Abstract
The coordinated regulation of chemokine responsiveness plays a critical role in the development of humoral immunity. After antigen challenge and B cell activation, the emerging plasma cells (PCs) undergo CXCL12-induced chemotaxis to the bone marrow, where they produce Ab and persist. Here we show that PCs, but not B cells or T cells from lupus-prone NZM mice, are deficient in CXCL12-induced migration. PC unresponsiveness to CXCL12 results in a marked accumulation of PCs in the spleen of mice, and a concordant decrease in bone marrow PCs. Unlike normal mice, in NZM mice, a majority of the splenic PCs are long-lived. This deficiency is a consequence of the genetic interactions of multiple systemic lupus erythematosus susceptibility loci.
Long-lived humoral immune responses are a hallmark of thymus-dependent (TD) immunity (1, 2). The cellular basis for enduring Ab-mediated immunity is that of long-lived memory B cells and plasma cells (PCs). Both of these cell populations acquire longevity as a result of antigen (Ag)-specific, cognate interactions with helper T cells within germinal centers (GCs). PC precursors that emerge from these GC reactions seed the bone marrow (BM) and terminally differentiate to endstage Ab-secreting cells. Recent studies (3–6) have shown that long-lived PCs predominantly reside in the BM and can produce Abs for months, or even years, after initial Ag exposure. The importance of long-lived PCs is underscored by the classic studies by Benner and colleagues (7, 8), who established that up to 80% of the serum Ig produced is derived from BM PCs.
The specific microenvironment occupied by PCs has been implicated as a determinant in cellular lifespan, survival, and, hence, the capacity to produce Ig for long periods of time (2, 9, 10). Migration to specific microenvironmental niches is controlled by chemokines and their receptors. It is known that chemokines control the coordinated migration of Ag-reactive B cells to precise anatomical locations within secondary lymphoid organs and between lymphoid compartments. Studies have firmly established that B cell homing to follicles, periarteriolar lymphoid sheath, and the red pulp in spleen is orchestrated by the action of specific chemokines and their receptors. B cell entry into the follicles depends on the expression of CXCR5 by B cells, and by binding its ligand, CXCL13, which is produced by follicular dendritic cells (11, 12). After activation within the GC, the expression of CXCR5 and the CCL19 receptor (presumably CCR7) is reduced, relieving the GC retention of activated B cells (13). Concordant with this reduced GC retention, the activity of CXCR4 is enhanced and facilitates the departure of activated B cells from the GC. CXCL12, the ligand for CXCR4, is produced by BM stromal cells and guides the recruitment of postGC B cells to the BM (13–15). Having reached the BM, this environment supports the terminal differentiation and longevity of PCs.
Long-lived, affinity-matured humoral immune responses are essential for enduring protection of the host from pathogens. However, the persistent production of pathogenic autoAbs by the host mediates the chronic, destructive clinical manifestations of Ab-mediated autoimmune diseases, like systemic lupus erythematosus (SLE) (16–19). This article addresses the behavior of postGC B cells and PCs in a murine model of SLE. Contrary to the behavior of normal long-lived PCs, we show here that PCs from NZM2410 mice do not migrate to the BM, and, as such, accumulate in the spleen. Unexpectedly, although these pathogenic PCs are restricted to the splenic environment, the vast majority of them are long-lived. Based on ex vivo migration studies, a loss in responsiveness of NZM PCs to CXCL12, is at least one mechanism responsible for the lack of BM homing. Furthermore, we report that defective CXCL12-induced migration is not a trait expressed by resting lymphocytes, but is an acquired deficiency manifested by postGC B cells in the NZM2410 strain. Whereas not all lupus-prone strains of mice express this deficiency, this deficiency may contribute to the aggressive nature of the disease found in the NZM2410 strain. Evidence that this trait does cosegregate with disease is provided by observations that loss in CXCL12 responsiveness is the result of the interactions between Sle1, Sle2, and Sle3 susceptibility loci.
Materials and Methods
Mice. Ten- to 12-week old NZM2410 (Taconic Farms), C57/BL6, MRLlpr/lpr, BXSB, New Zealand white (NZW), New Zealand black (NZB), and Rag1–/– (The Jackson Laboratory) mice were maintained in the specific pathogen-free animal facility at Dartmouth Medical School. C57/BL6 congenic strains carrying the Sle1, Sle2, Sle3, and Sle123 susceptibility loci were generated as described (20).
Immunization. Mice were challenged i.p. with 100 μg of 4-hydroxy-3-nitrophenylacetyl-keyhole limpet hemocyanin (NP36-KLH) (Biosearch Technologies, Novato, CA) either emulsified in complete Freund's adjuvant (CFA) or CFA alone. For 2° responses, immune mice were rechallenged after 30 days with 100 μg of NP36-KLH Alum, and analyses were performed after an additional 30 days.
Enzyme-Linked Immunospot (ELISPOT) Assays. Spleen and BM IgG-secreting cells were enumerated by an Ag-specific ELISPOT assay as described (21). Plates were coated with calf thymus single-stranded DNA (Sigma), NP25-BSA, or NP4-BSA (Biosearch Technologies) to measure total and high-affinity Agspecific Abs. Data shown are the mean (± SD) values of three mice per group and are representative of at least three independent experiments.
