunopathology in TgH(KL25) Mice. To evaluate to which extent the accelerated antibody response influenced LCMV-induced T cell-mediated immunopathology, two different disease models were investigated, i.e., aggressive hepatitis and possible immune suppression of neutralizing B cells.
First, CTL-mediated liver pathology was assessed by monitoring serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In contrast to WT mice, which display drastically increased serum levels of AST and ALT upon infection with 2 × 106 pfu of LCMV-WE, the serum levels of the same liver enzymes remained normal throughout the infection of TgH(KL25) mice (Fig. 4C). Taking into consideration that high dose-infected TgH(KL25) mice displayed higher CTL activities (Fig. 8, which is published as supporting information on the PNAS web site), it can be concluded that early inducible neutralizing antibodies protected from immunopathology by reducing hematogenic viral spread to the liver.
The second model of LCMV-induced immunopathology tested was T cell-mediated immunosuppression of LCMV-neutralizing B cell responses. It has been speculated that neutralizing B cells may be preferentially infected by LCMV and thus become a target for antiviral CTL (9). To evaluate whether this applied to B cells derived from TgH(KL25) mice, supernatants of hybridomas prepared from day 4-infected TgH(KL25) mice were tested for the presence of LCMV. As shown in Fig. 1B, no preferential infection of transgenic neutralizing B cell hybridoma was observed. Because selection by mAbs leads to rapid outgrowth of neutralization-resistant viruses in vitro (42), it seems unlikely that virus was undetectable because of the presence of excess LCMV-neutralizing antibodies in the supernatant of neutralizing hybridomas. However, this possibility cannot be completely excluded based on these data.
Discussion
The Ig-transgenic mouse strains presented here permitted studies of protective neutralizing antibody responses and have revealed several interesting findings. First, the structural features of the LCMV glycoprotein comply with the requirements of thymus-independent antibody induction (i.e., rigidity and spacing). Thus, LCMV is highly immunogenic for neutralizing B cells. Second, VSV particles captured as noninfectious form by antibodies are sufficient to induce antiviral CD4+ T cells and B cells as long as 10–20 days after infection. Third, as for influenza virus, we confirm that the B cells responsible for the production of natural antibodies do not appear to participate in antigen-driven immune responses against VSV.
Analysis of gene-targeted mouse lines expressing the variable regions of virus-neutralizing antibodies revealed that whereas TgH(KL25) mice displayed only slightly elevated preimmune neutralizing titers, naïve TgH(VI10) mice expressed high preexisting titers. To explain this difference it could be argued that the KL25 heavy chain shows neutralizing activity only if paired with a very restricted set of endogenous light chains. In contrast, in TgH(VI10) mice a rather broad panel of light chains combined with the VI10 heavy chain could provide VSV-neutralizing activity. However, because frequencies of virus-specific B cells were found to be comparable in both TgH(KL25) and TgH(VI10) mice (5–10% and 10–20%, respectively), this explanation is not completely satisfactory. A better explanation could be that the different spontaneous levels of neutralizing antibodies in naïve TgH(KL25) and TgH(VI10) mice might reflect a potential cross-reactivity of VI10-BCRs to autoantigens or commensal bacteria, which would be absent for KL25-BCRs.
The impact of preimmune and/or rapidly inducible neutralizing antibodies on viral pathogenesis was distinct in both models. Preimmune antibodies in TgH(VI10) mice prevented VSV infection of the animal up to ≈106 pfu and interfered with the priming of CTL (Fig. 8 and ref. 43). However, our results provide evidence that primary antibodies and CD4+ T cell responses against VSV could be induced even in the presence of excess Ig of the same specificity. Moreover, because neutralizing antibody responses in TgH(VI10) mice displayed the kinetics of a primary response, we concluded that B cells responsible for the preimmune antibody titers apparently did not participate in virus-induced Ig responses. This notion is in accordance with previous findings using influenza virus where it has been shown that although B-1 B cells are mainly responsible for the production of natural IgM, they did not participate in antiviral responses (44, 45). However, using adoptive transfer experiments, we failed to demonstrate that B-1 B cells were the main source of preimmune titers in TgH(VI10) mice (data not shown).
