Abstract
The mechanisms that regulate mammalian cell size during development and homeostatic maintenance are poorly understood. The tumor suppressor Pten is required for correct maintenance of mammalian neuronal soma size. Selective inactivation of Pten in postnatal granule neurons of the cerebellum and dentate gyrus in mouse causes cell-autonomous hypertrophy as well as more complex phenotypes, including progressive macrocephaly, seizures, and premature death. To determine the contribution of mTor signaling to Pten-mediated growth regulation in the mammalian nervous system, we treated Pten conditional knockout mice with CCI-779, a specific mTor inhibitor. mTor inhibition decreased the seizure frequency and death rate in Pten mutant mice, prevented the increase in Pten-deficient neuronal soma size in young mice, and reversed neuronal soma enlargement in adult mice. mTor inhibition did not decrease the size of wild-type adult neurons. Thus, mTor is required for neuronal hypertrophy downstream of Pten deficiency, but is not required for maintenance of normal neuronal soma size. mTOR inhibitors may be useful therapeutic agents for diseases in brain resulting from PTEN deficiency such as Lhermitte–Duclos disease or glioblastoma multiforme.
The tumor supressor gene PTEN is inactivated by somatic mutation in a variety of human tumors, including 25–50% of glioblastoma multiforme (1). PTEN functions as a lipid phosphatase to dephosphorylate the 3′ position of phosphatidylinositol-3,4,5-trisphosphate (PIP3) and phosphatidylinositol-3,4-diphosphate (PIP2) (2), directly counteracting signals for growth, proliferation and survival transduced through the phosphatidylinositol 3-kinase (PI3K) pathway (3). Inherited mutation of PTEN occurs in the multiple hamartoma syndromes Bannayan–Zonana syndrome and Cowden disease and causes a wide spectrum of phenotypic abnormalities, including macrocephaly, hamartomas in multiple tissues, cancer predisposition, ataxia, seizures, and the cerebellar disorder Lhermitte–Duclos disease (4).
In vivo analysis of Pten function using conventional and conditional knockout mice reveals a complex and context-dependent role for Pten in mammalian development and homeostasis. Homozygous deletion of Pten causes embryonic lethality in mice, and Pten+/– mice have increased cancer incidence (5–7). Analyses of Pten conditional knockout mice include selective inactivation in neural precursor cells, T cells, B cells, keratinocytes, mammary gland, and cardiomyocytes (8–14). The consequences of Pten deficiency in vivo are diverse and include effects on cell size and cell migration as well as cell number through changes in proliferation and/or apoptosis and tumor development, depending on cell type and developmental context. PtenloxP/loxP; Gfap-cre mice, in which Pten was selectively inactivated in postnatal granule neurons of the dentate gyrus and cerebellum, developed seizures and died prematurely. Pten deficiency in neurons caused changes in cell size, but not cell number, and resulted in a dramatic cell-autonomous hypertrophy. In the cerebellum, this progressive hypertrophy, in combination with a migration defect in a subset of mutant cells, recapitulated the pathology observed in human Lhermitte– Duclos disease found in some patients with germ-line PTEN mutations (15, 16).
mTor is a central mediator of growth, and genetic studies indicate that dTOR functions downstream of dPTEN in Drosophila (17, 18). CCI-779, a rapamycin ester, is a specific inhibitor of mTOR developed for i.v. use. CCI-779 blocked proliferation of PTEN-deficient tumors in vitro and in a xenograft model and reduced the severity of endometrial complex atypical hyperplasia in Pten+/– mice (19, 20). In these models, Pten deficiency causes increased cell number. To determine the contribution of mTor to the hypertrophic growth abnormalities associated with Pten deficiency in brain, we treated PtenloxP/loxP; Gfap-cre mice (hereafter referred to as Pten mutant mice), with CCI-779.
Methods
Transgenic Mice and Drug Injection. Pten conditional knockout mice were described previously (15, 16), and were generated by breeding Gfap-cre mice (15) with PtenloxP mice (10), a gift from Tak Mak (University of Toronto, Toronto). CCI-779, a gift from Wyeth–Ayerst (Pearl River, NY), was dissolved immediately before injection in a vehicle solution containing 5% polyethyleneglycol 400, 5% Tween 80 (Sigma), and 4% ethyl alcohol. Mice were administered i.p. daily with CCI-779 or vehicle for 5 days per week. Mutant mice and their littermate controls were randomized into vehicle or drug groups. For analysis of adult animals, treatment began when mutant mice were clearly symptomatic, ranging in age from 6 to 16 weeks. A pair of age-matched mutant mice with equivalent symptoms was randomized for vehicle or CCI treatment. Littermate controls were included for each mutant mouse. As an estimate of seizure incidence, each mouse was observed independently by two investigators for ≈5 min at least twice per day, 6 days per week.