Immunohistochemistry. Frozen spleen sections (5 μm thick) were acetone-fixed, and stained in 1% BSA PBS with fluorochrome-coupled rat anti-mouse Abs. Samples were analyzed on a Bio-Rad 1024 confocal microscope. Images are representative of three experiments with >30 scanned images of three mice per group (objective magnification, ×10).
Adoptive Transfer. NZM, NZB, and C57/BL6 mice were immunized with NP36-KLH CFA or NP32-Ficoll. After 10 or 30 days as indicated, spleen and BM cells were T cell-depleted by using anti-Thy1.2 plus anti-CD4 Ab and rabbit complement (Accurate Chemicals, Westbury, NY), and transferred (20 × 106 cells) i.v. into Rag1–/– recipients. Initial PC numbers transferred from NZM, NZB, and B6 mice were 3.8 × 105, 6.6 × 105, and 4.2 × 105, respectively. As indicated, some recipients received splenic B cells that were treated with 50 μg/ml mitomycin C (Mito-C) (Sigma) in PBS for 20 min at 37°C. After treatment, cells were washed and viability was analyzed by Trypan blue exclusion. ELISPOT analyses of recipients were performed 30 days posttransfer.
Abs and Reagents. Staining mAbs to B220, CD138, VLA-4, CXCR4, and CD4 were purchased from Pharmingen. Streptavidin-phycoerythrin (PE) and FITC were obtained from Southern Biotechnology Associates.
Migration Assays. To quantify PC chemotaxis, 3 × 106 spleen cells were added to 5 μm of microporous 6.5-mm transwell plates (Corning), and were allowed to equilibrate in migration medium (0.5% BSA/10 mM Hepes/10% FCS in RPMI medium 1640) for 1 h at 37°C. Recombinant mouse CXCL12 or CXCL13 (R & D Systems) was added at 100 ng/ml to the wells, and cells were cultured for an additional 2 h. Cells remaining in the inserts and those that migrated to wells were collected, and flow cytometry or ELISPOT analyses were performed. Data shown are representative of at least six experiments.
Results
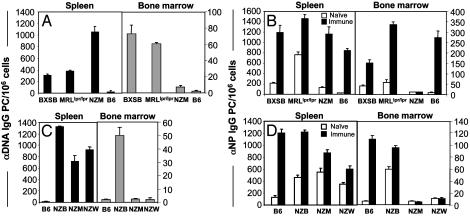
PCs Are Absent in the BM of NZM2410 Mice. It has been shown that autoreactive B cells clonally expand and differentiate to produce isotype-switched, high-affinity autoAbs, a process that mimics normal TD B cell responses (22). Because many autoAbs produced by lupus-prone mice are somatically mutated and isotype-switched, it has been assumed that high levels of persisting serum autoAbs are generated through the development of long-lived PCs residing in the BM. To evaluate this hypothesis, we determined the tissue distribution of anti-nuclear PCs from a cohort of autoimmune mice (Fig. 1A). Measurement of anti-DNA IgG PCs revealed high numbers of PCs in the spleens of NZM mice, with a concordant loss of BM PCs producing anti-DNA Abs. A similar loss of BM PCs was also observed for histone-specific PCs in NZM mice (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). To determine whether this dramatic loss of BM PCs in the NZM strain was manifested in other lupus-prone strains, we evaluated the distribution of PCs in BXSB and MRLlpr/lpr mice. Data show that both the BXSB and MRLlpr/lpr mice have high levels of anti-DNA PCs in the BM (Fig. 1 A). To account for potential differences in the frequency of anti-DNA PCs among the lupus models, and to determine whether the skewed distribution of PCs was restricted to antiself specificities, we immunized mice with NP-KLH to identify NP-specific PCs. Total anti-NP IgG PCs were quantified from the spleen and BM 30 days after Ag challenge, providing sufficient time for the migration of long-lived PCs to the BM (Fig. 1B and refs. 5 and 23). The presence of NP-specific PCs found almost exclusively in the spleen, but not in the BM of NZM mice, is striking, and indicates that the paucity of BM PCs is irrespective of Ag specificity.
Fig. 1.
PCs accumulate in the spleen but are absent in the BM of NZM mice. Spleen- and BM-derived lymphocytes from autoimmune mice were assessed for the generation of anti-DNA IgG PCs (A and C) or NP-specific IgG PCs (B and D) from mice that were immunized with NP36-KLH CFA (immune) or CFA alone (naïve) for 30 days.
Because NZM mice represent an inbred strain that has acquired specific disease susceptibility loci from the NZB and NZW genomes, we examined the parental strains to dissect the genetics associated with this homing deficiency. The absence of BM anti-DNA PCs was found to track with the NZW, rather than with the NZB parental strain (Fig. 1C). Moreover, immunization of mice with Ag confirmed these results, showing the presence of splenic anti-NP PCs in NZM and NZW mice, but a lack of Ag-specific PCs in the BM compared with NZB mice (Fig. 1D). These findings indicate that the genetic lesion responsible for deficient BM PCs is imparted from the NZW genome. This result is consistent with expectations, because the NZM2410 strain has derived ≈75% of its genome from NZW and 25% from NZB mice (24).