In TgH(KL25) mice and in contrast to WT mice, we observed a rapid induction of neutralizing antibodies within the first 3 days after infection. Thus, the increased precursor frequency of LCMV-neutralizing B cells enabled TgH(KL25) mice to mount an early neutralizing antibody response. Alternatively, it has been speculated that many specific neutralizing B cells may be destroyed initially by virus-specific CD8+ T cells (46). However, this hypothesis is not readily compatible with the findings presented here where preferential infection of LCMV-neutralizing B cells was not found in TgH(KL25) mice.
Although definitive conclusions cannot be drawn, it seems as if LCMV indeed takes advantage of a weak spot in either the B cell repertoire (i.e., low frequencies in WT mice) or in mechanisms of B cell induction to establish a persistent infection.
The early inducible antibodies observed in TgH(KL25) could neither prevent a systemic infection with low doses of LCMV-WE nor induction of CTL, but they drastically reduced the maximal viral load. As a consequence, KL25 gene-targeted mice cleared the virus more readily, remained free of liver immunopathology, and obviated the exhaustion of virus-specific CTL upon infection with a high dose of virus (Fig. 8). Because a systemic infection with LCMV was established despite the early and high neutralizing activity of serum, priming of virus-specific CTL did not appear to be substantially impaired in TgH(KL25) mice.
Interestingly, we found that LCMV-WE was able to elicit a TI B cell response in TgH(KL25) mice that is comparable to the response of WT mice against VSV. Two conclusions can be drawn from this observation: first, the LCMV-GP on the surface of virions must be presented as an organized rigid structure of 5- to 10-nm-spaced epitopes (38, 47). Second, sufficient antigen must reach the secondary lymphoid organs to induce a TI antibody response (41). Although the efficient spreading of LCMV to spleen and lymph nodes is well documented, the rigidity of the multimeric GP spikes on the virion surface is poorly investigated. However, results obtained by other groups speak against a T independence of LCMV-GP, because CD4-depleted mice fail to mount a neutralizing antibody response (37). Thus, the presented data suggest again that sufficient numbers of specific B cells must be present to permit detectable TI antibody responses. The results obtained for the TgH(VI10) strain confirm previous results that VSV is a potent TI B cell activator. Even in the presence of high neutralizing preimmune titers, TI activation of B cells was observed.
The models presented here take advantage of gene-targeted antibody transgenic mice to study protective antibody-dependent antiviral immunity. They should help to evaluate factors that determine levels of preimmune antibodies and importantly also enable investigation of activation processes and localizations of neutralizing antibody producing B cells during virus infections that lead to recovery from disease.
Supplementary Material
Acknowledgments
We thank Prof. Klaus Rajewsky for providing us with the construct piVhL2neor, Therese Uhr for technical assistance, Diana Cham at the Institute of Laboratory Animal Science and Lenka Vlk at the Institute of Clinical Pathology for excellent technical expertise, and Dr. Kathy McCoy and Dr. Nicola Harris for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation and the Kanton of Zurich.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTL, cytotoxic T lymphocytes; IgH, Ig heavy chain; IND, serotype Indiana; LCMV, lymphocytic choriomeningitis virus; pfu, plaque-forming units; TI, thymus independent; VSV, vesicular stomatitis virus.