Immunohistochemistry and Western Blotting. Brains were cut in half sagittally; half of the brain was fixed in 4% PFA for 2 days for immunohistochemistry, and cerebellum and hippocampal formation were dissected from the remaining half brain and snap-frozen for protein extraction. Fixed brains were embedded in paraffin and cut sagittally into 5-μm-thick sections. Cresyl violet staining was done in 0.02% cresyl violet (Sigma) for 2 h. For immunohistochemistry, we used antigen retrieval and primary antibodies against Pten (1:50, Neomarkers), P-Ser-473-Akt (1:50), or P-Ser-235/236-S6 (1:500, Cell Signaling). Antibodies were detected with biotinylated secondary antibodies followed by peroxidase-conjugated avidin (Elite ABC, Vector Laboratories), and DAB substrate in combination with hematoxylin counterstain (Vector Laboratories). For Western blotting, tissue was homogenized in Cell Lysis Buffer (Cell Signaling) containing 10 mM sodium fluoride, 1 mM sodium orthovanadate (Sigma), and protease inhibitors (Boehringer Mannheim). Fifty micrograms of protein extract was resolved on 4–12% NuPAGE gels (Novex), and Western blotting was performed as described (21). Primary antibodies used for Western blotting were against Pten (Neomarkers), P-Ser-473-Akt, Akt, P-Ser-235/236-S6, or S6 (Cell Signaling).
Soma Size Measurement. For soma measurements, investigators were blinded as to whether animals were treated with CCI-779 or vehicle. The Bioquant Image Analysis system (R & M Biometrics) was used to measure cells. In the dentate gyrus, we measured soma diameters of Pten-positive and -negative granule cells from matched sections from mutant and control brains immunostained for Pten. In the cerebellum, because of the almost complete inactivation of Pten in cerebellar granule cells, there were insufficient numbers of Pten-positive granule cells in mutant mice to use the same approach used in the dentate gyrus. Therefore, we compared cerebellar granule neurons from mutant and control brains that had been stained with cresyl violet, a Nissl stain used to visualize all cells. We measured granule cell soma diameters in two 2,000-μm2 areas in lobule X of matched sections from mutant and control cerebella.
Results
CCI-779 Selectively Blocked the mTor Pathway in the Dentate Gyrus of Young Mice. Pten mutant mice appear normal at 2 weeks of age, but show striking hypertrophy of Pten-deficient neuronal soma in the dentate gyrus by 4 weeks of age (15). To determine whether inhibition of mTor could prevent the Pten-deficient phenotype, we treated Pten mutant and littermate control mice with CCI-779 from postnatal day 10 (P10), before the onset of the phenotype, through P28, when the phenotype in the dentate gyrus was easily detectable. At this age, a dosage of 10 mg/kg, which is well tolerated in adult animals, caused significant morbidity and mortality (data not shown), so we used 7.5 mg/kg. Wild-type and mutant mice treated with CCI-779 were healthy, but smaller, consistent with a central role for mTor in growth regulation. Rapamycin or CCI-779 inhibition of mTor activity cannot be directly measured, because it does not directly change phosphorylation or activity of mTor substantially (22, 23). Therefore, to verify the efficacy and specificity of mTor inhibition, we evaluated activation of downstream and upstream components of the mTor signaling pathway in treated mice. mTor is required for full activation of S6k, which in turn, phosphorylates S6 (24). Therefore, we evaluated levels of phospho-S6 (P-S6) as an indicator of inhibition of the mTor pathway. In the vehicle-treated group, endogenous P-S6 signals were detected in control dentate gyrus, and the signals were increased in mutant dentate gyrus, consistent with Pten deficiency activating the mTor pathway (Fig. 1 a and b). CCI-779 treatment almost completely ablated P-S6 signals in the dentate gyrus and hippocampus from both control and mutant mice, indicating that the drug treatment efficiently blocked the mTor pathway in these regions (Fig. 1 c and d). This effect was specific because CCI-779 treatment did not affect levels of phospho-Akt (P-Akt), which were similarly elevated in the dentate gyrus of CCI-779 and vehicle-treated mutant mice (Fig. 1 f and h), compared with control mice (Fig. 1 e and g).
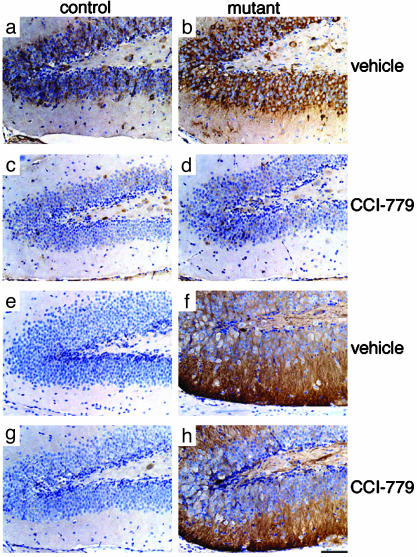
Fig. 1.