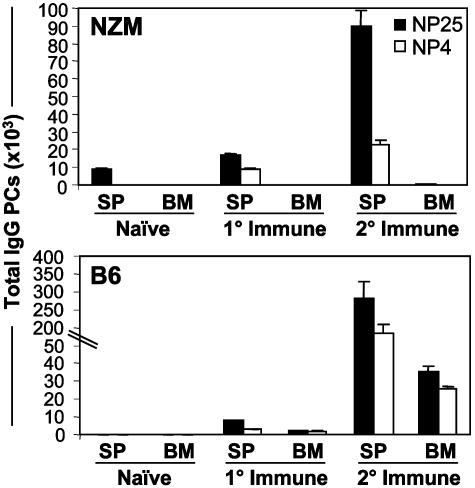
PC migration from the spleen to the BM is especially pronounced during secondary immune responses (25, 26). To determine whether the tissue distribution of PCs in NZM mice would change under these circumstances, ELISPOT analyses were performed after secondary immunization. Whereas the number of splenic Ag-specific PCs in NZM mice was significantly elevated after rechallenge, virtually no PCs were observed in the BM, compared with B6 controls (Fig. 2).
Fig. 2.
PCs are absent in the BM of NZM mice on secondary immunization. NP-specific ELISPOT was performed on spleen and BM cells from naïve NZM and B6 mice and those that were immunized for 30 days (1°) or rechallenged with Ags for 30 days (2°). Total Ag-specific IgG PCs were quantified from individual organs, with the mean (±SD) values from three mice per group shown.
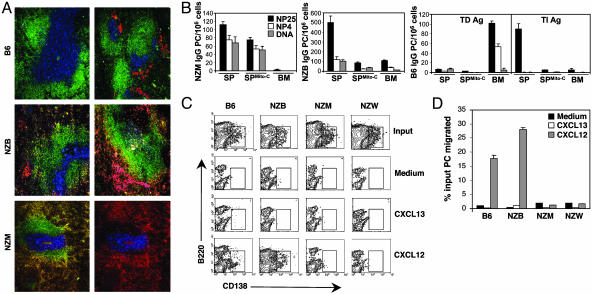
Accumulating PCs in the Spleen of NZM Mice Are Long-Lived and Show Impaired CXCL12 Responsiveness. Phenotypic markers can be used to distinguish PCs from naïve and GC B cells. Expression of CD138 is observed on differentiating PCs, but not on other mature B cells (27–30), and is accompanied by reduced levels of B220 expression (29, 31–33). By using three-color laser scanning confocal microscopy, we can identify PCs as CD138+ (Fig. 3A). Spleen sections from NZM and NZB mice revealed copious numbers of PCs distributed throughout the red pulp, regardless of immunization. In contrast, splenic PCs from nonautoimmune B6 mice challenged with NP-KLH clustered in defined areas outside B:T cell zones. It has been well established that during TD responses, Ag-activated B cells differentiate into PCs within the extrafollicular space of splenic red pulp (34–37). Because many of these splenic PCs are short-lived and rapidly decline by days 8–10 after immunization (25, 38), this result raises the question as to whether splenic PCs in NZM mice are short- or long-lived. To determine whether the splenic PCs were long-lived, immune spleen cells from NZM, NZB, and B6 mice were isolated, treated with the DNA synthesis inhibitor, Mito-C, and adoptively transferred into Rag1–/– recipients (Fig. 3B). Under these conditions, only long-lived PCs will continue to produce Ig due to their quiescent state (5, 23, 39). ELISPOT analysis of recipient mice demonstrated that the majority of NZM splenic PCs were long-lived by virtue of their resistance to Mito-C and capacity to produce high-affinity NP-specific IgG, comprising 75% of total NP PCs. As expected, the transfer of NZM BM cells into recipients failed to confer PCs. The slight reduction in numbers from recipients that received inhibitor-treated cells is likely due to the loss of short-lived PCs. In contrast, the transfer of NZB splenic PCs revealed that the majority of these cells were short-lived. Transfer studies using cells from nonautoimmune B6 mice immunized with NP-KLH (TD Ag) demonstrated that after 30 days the majority of long-lived PCs were found in the BM and produced high-affinity Abs. Results from parallel experiments using cells from B6 mice immunized with NP-Ficoll [thymus-independent (TI) AG] demonstrated the short-lived nature of splenic PCs by their production of low-affinity Abs, susceptibility to Mito-C treatment and absence in the BM. The findings show that, unlike PCs from normal mice, PCs from NZM mice accumulate in the spleen, fail to home to the BM, and attain a long-lived phenotype.
Fig. 3.