References
- 1.Cole, G. A., Nathanson, N. & Prendergast, R. A. (1972) Nature 238 335–337. [DOI] [PubMed] [Google Scholar]
- 2.Moskophidis, D., Cobbold, S. P., Waldmann, H. & Lehmann-Grube, F. (1987) J. Virol. 61 1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kagi, D., Ledermann, B., Burki, K., Seiler, P., Odermatt, B., Olsen, K. J., Podack, E. R., Zinkernagel, R. M. & Hengartner, H. (1994) Nature 369 31–37. [DOI] [PubMed] [Google Scholar]
- 4.Ochsenbein, A. F., Fehr, T., Lutz, C., Suter, M., Brombacher, F., Hengartner, H. & Zinkernagel, R. M. (1999) Science 286 2156–2159. [DOI] [PubMed] [Google Scholar]
- 5.Gobet, R., Cerny, A., Ruedi, E., Hengartner, H. & Zinkernagel, R. M. (1988) Exp. Cell Biol. 56 175–180. [DOI] [PubMed] [Google Scholar]
- 6.Leist, T. P., Cobbold, S. P., Waldmann, H., Aguet, M. & Zinkernagel, R. M. (1987) J. Immunol. 138 2278–2281. [PubMed] [Google Scholar]
- 7.Ciurea, A., Klenerman, P., Hunziker, L., Horvath, E., Senn, B. M., Ochsenbein, A. F., Hengartner, H. & Zinkernagel, R. M. (2000) Proc. Natl. Acad. Sci. USA 97 2749–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerny, A., Sutter, S., Bazin, H., Hengartner, H. & Zinkernagel, R. M. (1988) J. Virol. 62 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Planz, O., Ehl, S., Furrer, E., Horvath, E., Brundler, M. A., Hengartner, H. & Zinkernagel, R. M. (1997) Proc. Natl. Acad. Sci. USA 94 6874–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomsen, A. R., Johansen, J., Marker, O. & Christensen, J. P. (1996) J. Immunol. 157 3074–3080. [PubMed] [Google Scholar]
- 11.Seiler, P., Kalinke, U., Rulicke, T., Bucher, E. M., Bose, C., Zinkernagel, R. M. & Hengartner, H. (1998) J. Virol. 72 2253–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senn, B. M., López-Macías, C., Kalinke, U., Lamarre, A., Isibasi, A., Zinkernagel, R. M. & Hengartner, H. (2003) Eur. J. Immunol. 33 950–961. [DOI] [PubMed] [Google Scholar]
- 13.Sonoda, E., Pewzner-Jung, Y., Schwers, S., Taki, S., Jung, S., Eilat, D. & Rajewsky, K. (1997) Immunity 6 225–233. [DOI] [PubMed] [Google Scholar]
- 14.Bertrand, F. E., Golub, R. & Wu, G. E. (1998) Eur. J. Immunol. 28 3362–3370. [DOI] [PubMed] [Google Scholar]
- 15.Cascalho, M., Ma, A., Lee, S., Masat, L. & Wabl, M. (1996) Science 272 1649–1652. [DOI] [PubMed] [Google Scholar]
- 16.Cascalho, M., Wong, J. & Wabl, M. (1997) J. Immunol. 159 5795–5801. [PubMed] [Google Scholar]
- 17.Chen, C., Nagy, Z., Prak, E. L. & Weigert, M. (1995) Immunity 3 747–755. [DOI] [PubMed] [Google Scholar]
- 18.Taki, S., Schwenk, F. & Rajewsky, K. (1995) Eur. J. Immunol. 25 1888–1896. [DOI] [PubMed] [Google Scholar]
- 19.Pelanda, R., Schwers, S., Sonoda, E., Torres, R. M., Nemazee, D. & Rajewsky, K. (1997) Immunity 7 765–775. [DOI] [PubMed] [Google Scholar]
- 20.Phan, T. G., Amesbury, M., Gardam, S., Crosbie, J., Hasbold, J., Hodgkin, P. D., Basten, A. & Brink, R. (2003) J. Exp. Med. 197 845–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedmann, D., Yachimovich, N., Mostoslavsky, G., Pewzner-Jung, Y., Ben-Yehuda, A., Rajewsky, K. & Eilat, D. (1999) J. Immunol. 162 4406–4416. [PubMed] [Google Scholar]
- 22.Pewzner-Jung, Y., Friedmann, D., Sonoda, E., Jung, S., Rajewsky, K. & Eilat, D. (1998) J. Immunol. 161 4634–4645. [PubMed] [Google Scholar]