CCI-779 effectively blocked mTor signaling in granule neurons of the dentate gyrus. Dentate gyri from 4-week-old control (a, c, e, and g) and mutant (b, d, f, and h) mice treated with vehicle (a, b, e, and f) or CCI-779 (c, d, g, and h) from ages P10 to P28 were immunostained with an antibody specific for P-S6 (a–d), a downstream indication of mTor signaling, and an antibody specific for P-Akt (e–h), an indicator of Akt activation that occurs upstream of mTor. CCI-779 treatment blocked S6 phosphorylation (c and d) without affecting the dramatic increase in P-Akt observed in Pten-deficient cells (f and h). (Scale bar is 100 μm.)
CCI-779 Prevented Hypertrophy of Pten-Deficient Neurons in the Dentate Gyrus. Pten deletion occurs in most, but not all, neurons of the dentate gyrus in Pten mutant mice because of incomplete expression of cre activity in this cell population (15). To assess the effect of CCI-779 on cell-autonomous hypertrophy, we immunostained for Pten and evaluated the soma size of Ptendeficient neurons, compared with Pten-expressing neurons, with or without CCI-779 treatment. In vehicle-treated mice, most granule neurons expressed Pten and showed relatively uniform soma size in the dentate gyrus from control mice (Fig. 2a). In mutant mice, Pten-deficient granule cell soma were significantly bigger than Pten-positive, normal-sized cells (Fig. 2b). In contrast, after CCI-779 treatment, the soma size of Pten-deficient cells were similar to those of Pten-positive cells in mutant or control mice (Fig. 2 c and d). To quantitate this effect, we separately measured soma diameters of Pten-positive and -negative granule cells in the dentate gyrus. CCI-779 treatment significantly prevented the cell-autonomous hypertrophy of Pten-deficient neurons in the dentate gyrus (P < 0.005, Fig. 2e). Changes in nuclear size paralleled changes in soma size (Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). Neuronal hypertrophy resumed after cessation of CCI-779 (Fig. 8, which is published as supporting information on the PNAS web site), indicating that chronic treatment is required to maintain the corrective effects of mTor inhibition.
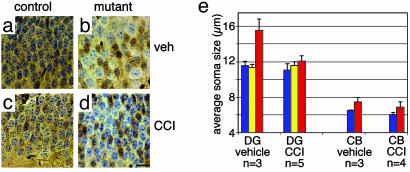
Fig. 2.
CCI-779 treatment prevented granule cell soma hypertrophy in the dentate gyrus. Pten mutant mice and control mice were treated with 7.5 mg/kg five times per week from age P10 to P28, and mice were killed at P28. Brain sections were immunostained for Pten. In the dentate gyrus from vehicle-treated 4-week-old control mice, all granule cells were Pten-positive and uniform in size (a), whereas in mutant dentate gyrus, Pten-negative granule cells were enlarged compared with adjacent Pten-positive cells (b). CCI-779 treatment prevented granule cell enlargement, resulting in Pten-negative granule cells of similar size to Pten-positive cells from mutant (d) and control (c) mice. (Scale bar is 20 μm.) (e) In the dentate gyrus (DG), soma diameters of granule cells from control mice (blue bars) and Pten-positive or -negative granule cells from Pten mutant mice (yellow and red bars, respectively) were separately measured. CCI-779 treatment significantly inhibited the enlargement of Pten-deficient neurons (P < 0.005). In the cerebellum (CB), the diameter of granule neuron soma from control (blue bars) or Pten mutant (red bars) was measured from sections stained with cresyl violet. CCI-779 treatment did not substantially affect the size of Pten-deficient neurons compared with control neurons (P = 0.23).
CCI-779 Treatment Did Not Rescue Cerebellar Abnormalities in Young Mutant Mice. In mutant mice, Pten is inactivated more uniformly in cerebellar granule neurons, where deletion occurs in almost all cells within this population by 4 weeks of age. Cerebellar granule cell soma enlargement is much more pronounced in adult mice; however, it is detectable at 4 weeks of age (15). At this age, mutant mice also show ectopic granule neurons that failed to migrate correctly during development. We evaluated mice treated with vehicle or CCI-779 from P10 to P28 to see whether these cerebellar abnormalities could also be prevented. In the vehicle-treated group, the average soma diameter of mutant cerebellar granule cells was significantly larger than that of control, and this difference was not substantially changed by CCI-779 treatment (Fig. 2e). CCI-779 treatment also failed to prevent the migration defect resulting in some ectopic granule cells. In contrast to the dentate gyrus, CCI-779 treatment did not substantially decrease levels of P-S6 in cerebellum, indicating that the inhibition of the mTor pathway was incomplete (Fig. 9, which is published as supporting information on the PNAS web site). The differential response to CCI-779 could imply regional variation in efficacy or penetrance of the drug. Because of dose-limiting toxicity in young mice, we were unable to evaluate the effects of higher CCI-779 dosage in mice <4 weeks of age.