The deficit of BM PCs in NZM mice tracks with the NZW parental strain and show aberrant chemokine responsiveness. (A) Immunohistological analysis was performed by staining spleen cryosections with allophycocyanin (APC)-CD4, FITC-B220, and PE-CD138. The localization of CD4+ T cells (blue), follicular B cells (green), and PCs (red) are shown from naïve (Left) and immune (Right) B6 and NZB mice. The accumulation of CD138+B220lo PCs in the splenic red pulp of NZM mice is evidenced by areas of yellow (Left). The exclusion of NZM PCs from B cell follicles is shown by CD4 and CD138 staining only (Right). (B) Spleen (SP) or BM cells from TD immune NZM, NZB, and B6 mice were adoptively transferred into Rag1–/– recipients. Cells from TI immune B6 mice were also transferred. As indicated, recipients also received splenic B cells that were treated with Mito-C. Affinity maturation and long-lived nature of the transferred PCs was measured after 30 days by performing ELISPOT on splenocytes from recipients. (C) Ex vivo chemokine migration of PCs from immune mice to the chemokines, CXCL12 and CXCL13, was examined by flow cytofluorimetric analysis. Migrating cells were collected and stained with fluorochrome-coupled Abs to B220 and CD138. (D) The percentage of input NP-specific IgG PCs that migrated in response to chemokine was measured from the indicated mice by performing ELISPOT on nonmigrating cells (inserts) and those that migrated (wells).
Changing patterns of chemokine receptor expression and function coordinate PC emigration from the spleen to the BM. Affinity-matured, long-lived PCs emigrate from GCs due to their loss in chemokine responsiveness to CCL19, CCL21, and CXCL13, which are involved in the follicular retention of mature B cells (13, 40). In contrast, expression of CXCR4 on PCs imparts responsiveness to CXCL12, a chemokine produced by stromal cells in the BM and is critical for homing to this organ (13). To determine whether splenic PCs from NZM mice were responsive to CXCL12-induced migration, ex vivo chemotaxis assays on spleen cells from immune mice were performed. Flow cytometric analysis of responding PCs (B220loCD138+) showed that migration of NZM PCs to CXCL12 was deficient compared with nonautoimmune B6 controls (Fig. 3C). Moreover, this trait segregated with NZW but not NZB PCs. Anti-NP ELISPOT analysis was also performed to functionally assess the chemokine responsiveness of splenic PCs (Fig. 3D). These studies confirmed the phenotypic data showing that CXCL12-induced chemotaxis was impaired in NZM PCs and tracked with the NZW parental strain. These results were identical to those obtained by using greater concentrations of CXCL12, excluding the possibility that NZM PCs require a higher threshold of chemokine for CXCR4 signaling (see Fig. 7, which is published as supporting information on the PNAS web site).
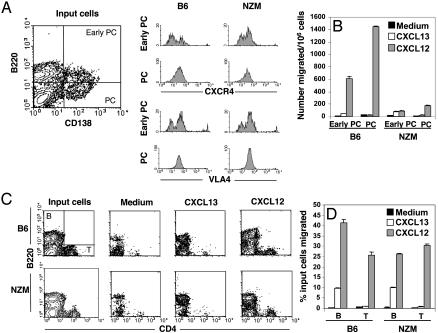
To verify that the impaired chemokine responsiveness of NZM PCs was not due to differential levels of CXCR4 expression, PCs from NZM mice were compared with B6 controls (Fig. 4A). Both early differentiating PCs (B220hiCD138+) and mature PCs (B220loCD138+) from NZM mice showed identical CXCR4 expression levels compared with B6 PCs. Sequence analysis of the CXCR4 gene did not reveal any single-nucleotide polymorphisms that might confer an alteration in protein structure (data not shown). Among the integrin family of adhesion molecules, VLA-4 is principally expressed on mature PCs and is involved in the retention of PCs in BM (23). Levels of VLA-4 expression were identical between B6 and NZM PCs, indicating that the phenotype of differentiating NZM PCs appeared normal. Migration assays further revealed that both early and mature NZM PCs were defective in chemotaxis (Fig. 4B). Of significance, however, is that mature naïve B cells and T cells from NZM mice were fully capable of responding to CXCL12 compared with B6 controls (Fig. 4 C and D). Thus, the inadequacy of NZM PCs to migrate in response to CXCL12 is acquired at the early stage of plasmacytic differentiation, is not a result of lower CXCR4 surface expression, and is selective to B lymphocytes committed to a PC fate.
Fig. 4.
Deficient chemokine responsiveness to CXCL12 is restricted to B cells differentiating to a PC fate. (A) Ex vivo flow cytofluorimetric analysis was performed on input spleen cells from immune B6 and NZM before chemotaxis assays. Cells were stained with mAbs to B220, CD138, CXCR4, and VLA4. Fluorescence intensity of CXCR4 and VLA4 are shown on gated early differentiating PCs (B220+CD138+) and mature PCs (B220loCD138+). (B) Migration of early and mature PCs in response to CXCL12 and CXCL13 were quantified, based on their respective phenotype. (C and D) The selective distortion in CXCL12-induced migration of NZM PCs was further shown by measuring the chemotaxis of mature B and CD4+ T cells from identical assays. Profiles shown represent input B (B220+) and T (CD4+) cells and those that migrated, in response to medium alone or chemokines. The percentage of input B and T cells that migrated in response to chemokine was quantified by performing flow cytofluorimetric analysis on nonmigrating cells (inserts) and those that migrated (wells).