- 23.Litzenburger, T., Fassler, R., Bauer, J., Lassmann, H., Linington, C., Wekerle, H. & Iglesias, A. (1998) J. Exp. Med. 188 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lafaille, M. A., Muriglan, S., Sunshine, M. J., Lei, Y., Kutchukhidze, N., Furtado, G. C., Wensky, A. K., Olivares-Villagomez, D. & Lafaille, J. J. (2001) J. Exp. Med. 194 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruns, M., Cihak, J., Muller, G. & Lehmann-Grube, F. (1983) Virology 130 247–251. [DOI] [PubMed] [Google Scholar]
- 26.Kalinke, U., Bucher, E. M., Ernst, B., Oxenius, A., Roost, H. P., Geley, S., Kofler, R., Zinkernagel, R. M. & Hengartner, H. (1996) Immunity 5 639–652. [DOI] [PubMed] [Google Scholar]
- 27.Roost, H. P., Bachmann, M. F., Haag, A., Kalinke, U., Pliska, V., Hengartner, H. & Zinkernagel, R. M. (1995) Proc. Natl. Acad. Sci. USA 92 1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cobbold, S. P., Jayasuriya, A., Nash, A., Prospero, T. D. & Waldmann, H. (1984) Nature 312 548–551. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann, M. F., Odermatt, B., Hengartner, H. & Zinkernagel, R. M. (1996) J. Exp. Med. 183 2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott, D. W. & Gershon, R. K. (1970) Clin. Exp. Immunol. 6 313–316. [PMC free article] [PubMed] [Google Scholar]
- 31.Charan, S. & Zinkernagel, R. M. (1986) J. Immunol. 136 3057–3061. [PubMed] [Google Scholar]
- 32.Battegay, M., Cooper, S., Althage, A., Banziger, J., Hengartner, H. & Zinkernagel, R. M. (1991) J. Virol. Methods 33 191–198. [DOI] [PubMed] [Google Scholar]
- 33.Taki, S., Meiering, M. & Rajewsky, K. (1993) Science 262 1268–1271. [DOI] [PubMed] [Google Scholar]
- 34.Martin, F. & Kearney, J. F. (2002) Nat. Rev. Immunol. 2 323–335. [DOI] [PubMed] [Google Scholar]
- 35.Roost, H. P., Charan, S. & Zinkernagel, R. M. (1990) Eur. J. Immunol. 20 2547–2554. [DOI] [PubMed] [Google Scholar]
- 36.Gupta, S. C., Hengartner, H. & Zinkernagel, R. M. (1986) Proc. Natl. Acad. Sci. USA 83 2604–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed, R., Butler, L. D. & Bhatti, L. (1988) J. Virol. 62 2102–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachmann, M. F., Rohrer, U. H., Kundig, T. M., Burki, K., Hengartner, H. & Zinkernagel, R. M. (1993) Science 262 1448–1451. [DOI] [PubMed] [Google Scholar]
- 39.Bachmann, M. F., Hengartner, H. & Zinkernagel, R. M. (1995) Eur. J. Immunol. 25 3445–3451. [DOI] [PubMed] [Google Scholar]
- 40.Ochsenbein, A. F., Pinschewer, D. D., Odermatt, B., Carroll, M. C., Hengartner, H. & Zinkernagel, R. M. (1999) J. Exp. Med. 190 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochsenbein, A. F., Pinschewer, D. D., Odermatt, B., Ciurea, A., Hengartner, H. & Zinkernagel, R. M. (2000) J. Immunol. 164 6296–6302. [DOI] [PubMed] [Google Scholar]
- 42.Seiler, P., Senn, B. M., Brundler, M. A., Zinkernagel, R. M., Hengartner, H. & Kalinke, U. (1999) J. Immunol. 162 4536–4541. [PubMed] [Google Scholar]
- 43.Seiler, P., Brundler, M. A., Zimmermann, C., Weibel, D., Bruns, M., Hengartner, H. & Zinkernagel, R. M. (1998) J. Exp. Med. 187 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumgarth, N., Herman, O. C., Jager, G. C., Brown, L. & Herzenberg, L. A. (1999) Proc. Natl. Acad. Sci. USA 96 2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumgarth, N., Herman, O. C., Jager, G. C., Brown, L. E., Herzenberg, L. A. & Chen, J. (2000) J. Exp. Med. 192 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Planz, O., Seiler, P., Hengartner, H. & Zinkernagel, R. M. (1996) Nature 382 726–729. [DOI] [PubMed] [Google Scholar]
- 47.Dintzis, H. M., Dintzis, R. Z. & Vogelstein, B. (1976) Proc. Natl. Acad. Sci. USA 73 3671–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.