CCI-779 Efficacy Varied in Different Brain Regions. To identify a dosage of CCI-779 that would effectively block the mTor/S6k pathway in adult cerebellum, we treated 8-week-old wild-type mice with vehicle or 10, 25, or 50 mg/kg CCI-779 and measured levels of endogenous P-S6 by Western blotting. In vehicle-treated mice, P-S6 was detected in the hippocampal formation and the cerebellum. Treatment with 10 mg/kg CCI-779 effectively blocked phosphorylation of S6 in the hippocampal formation, but only marginally decreased P-S6 in the cerebellum. However, increasing the CCI-779 dosage to 25 or 50 mg/kg effectively blocked S6 phosphorylation in cerebellum as well as in the hippocampal formation (Fig. 3), revealing a previously unrecognized regional difference in sensitivity to CCI-779. Immunohistochemical detection of P-S6 confirmed Western blotting results, and revealed the hippocampal region to have a greater sensitivity to CCI-779 treatment than most other regions of the brain (data not shown). Hippocampal neurons were not more sensitive than cerebellar granule neurons when treated with CCI-779 in vitro (Fig. 10, which is published as supporting information on the PNAS web site), suggesting the differences observed in vivo are likely to be caused by regional drug uptake rather than cell-intrinsic differences.
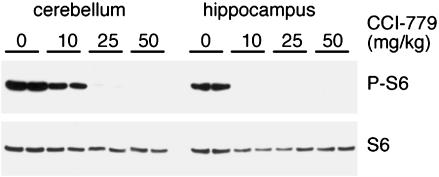
Fig. 3.
CCI-779 sensitivity varied between the dentate gyrus and cerebellum. To determine the optimal dosage to block mTor signaling in cerebellum compared with hippocampus, duplicate pairs of wild-type mice were treated with vehicle (–), 10, 25, or 50 mg/kg daily for 5 days, and protein extracts from hippocampal formation and cerebellum were analyzed by Western blotting for P-S6 and total S6 protein.
CCI-779 Treatment Reduced Seizure Frequency and Death Rate in Adult Mutant Mice. Adult Pten mutant mice displayed tonic-clonic seizures and ataxia and died before the first year of life. This phenotype was observed in all mutant mice and none of the control mice (15). We treated symptomatic adult mutant mice with 10 mg/kg CCI-779 for 4–8 weeks to determine whether phenotypic abnormalities could be reversed, and whether the lifespan of mutant mice could be extended. As seen in untreated adult mutant mice (15), vehicle-treated adult mutant mice displayed seizures and many of them died during the 8 weeks of treatment (Fig. 4). In contrast, in the CCI-779-treated group, the frequency of seizures decreased dramatically from the second week of drug injection (Fig. 4a). In addition, CCI-779 significantly decreased mortality of mutant mice (P = 0.0002, Fig. 4b). Thus, 10 mg/kg CCI-779 effectively reduced the seizure frequency and death rate of adult mutant mice. No seizures or mortality were observed in littermate controls treated with vehicle or CCI-779.
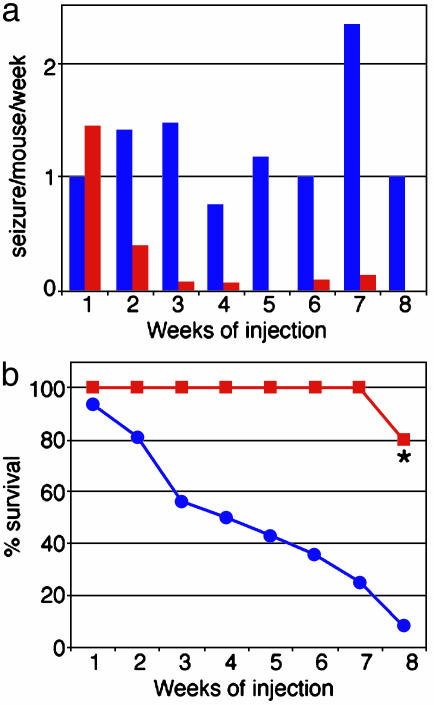
Fig. 4.