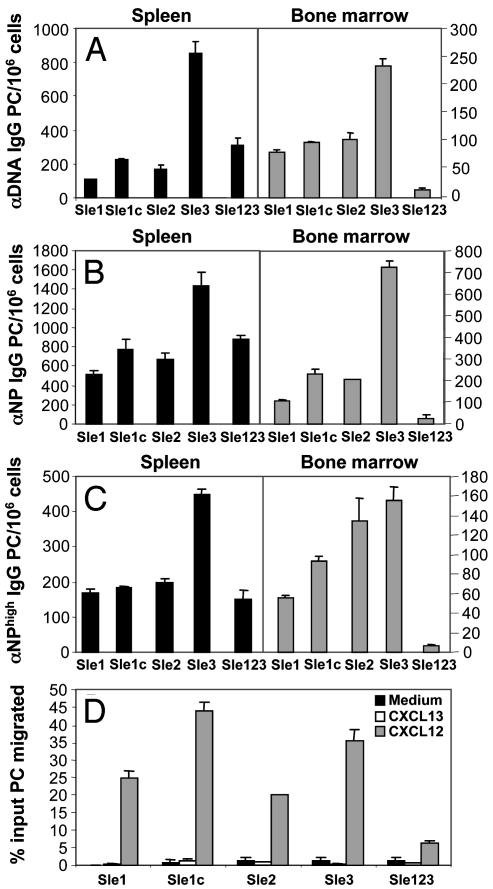
Multiple Autoimmune Susceptibility Loci Are Required for Deficient CXCL12 Migration. Linkage studies of the NZB/W, NZM2410, MRL, and BXSB mouse models have identified multiple susceptibility loci that are critical to the development of severe lupus nephritis (41–43). Three recessive loci strongly associated with SLE susceptibility have been identified in the NZM2410 strain (Sle1, Sle2, and Sle3, which are located on chromosomes 1, 4, and 7, respectively; ref. 44). B6 congenic mice carrying these susceptibility loci individually (B6.Sle1, B6.Sle2, and B6.Sle3) and combined (B6.Sle123) have been generated (20). The tricongenic strain reconstitutes the autoimmune pathology of NZM2410, whereas the single-congenic mice have only partial, nonpathogenic phenotypes (45). A subcongenic strain (B6.Sle1c) has also been generated that contains within its interval the gene, Cr2, and has been shown to encode dysfunctional complement receptors 1 and 2 (46). In an effort to dissect the susceptibility interval responsible for the deficiency in BM PCs, we performed ELISPOT analysis on each of the congenic strains (Fig. 5 A–C). Results showed that anti-DNA PCs were present in the spleen and BM from each of the single congenic lines. In contrast, the tricongenic strain contained very few BM PCs. Data from NP-KLH immune congenic mice showed similar results for both total Ag-specific IgG PCs and high-affinity anti-NP PCs. Chemotaxis assays revealed that splenic PCs from single-congenic mice strongly responded to CXCL12, whereas nominal migration was observed from tricongenic PCs (Fig. 5D). Therefore, functional analyses of these mice show that an individual locus does not confer impaired chemotaxis of PCs, but it is the epistatic interactions of Sle1, Sle2i, and Sle3 that mediate this deficiency.
Fig. 5.
Multiple autoimmune susceptibility loci are required for distorted chemokine responsiveness of NZM PCs. (A–C) Spleen and BM cells from B6 congenic mice that singly express the SLE susceptibility locus Sle1, Sle2, and Sle3, or in combination, were assessed for the production of anti-DNA, total NP, and high-affinity NP IgG-secreting PCs by ELISPOT. (D) The percentage of input autoreactive PCs that migrated in response to chemokine was measured from the indicated mice by performing ELISPOT as described (21).
Discussion
The specific microenvironment occupied by PCs has been implicated as a determinant in cellular survival and function (2). Accordingly, the anatomical niche that PCs occupy ultimately controls the intensity and longevity of the humoral immune response. This article focuses on the abnormalities observed in the migration and function of PCs in the NZM mouse strain. First, our data show that the inheritance of SLE susceptibility loci results in fundamental changes in the behavior of PCs with regard to their migratory patterns in vivo. The synergy between these loci incapacitates the ability of postGC B cells to migrate to the BM. Second, we show that, unlike normal splenic PCs, PCs in the spleen of NZM mice are long-lived, establishing that the spleen can support long-lived PCs. Third, we show that splenic NZM PCs are unresponsive to CXCL12-induced chemotaxis. This observation explains the basis for the inability of NZM postGC B cells to exit the spleen. Finally, we report that resting NZM B cells are responsive to CXCL12 migration, even though postGC B cells are unresponsive. Thus, unresponsiveness to CXCL12 in NZM mice is only expressed after GC activation.
