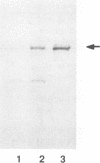
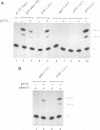
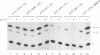
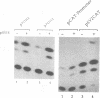
Abstract
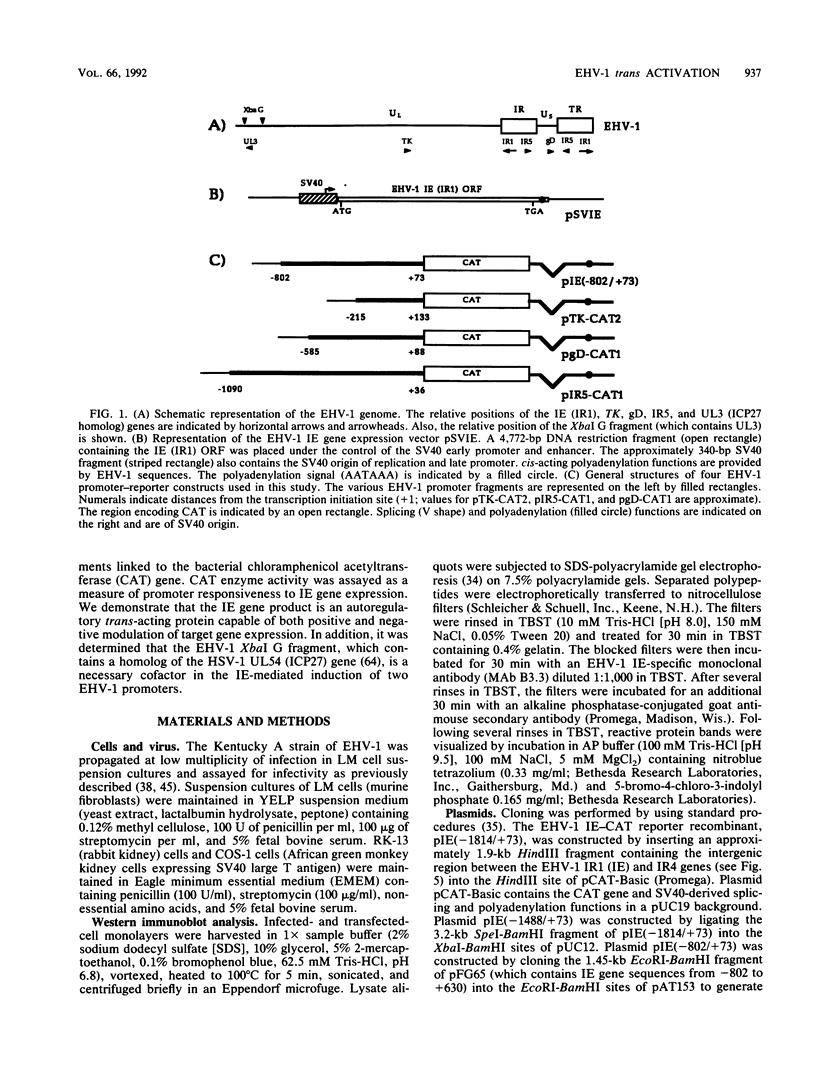
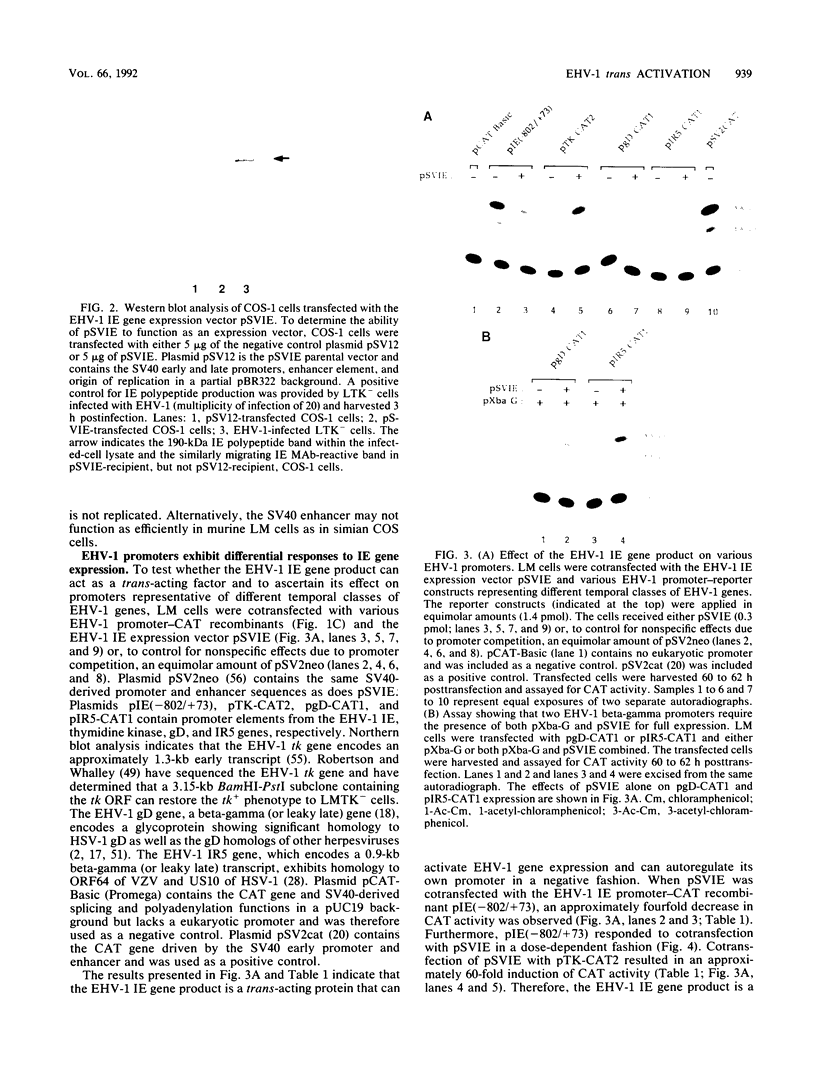
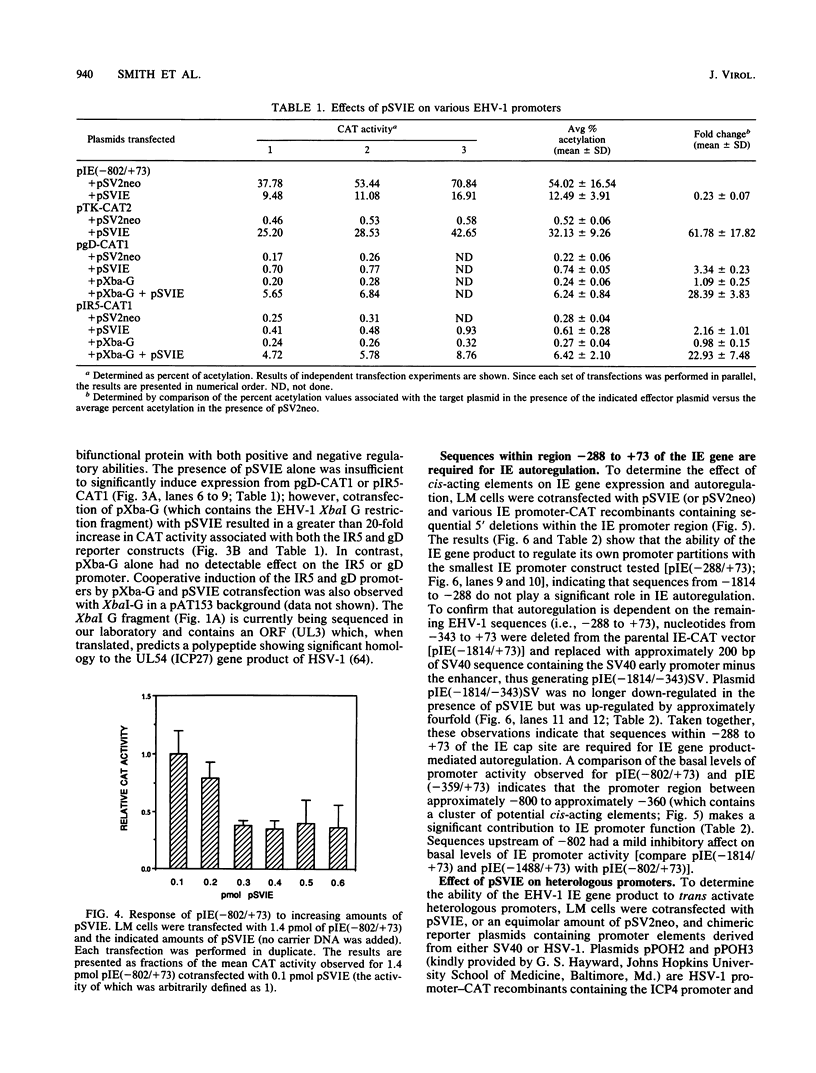
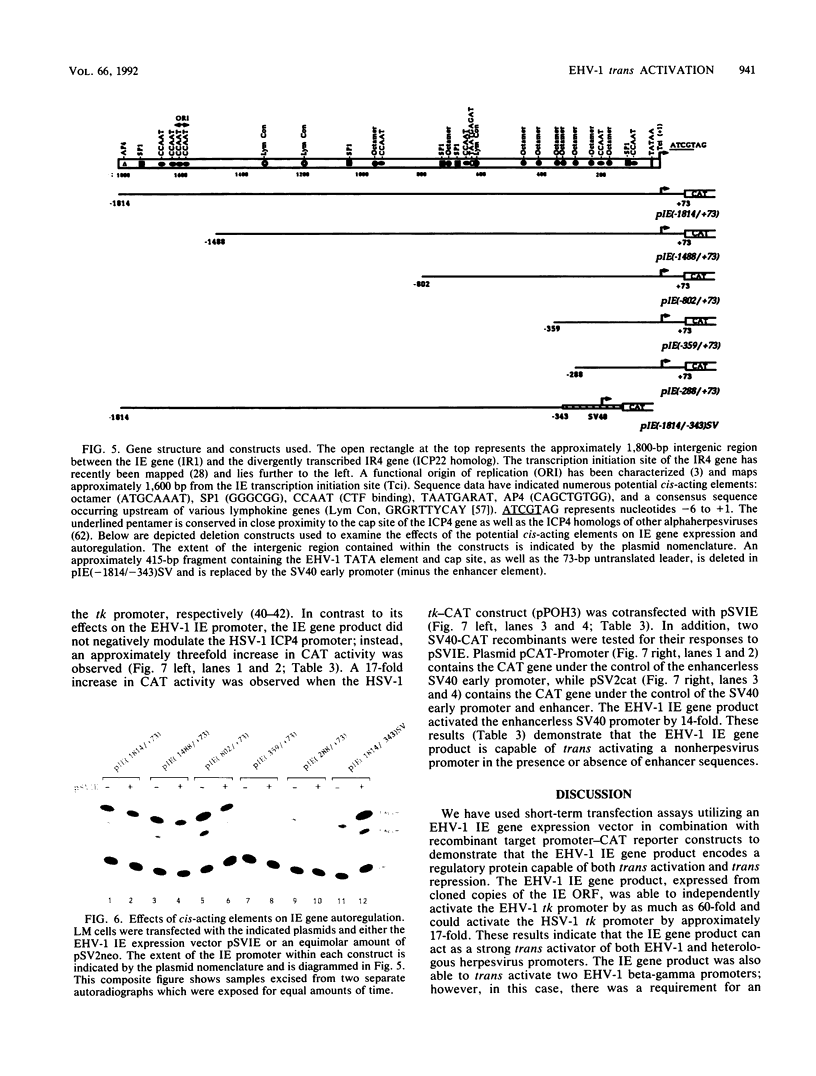
Use of the translation-inhibiting drug cycloheximide has indicated that the equine herpesvirus 1 (EHV-1) immediate-early (IE) gene, the sole EHV-1 IE gene, encodes a major viral regulatory protein since IE mRNA translation is a prerequisite for all further viral gene expression (W.L. Gray, R. P. Baumann, A. T. Robertson, G. B. Caughman, D. J. O'Callaghan, and J. Staczek, Virology 158:79-87, 1987). An EHV-1 IE gene expression vector (pSVIE) in combination with chimeric EHV-1 promoter-chloramphenicol acetyltransferase (CAT) reporter constructs was used in transient transfection assays to characterize the regulatory functions of the IE gene product. These experiments demonstrated that (i) the EHV-1 IE gene product is a bifunctional protein capable of both positive and negative modulation of gene expression; (ii) the IE gene product possesses