CCI-779 treatment reduced seizure frequency and death rate of adult mutant mice. CCI-779 treatment dramatically decreased the incidence of seizures (a) and mortality (b)of Pten mutant mice (n = 18 for the first 4 weeks, n = 11 continued for 6 weeks, n = 6 continued for 8 weeks, red bars and lines), compared with vehicle-treated mutant mice (n = 20 at first week, blue bars and lines). *, The only death in the CCI-779-treated group was caused by aggressive attacks from a littermate mutant mouse treated with CCI-779 and did not display symptoms typically associated with premature death in mutant mice.
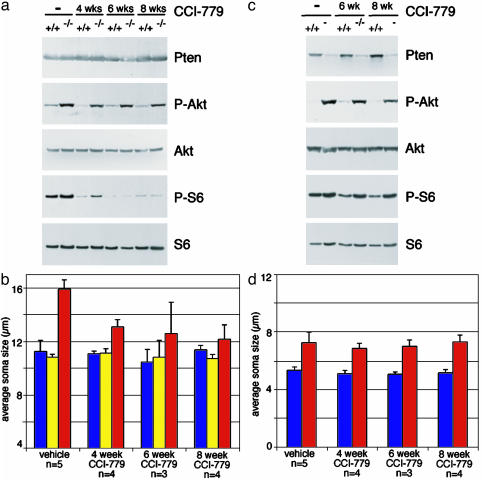
Lower-Dose CCI-779 Treatment Selectively Reversed Neuronal Hypertrophy in the Dentate Gyrus. We assessed the efficacy and specificity of CCI-779 action in hippocampal formation and cerebellum of adult mice by Western blotting. The hippocampal formation is comprised of granule neurons of the dentate gyrus, in which Pten deletion is substantial but incomplete, as well as hippocampal neurons and glia in which there is no significant deletion of Pten. Therefore, decreased Pten levels were not obvious by Western blot of this mixed cell population. However, increased levels of P-Akt and P-S6 were observed in vehicle-treated mutant mice (Fig. 5a). As expected, CCI-779 treatment did not significantly alter Akt phosphorylation, but almost completely blocked S6 phosphorylation in the hippocampal formation from adult mutant mice (Fig. 5a). Immunohistochemistry confirmed the absence of P-S6 in dentate gyri from mice treated with CCI-779 (data not shown).
Fig. 5.
Lower-dose CCI-779 treatment blocked S6 phosphorylation and reversed neuronal hypertrophy in the dentate gyrus, but not cerebellum, of mutant mice. (a) Western blotting of extracts from adult hippocampal formation from control (+/+) and Pten mutant (–/–) mice treated with vehicle (–)or10mg/kg five times a week CCI-779 for 4, 6, or 8 weeks. CCI-779 treatment specifically decreased downstream phosphorylation of S6 but did not alter levels of P-Akt, Pten, or total S6 protein. (b) Sections were immunostained for Pten, Pten-negative (red bars) and -positive (yellow bars) granule cells in mutant dentate gyrus were separately measured, and the average soma diameter was compared with dentate gyrus granule cells from littermate control mice (blue bars). CCI-779 treatment significantly decreased the size of Pten-deficient granule neurons compared with vehicle-treated mice (P < 0.0005 for 4 and 8 weeks of treatment, P < 0.05 for 6 weeks of treatment). (c) Western blotting of extracts from adult cerebellum from control (+/+) and Pten mutant (–/–) mice treated with vehicle (–) or 10 mg/kg CCI-779 for 6 or 8 weeks. CCI-779 treatment only slightly decreased downstream phosphorylation of S6 compared with effects in the dentate gyrus (a), and did not alter levels of P-Akt, Pten, or total S6 protein. (d) In the cerebellum, the average diameter of cresyl violet-stained granule cells from mutant (red bars) and littermate controls (blue bars) are shown. The size of Pten-deficient granule neurons was not significantly different from mice treated with CCI-779 compared with vehicle (P > 0.2 for all lengths of treatment).
To assess the effect of CCI-779 treatment on hypertrophy of Pten-deficient neurons in adult mutant mice, we measured soma diameters of Pten-positive and -negative granule neurons in the dentate gyrus. In the vehicle-treated group, as expected, the average soma diameter of Pten-negative cells from mutant mice was ≈40% larger than those of Pten-positive cells from both control and mutant mice (Fig. 5b). After 4 weeks of CCI-779 treatment, the average soma diameter of Pten-negative cells was significantly reduced in mutant mice (P < 0.0005), whereas the average soma diameters of Pten-positive cells were not significantly changed in mutant or control mice. Similar decreases in the hypertrophy of Pten-negative cells in adult mutant mice were observed after 6 and 8 weeks of CCI-779 treatment. The diameter of Pten-deficient granule neurons were smaller in adult mice treated with CCI-779 than in vehicle-treated mutant mice at 4 weeks of age, indicating that CCI-779 treatment was able to reverse neuronal hypertrophy.