PC longevity is believed to be controlled by specific survival signals within their microenvironment. The lack of PC migration to the BM early in life has been linked to the short duration of humoral immunity in the newborn (47). Furthermore, the general short-lived nature of splenic PCs is believed to be due to the fact that the splenic red pulp does not provide the appropriate survival signals for PC longevity (37). It must be noted that whereas most of the PCs in the spleen are short-lived, MacClennan and coworkers (37) have shown that there is a subset of splenic PCs that are long-lived. Studies presented herein show that the spleen can be a site that supports the survival of long-lived PCs. It could be argued that one contribution of an Sle loci is to change the splenic microenvironment to support PC longevity, with this genetic trait being distinct from those genes involved in controlling CXCL12 responsiveness. It is of interest to note that B6.Sle3 mice appear to have an increased number of PCs in the spleen compared with the other congenic strains, however, whether this is due to increased B cell hyperactivity or to increased longevity is not known. Another important issue is that it has been suspected that CXCR4 triggering may elicit PC longevity. Data presented here strongly argue that CXCL12 is not an essential factor for PC longevity, because NZM PCs are long-lived and are unresponsive to CXCL12-induced migration.
Even within the PC compartment, the issue of PC responsiveness to CXCL12 is complex. A number of groups have recently shown that early PC emigrants into the BM are CXCL12-responsive (13, 48). However, Manz and coworkers (48) have established that there is a progressive loss of CXCL12 responsiveness that naturally occurs as long-lived PCs “age” within the BM compartment, even though CXCR4 expression is still evident. This finding is intriguing in light of our data showing that naïve NZM B cells are responsive to CXCL12-induced migration, but postGC B cells are not responsive. Because newly emerging PCs in the spleen of NZM mice are deficient in CXCL12 chemotaxis, it is possible that PC precursors prematurely terminate responsiveness toward CXCL12. Thus, the aging of PCs may be hyperaccelerated in NZM mice. Wakeland and coworkers (45, 49) have recently shown that Sle1 and Sle3 confer susceptibility to lupus nephritis through hyperactivity of B cells in the NZM2410 strain. These distinct traits of Sle1 and Sle3 may fit with the hypothesis that terminal differentiation in NZM mice is hyperaccelerated. Further studies using bicongenic mice to address the role of these susceptibility loci on PC migration are warranted.
The fact that the deficiency in CXCL12 responsiveness depends on multiple Sle loci suggests that this phenotype is associated with disease development. We have compared the frequency of splenic and BM PCs from NZM mice ranging from 5 to 24 weeks of age to determine whether the homing properties of PCs changes over time. Whereas results from these studies demonstrated an increased number of splenic PCs as the mice aged, there remained no difference in the paucity of BM PCs regardless of Ag specificity (data not shown). Thus, at this time, how the loss in PC homing to the BM contributes to lupus is unclear. The discovery that PCs in the NZM model are sustained in the spleen, but are deficient in the BM, reveals that persisting systemic Ab responses can be achieved without the accumulation of long-lived PCs in the BM compartment. One potential consequence of this deficiency in the homing properties of PCs may be preventing the natural selection of higher-affinity longlived PCs in the BM, thereby contributing to the loss of selftolerance. Recently, it has been shown that the inappropriate distribution of B cells outside the GC may result in the loss of censoring the development of autoreactive B lymphocytes (50). Work by Kelsoe and coworkers (51) have shown that affinity selection can occur outside the GC. Whereas it is clear that the BM plays a critical role in positive and negative selection during B cell ontogeny, the question emerges as to whether the BM also plays a role in the censorship of affinity-matured B cells before terminal differentiation to PCs. As linkage analyses of human SLE have demonstrated striking similarities to the genetics of disease susceptibility with animal models (52–55), it will be of significant interest to learn whether defects in chemokine responsiveness is manifested by PCs in humans with lupus.
Supplementary Material
Acknowledgments
We thank B. Kotzin and T. Waldschmidt for critical reading of the manuscript. Confocal microscopy and flow cytometry were performed at the Herbert C. Englert Cell Analysis Laboratory, Dartmouth Medical School, which was established by equipment grants from the Fannie E. Rippel Foundation and by National Cancer Institute Core Grant CA23108. This work was supported by National Institutes of Health Grants AI26296 and AI42234 (to R.J.N.), Centers of Biomedical Research Excellence Program of the National Center for Research Resources Grant P20 RR16437 (to L.D.E.), and American Cancer Society Postdoctoral Fellowship LIB-0202101 (to L.D.E.) and Research Project Grant LIB-100205 (to L.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PC, plasma cell; BM, bone marrow; GC, germinal center; SLE, systemic lupus erythematosus; TD, thymus-dependent; CFA, complete Freund's adjuvant; ELISPOT, enzyme-linked immunospot; Ag, antigen; NZB, New Zealand black; NZW, New Zealand white; Mito-C, mitomycin C.
References
- 1.Slifka, M. K. & Ahmed, R. (1998) Curr. Opin. Immunol. 10 252–258. [DOI] [PubMed] [Google Scholar]
- 2.Manz, R. A., Arce, S., Cassese, G., Hauser, A. E., Hiepe, F. & Radbruch, A. (2002) Curr. Opin. Immunol. 14 517–521. [DOI] [PubMed] [Google Scholar]
- 3.Jacob, J. & Kelsoe, G. (1992) J. Exp. Med. 176 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nossal, G. J. V. (1992) Cell 68 1–2. [DOI] [PubMed] [Google Scholar]
- 5.Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. (1998) Immunity 8 363–372. [DOI] [PubMed] [Google Scholar]
- 6.Manz, R. A., Thiel, A. & Radbruch, A. (1997) Nature 388 133–134. [DOI] [PubMed] [Google Scholar]
- 7.Koch, G., Osmond, D. G., Julius, M. H. & Benner, R. (1981) J. Immunol. 126 1447–1451. [PubMed] [Google Scholar]
- 8.Benner, R., Hijmans, W. & Haaijman, J. J. (1981) Clin. Exp. Immunol. 46 1–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Freitas, A. A. & Rocha, B. (2000) Annu. Rev. Immunol. 18 83–111. [DOI] [PubMed] [Google Scholar]
- 10.Antia, R., Pilyugin, S. S. & Ahmed, R. (1998) Proc. Natl. Acad. Sci. USA 95 14926–14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forster, R., Mattis, A. E., Kremmer, E., Wolf, E., Brem, G. & Lipp, M. (1996) Cell 87 1037–1047. [DOI] [PubMed] [Google Scholar]
- 12.Ansel, K. M., Ngo, V. N., Hyman, P. L., Luther, S. A., Forster, R., Sedgwick, J. D., Browning, J. L., Lipp, M. & Cyster, J. G. (2000) Nature 406 309–314. [DOI] [PubMed] [Google Scholar]
- 13.Hargreaves, D. C., Hyman, P. L., Lu, T. T., Ngo, V. N., Bidgol, A., Suzuki, G., Zou, Y. R., Littman, D. R. & Cyster, J. G. (2001) J. Exp. Med. 194 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawabata, K., Ujikawa, M., Egawa, T., Kawamoto, H., Tachibana, K., Iizasa, H., Katsura, Y., Kishimoto, T. & Nagasawa, T. (1999) Proc. Natl. Acad. Sci. USA 96 5663–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma, Q., Jones, D. & Springer, T. A. (1999) Immunity 10 463–471. [DOI] [PubMed] [Google Scholar]
- 16.Odendahl, M., Jacobi, A., Hansen, A., Feist, E., Hiepe, F., Burmester, G. R., Lipsky, P. E., Radbruch, A. & Dorner, T. (2000) J. Immunol. 165 5970–5979. [DOI] [PubMed] [Google Scholar]
- 17.Putterman, C., Deocharan, B. & Diamond, B. (2000) J. Immunol. 164 2542–2549. [DOI] [PubMed] [Google Scholar]
- 18.Jacobi, A. M., Hansen, A., Burmester, G. R., Dorner, T. & Lipsky, P. E. (2000) Autoimmunity 33 61–76. [DOI] [PubMed] [Google Scholar]
- 19.Manheimer-Lory, A. J., Zandman-Goddard, G., Davidson, A., Aranow, C. & Diamond, B. (1997) J. Clin. Invest. 100 2538–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morel, L., Yu, Y., Blenman, K. R., Caldwell, R. A. & Wakeland, E. K. (1996) Mamm. Genome 7 335–339. [DOI] [PubMed] [Google Scholar]
- 21.Erickson, L. D., Durell, B. G., Vogel, L. A., O'Connor, B. P., Cascalho, M., Yasui, T., Kikutani, H. & Noelle, R. J. (2002) J. Clin. Invest. 109 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radic, M. Z., Mackle, J., Erikson, J., Mol, C., Anderson, W. F. & Weigert, M. (1993) J. Immunol. 150 4966–4977. [PubMed] [Google Scholar]
- 23.O'Connor, B. P., Cascalho, M. & Noelle, R. J. (2002) J. Exp. Med. 195 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morel, L. & Wakeland, E. K. (2000) Int. Rev. Immunol. 19 423–446. [DOI] [PubMed] [Google Scholar]
- 25.Smith, K. G., Hewitson, T. D., Nossal, G. J. & Tarlinton, D. M. (1996) Eur. J. Immunol. 26 444–448. [DOI] [PubMed] [Google Scholar]
- 26.Dilosa, R. M., Maeda, K., Masuda, A., Szakal, A. K. & Tew, J. G. (1991) J. Immunol. 146 4071–4077. [PubMed] [Google Scholar]
- 27.Sanderson, R. D., Lalor, P. & Bernfield, M. (1989) Cell Regul. 1 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHeyzer-Williams, M. G., McLean, M. J., Lalor, P. A. & Nossal, G. J. (1993) J. Exp. Med. 178 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalor, P. A., Nossal, G. J., Sanderson, R. D. & McHeyzer-Williams, M. G. (1992) Eur. J. Immunol. 22 3001–3011. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi, K., Hayashi, M., Jalkanen, M., Firestone, J. H., Trelstad, R. L. & Bernfield, M. (1987) J. Histochem. Cytochem. 35 1079–1088. [DOI] [PubMed] [Google Scholar]