In the mutant cerebellum, Pten was deleted in the vast majority of granule neurons, which outnumber other cell types in the tissue. Therefore, mutant mice showed obviously decreased levels of Pten and corresponding increases in P-Akt in protein extracts from cerebellum (Fig. 5c). As expected from the decreased sensitivity observed in cerebellum, CCI-779 treatment only moderately decreased the level of P-S6 (Fig. 5c). Furthermore, CCI-779 treatment did not affect the substantial increase in soma size observed in cerebellar granule cells from mutant mice compared with control mice (Fig. 5d). Thus, 10 mg/kg CCI-779 treatment selectively blocked the mTor pathway and reversed neuronal hypertrophy in dentate gyrus, but not in cerebellum of Pten mutant mice.
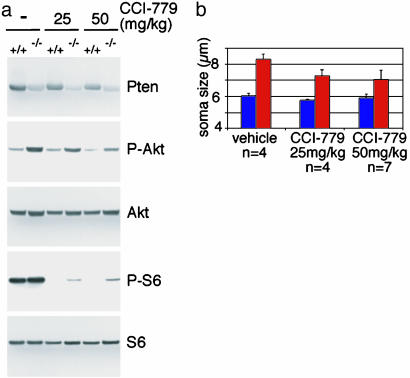
Increased CCI-779 Dosage Reversed Hypertrophy of Pten-Deficient Neurons in the Cerebellum. Adult mice were treated with 25 or 50 mg/kg CCI-779 for 4 weeks, to see whether increased dosages of CCI-779 could reverse neuronal hypertrophy in the cerebellum. Western blotting showed that CCI-779 treatment effectively blocked mTor signaling in the cerebellum as it dramatically decreased levels of P-S6 compared with vehicle treatment (Fig. 6a). The residual level of P-S6 is substantially lower than in vehicle-treated control mice, and therefore unlikely to drive hypertrophy. Measurements of cerebellar granule cells from these treated mice showed that this effective dosage of CCI-779 also caused a significant, but incomplete reversion of the cerebellar granule cell hypertrophy observed in mutant mice (Fig. 6b).
Fig. 6.
Higher-dose CCI-779 treatment reversed Pten-deficient hypertrophy in cerebellum. (a) Western blotting of extracts from adult cerebellum from control (+/+) and Pten mutant (–/–) mice treated with vehicle (–), 25, or 50 mg/kg CCI-779 for 4 weeks. At these dosages, CCI-779 treatment effectively decreased downstream phosphorylation of S6. (b) The diameter of granule neuron soma from control (blue bars) or Pten mutant (red bars) was measured from sections stained with cresyl violet. CCI-779 treatment caused a significant reduction in the size of Pten-deficient neurons compared with vehicle treatment (P < 0.05).
Discussion
The ability of CCI-779 to prevent or reverse abnormalities caused by Pten deficiency was directly related to the efficacy of mTor inhibition, providing compelling evidence that mTor is a key effector in hypertrophic growth signaling in neurons. This finding is consistent with the role for TOR downstream of insulin/PI3K growth signaling in Drosophila models (25), and also downstream of constitutive Akt signaling in an in vivo mammalian model of muscle hypertrophy (26, 27). In addition to a ubiquitous and conserved function in growth signaling, PI3k and Akt are also implicated in a number of specialized and complex processes in the mammalian nervous system, including neuronal survival, synaptic plasticity, and axonal branching and caliber (28). mTor also contributes to long-term synaptic plasticity (29). The precise cause of the seizures in Pten mutant mice is unclear; however, mTor inhibition dramatically affected this complex phenotype as well as the cell-autonomous growth defects.
The normal maintenance of neuronal soma size is not mTor-dependent, because CCI-779 treatment did not cause a significant decrease in adult soma size. Furthermore, although CCI-779, when used at appropriate dosages, induced a substantial reversal of hypertrophy in both the dentate gyrus and cerebellum, the growth abnormalities were not completely corrected. This is likely to indicate that a component of the Pten-deficient hypertrophic signal is mTor-independent, consistent with in vitro experiments that showed that mTor is not required for PI3k-dependent survival of cerebellar granule neurons (30, 31). The concept of multiple downstream effectors in insulin-PI3K signaling is also supported by recent genetic studies in Drosophila and biochemical studies in mammalian culture systems that demonstrate multiple parallel pathways rather than a simple linear progression for insulin-dependent growth signaling (32). Reversion of hypertrophy was less significant in cerebellum than in dentate gyrus; thus, mTor-independent pathways may play a greater role in growth regulation in cerebellar granule cells. Alternatively, it may be more difficult to reverse the established hypertrophic abnormality that includes secondary changes such as compensatory elongation of glia in the enlarged cerebellum, atrophy of Purkinje neurons that synapse with granule neurons (15) and increased myelination (16). We were unable to determine the contribution of mTor to defective cell migration in developing cerebellum because the dosage of CCI-779 required to inhibit mTor activity in the cerebellum was toxic at this stage of development. Local administration of CCI-779 directly to the cerebellum of developing mice could circumvent this problem.