- 31.Dustin, L. B., Bullock, E. D., Hamada, Y., Azuma, T. & Loh, D. Y. (1995) J. Immunol. 154 4936–4949. [PubMed] [Google Scholar]
- 32.Jensen, G. S., Poppema, S., Mant, M. J. & Pilarski, L. M. (1989) Int. Immunol. 1 229–236. [DOI] [PubMed] [Google Scholar]
- 33.Oliver, A. M., Martin, F. & Kearney, J. F. (1997) J. Immunol. 158 1108–1115. [PubMed] [Google Scholar]
- 34.Jacob, J., Kassir, R. & Kelsoe, G. (1991) J. Exp. Med. 173 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, Y. J., Zhang, J., Lane, P. J., Chan, E. Y. & MacLennan, I. C. (1991) Eur. J. Immunol. 21 2951–2962. [DOI] [PubMed] [Google Scholar]
- 36.Toellner, K. M., Gulbranson-Judge, A., Taylor, D. R., Sze, D. M. & MacLennan, I. C. (1996) J. Exp. Med. 183 2303–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sze, D. M., Toellner, K. M., Garcia de Vinuesa, C., Taylor, D. R. & MacLennan, I. C. (2000) J. Exp. Med. 192 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho, F., Lortan, J. E., MacLennan, I. C. & Khan, M. (1986) Eur. J. Immunol. 16 1297–1301. [DOI] [PubMed] [Google Scholar]
- 39.Slifka, M. K. & Ahmed, R. (1996) J. Immunol. Methods 199 37–46. [DOI] [PubMed] [Google Scholar]
- 40.Wehrli, N., Legler, D. F., Finke, D., Toellner, K. M., Loetscher, P., Baggiolini, M., MacLennan, I. C. & Acha-Orbea, H. (2001) Eur. J. Immunol. 31 609–616. [DOI] [PubMed] [Google Scholar]
- 41.Wakeland, E. K., Wandstrat, A. E., Liu, K. & Morel, L. (1999) Curr. Opin. Immunol. 11 701–707. [DOI] [PubMed] [Google Scholar]
- 42.Mohan, C. (2001) Curr. Opin. Rheumatol. 13 352–360. [DOI] [PubMed] [Google Scholar]
- 43.Vyse, T. J. & Kotzin, B. L. (1998) Annu. Rev. Immunol. 16 261–292. [DOI] [PubMed] [Google Scholar]
- 44.Morel, L., Rudofsky, U. H., Longmate, J. A., Schiffenbauer, J. & Wakeland, E. K. (1994) Immunity 1 219–229. [DOI] [PubMed] [Google Scholar]
- 45.Morel, L., Croker, B. P., Blenman, K. R., Mohan, C., Huang, G., Gilkeson, G. & Wakeland, E. K. (2000) Proc. Natl. Acad. Sci. USA 97 6670–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boackle, S. A., Holers, V. M., Chen, X., Szakonyi, G., Karp, D. R., Wakeland, E. K. & Morel, L. (2001) Immunity 15 775–785. [DOI] [PubMed] [Google Scholar]
- 47.Pihlgren, M., Schallert, N., Tougne, C., Bozzotti, P., Kovarik, J., Fulurija, A., Kosco-Vilbois, M., Lambert, P. H. & Siegrist, C. A. (2001) Eur. J. Immunol. 31 939–946. [DOI] [PubMed] [Google Scholar]
- 48.Hauser, A. E., Debes, G. F., Arce, S., Cassese, G., Hamann, A., Radbruch, A. & Manz, R. A. (2002) J. Immunol. 169 1277–1282. [DOI] [PubMed] [Google Scholar]
- 49.Mohan, C., Morel, L., Yang, P., Watanabe, H., Croker, B., Gilkeson, G. & Wakeland, E. K. (1999) J. Clin. Invest. 103 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.William, J., Euler, C., Christensen, S. & Shlomchik, M. J. (2002) Science 297 2066–2070. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi, Y., Dutta, P. R., Cerasoli, D. M. & Kelsoe, G. (1998) J. Exp. Med. 187 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shai, R., Quismorio, F. P., Jr., Li, L., Kwon, O. J., Morrison, J., Wallace, D. J., Neuwelt, C. M., Brautbar, C., Gauderman, W. J. & Jacob, C. O. (1999) Hum. Mol. Genet. 8 639–644. [DOI] [PubMed] [Google Scholar]
- 53.Gaffney, P. M., Kearns, G. M., Shark, K. B., Ortmann, W. A., Selby, S. A., Malmgren, M. L., Rohlf, K. E., Ockenden, T. C., Messner, R. P., King, R. A., et al. (1998) Proc. Natl. Acad. Sci. USA 95 14875–14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moser, K. L., Neas, B. R., Salmon, J. E., Yu, H., Gray-McGuire, C., Asundi, N., Bruner, G. R., Fox, J., Kelly, J., Henshall, S., et al. (1998) Proc. Natl. Acad. Sci. USA 95 14869–14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsao, B. P., Cantor, R. M., Kalunian, K. C., Chen, C. J., Badsha, H., Singh, R., Wallace, D. J., Kitridou, R. C., Chen, S. L., Shen, N., et al. (1997) J. Clin. Invest. 99 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.