mTOR is a key regulator of mammalian cell growth and size, and therefore is a relevant therapeutic target for pathological growth including hypertrophic conditions and cancer. CCI-779 is currently in clinical trials for use as an anticancer agent. Our findings have obvious therapeutic implications for disorders resulting from PTEN deficiency in brain, such as Lhermitte–Duclos disease, ataxia and seizures associated with germline mutations in PTEN, and glioblastoma multiforme, in which PTEN mutation and AKT activation occur frequently. Substantially higher doses of CCI-779 were required to inhibit mTor in brain compared with circulating T cells, and the effective dose for brain varied between regions. The relative drug sensitivities of different neuronal populations in vivo was not apparent in vitro. Therefore, Pten conditional knockout mice may be a useful in vivo model system to test other therapeutic agents under consideration for PTEN-deficient brain disorders.
Supplementary Material
Acknowledgments
We thank Drs. Michisuki Yuzaki and Keiko Matsuda for hippocampal cultures; Drs. Peter Houghton, Tom Curran, Peter McKinnon, and Richard Gilbertson for helpful discussions; Dr. Tak Mak for PtenloxP mice; and Philip Frost, James Gibbons, and Wyeth–Ayerst for CCI-779. This work was supported in part by a National Institutes of Health Cancer Center Support CORE grant, National Institutes of Health Grants NS44172, CA096832, and CA92117 (to S.J.B.), and the American Lebanese Syrian Associated Charities.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Pn, postnatal day n; PI3K, phosphatidylinositol 3-kinase; P-S6, phospho-S6; P-Akt, phospho-Akt.
References
- 1.Ali, I. U., Schriml, L. M. & Dean, M. (1999) J. Natl. Cancer Inst. 91 1922–1932. [DOI] [PubMed] [Google Scholar]
- 2.Maehama, T. & Dixon, J. E. (1998) J. Biol. Chem. 273 13375–13378. [DOI] [PubMed] [Google Scholar]
- 3.Cantley, L. C. (2002) Science 296 1655–1657. [DOI] [PubMed] [Google Scholar]
- 4.Marsh, D. J., Kum, J. B., Lunetta, K. L., Bennett, M. J., Gorlin, R. J., Ahmed, S. F., Bodurtha, J., Crowe, C., Curtis, M. A., Dasouki, M., et al. (1999) Hum. Mol. Genet. 8 1461–1472. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki, A., de la Pompa, J. L., Stambolic, V., Elia, A. J., Sasaki, T., del Barco Barrantes, I., Ho, A., Wakeham, A., Itie, A., Khoo, W., et al. (1998) Curr. Biol. 8 1169–1178. [DOI] [PubMed] [Google Scholar]
- 6.Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P. P. (1998) Nat. Genet. 19 348–355. [DOI] [PubMed] [Google Scholar]
- 7.Podsypanina, K., Ellenson, L. H., Nemes, A., Gu, J., Tamura, M., Yamada, K. M., Cordon-Cardo, C., Catoretti, G., Fisher, P. E. & Parsons, R. (1999) Proc. Natl. Acad. Sci. USA 96 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groszer, M., Erickson, R., Scripture-Adams, D. D., Lesche, R., Trumpp, A., Zack, J. A., Kornblum, H. I., Liu, X. & Wu, H. (2001) Science 294 2186–2189. [DOI] [PubMed] [Google Scholar]
- 9.Marino, S., Krimpenfort, P., Leung, C., van der Korput, H. A., Trapman, J., Camenisch, I., Berns, A. & Brandner, S. (2002) Development (Cambridge, U.K.) 129 3513–3522. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki, A., Yamaguchi, M. T., Ohteki, T., Sasaki, T., Kaisho, T., Kimura, Y., Yoshida, R., Wakeham, A., Higuchi, T., Fukumoto, M., et al.(2001) Immunity 14 523–534. [DOI] [PubMed] [Google Scholar]
- 11.Anzelon, A. N., Wu, H. & Rickert, R. C. (2003) Nat. Immunol. 4 287–294. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki, A., Itami, S., Ohishi, M., Hamada, K., Inoue, T., Komazawa, N., Senoo, H., Sasaki, T., Takeda, J., Manabe, M., et al. (2003) Cancer Res. 63 674–681. [PubMed] [Google Scholar]
- 13.Li, G., Robinson, G. W., Lesche, R., Martinez-Diaz, H., Jiang, Z., Rozengurt, N., Wagner, K. U., Wu, D. C., Lane, T. F., Liu, X., et al. (2002) Development (Cambridge, U.K.) 129 4159–4170. [DOI] [PubMed] [Google Scholar]
- 14.Crackower, M. A., Oudit, G. Y., Kozieradzki, I., Sarao, R., Sun, H., Sasaki, T., Hirsch, E., Suzuki, A., Shioi, T., Irie-Sasaki, J., et al. (2002) Cell 110 737–749. [DOI] [PubMed] [Google Scholar]
- 15.Kwon, C. H., Zhu, X., Zhang, J., Knoop, L. L., Tharp, R., Smeyne, R. J., Eberhart, C. G., Burger, P. C. & Baker, S. J. (2001) Nat. Genet. 29 404–411. [DOI] [PubMed] [Google Scholar]
- 16.Backman, S. A., Stambolic, V., Suzuki, A., Haight, J., Elia, A., Pretorius, J., Tsao, M. S., Shannon, P., Bolon, B., Ivy, G. O. & Mak, T. W. (2001) Nat. Genet. 29 396–403. [DOI] [PubMed] [Google Scholar]
- 17.Oldham, S., Montagne, J., Radimerski, T., Thomas, G. & Hafen, E. (2000) Genes Dev. 14 2689–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, H., Stallock, J. P., Ng, J. C., Reinhard, C. & Neufeld, T. P. (2000) Genes Dev. 14 2712–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neshat, M. S., Mellinghoff, I. K., Tran, C., Stiles, B., Thomas, G., Petersen, R., Frost, P., Gibbons, J. J., Wu, H. & Sawyers, C. L. (2001) Proc. Natl. Acad. Sci. USA 98 10314–10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podsypanina, K., Lee, R. T., Politis, C., Hennessy, I., Crane, A., Puc, J., Neshat, M., Wang, H., Yang, L., Gibbons, J., et al. (2001) Proc. Natl. Acad. Sci. USA 98 10320–10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu, X., Kwon, C. H., Schlosshauer, P. W., Ellenson, L. H. & Baker, S. J. (2001) Cancer Res. 61 4569–4575. [PubMed] [Google Scholar]
- 22.Gingras, A. C., Raught, B. & Sonenberg, N. (2001) Genes Dev. 15 807–826. [DOI] [PubMed] [Google Scholar]
- 23.Schmelzle, T. & Hall, M. N. (2000) Cell 103 253–262. [DOI] [PubMed] [Google Scholar]
- 24.Saitoh, M., Pullen, N., Brennan, P., Cantrell, D., Dennis, P. B. & Thomas, G. (2002) J. Biol. Chem. 277 20104–20112. [DOI] [PubMed] [Google Scholar]
- 25.Oldham, S. & Hafen, E. (2003) Trends Cell Biol. 13 79–85. [DOI] [PubMed] [Google Scholar]
- 26.Rommel, C., Bodine, S. C., Clarke, B. A., Rossman, R., Nunez, L., Stitt, T. N., Yancopoulos, G. D. & Glass, D. J. (2001) Nat. Cell Biol. 3 1009–1013. [DOI] [PubMed] [Google Scholar]
- 27.Bodine, S. C., Stitt, T. N., Gonzalez, M., Kline, W. O., Stover, G. L., Bauerlein, R., Zlotchenko, E., Scrimgeour, A., Lawrence, J. C., Glass, D. J. & Yancopoulos, G. D. (2001) Nat. Cell Biol. 3 1014–1019. [DOI] [PubMed] [Google Scholar]
- 28.Markus, A., Zhong, J. & Snider, W. D. (2002) Neuron 35 65–76. [DOI] [PubMed] [Google Scholar]
- 29.Tang, S. J., Reis, G., Kang, H., Gingras, A. C., Sonenberg, N. & Schuman, E. M. (2002) Proc. Natl. Acad. Sci. USA 99 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams, E. J. & Doherty, P. (1999) Mol. Cell. Neurosci. 13 272–280. [DOI] [PubMed] [Google Scholar]
- 31.Gunn-Moore, F. J., Williams, A. G., Toms, N. J. & Tavare, J. M. (1997) Biochem. J. 324 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozma, S. C. & Thomas, G. (2002) BioEssays 24 65–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.