an autoregulatory function which represses the IE promoter; (iii) IE autoregulation is dependent on IE promoter sequences mapping within positions -288 to +73 relative to the transcription initiation site (+1) of the IE gene; (iv) the IE gene product can independently activate the EHV-1 tk promoter (an early promoter) by as much as 60-fold; (v) two EHV-1 beta-gamma (leaky late) promoters, those of IR5 (gene 5 in the inverted repeat) and the glycoprotein D gene, demonstrate a requirement for both the IE gene product as well as a gene product encoded within the EHV-1 XbaI G fragment for significant activation; and (vi) the IE gene product is capable of activating heterologous viral promoters.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., Bryans J. T. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog Vet Microbiol Immunol. 1986;2:78–144. [PubMed] [Google Scholar]
- Audonnet J. C., Winslow J., Allen G., Paoletti E. Equine herpesvirus type 1 unique short fragment encodes glycoproteins with homology to herpes simplex virus type 1 gD, gI and gE. J Gen Virol. 1990 Dec;71(Pt 12):2969–2978. doi: 10.1099/0022-1317-71-12-2969. [DOI] [PubMed] [Google Scholar]
- Baumann R. P., Yalamanchili V. R., O'Callaghan D. J. Functional mapping and DNA sequence of an equine herpesvirus 1 origin of replication. J Virol. 1989 Mar;63(3):1275–1283. doi: 10.1128/jvi.63.3.1275-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabirac G. F., Mahalingam R., Wellish M., Gilden D. H. Trans-activation of viral tk promoters by proteins encoded by varicella zoster virus open reading frames 61 and 62. Virus Res. 1990 Jan;15(1):57–68. doi: 10.1016/0168-1702(90)90013-2. [DOI] [PubMed] [Google Scholar]
- Caughman G. B., Robertson A. T., Gray W. L., Sullivan D. C., O'Callaghan D. J. Characterization of equine herpesvirus type 1 immediate early proteins. Virology. 1988 Apr;163(2):563–571. doi: 10.1016/0042-6822(88)90297-8. [DOI] [PubMed] [Google Scholar]
- Caughman G. B., Staczek J., O'Callaghan D. J. Equine herpesvirus type 1 infected cell polypeptides: evidence for immediate early/early/late regulation of viral gene expression. Virology. 1985 Aug;145(1):49–61. doi: 10.1016/0042-6822(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Perdue M. L., Randall C. C., O'Callaghan D. J. Herpesvirus transcription: altered regulation induced by FUdR. Virology. 1977 Feb;76(2):621–633. doi: 10.1016/0042-6822(77)90244-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Randall C. C., O'Callaghan D. J. Transcription of equine herpesvirus type 1: evidence for classes of transcripts differing in abundance. Virology. 1975 Dec;68(2):561–565. doi: 10.1016/0042-6822(75)90299-8. [DOI] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988 Mar;62(3):732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney G. H., McKee T. A., Preston C. M., Everett R. D. The product of varicella-zoster virus gene 62 autoregulates its own promoter. J Gen Virol. 1990 Dec;71(Pt 12):2999–3003. doi: 10.1099/0022-1317-71-12-2999. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Dunlop M. Trans activation of plasmid-borne promoters by adenovirus and several herpes group viruses. Nucleic Acids Res. 1984 Aug 10;12(15):5969–5978. doi: 10.1093/nar/12.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986 Nov;67(Pt 11):2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber S. W., Wilcox K. W. Association of the herpes simplex virus regulatory protein ICP4 with specific nucleotide sequences in DNA. Nucleic Acids Res. 1986 Aug 11;14(15):6067–6083. doi: 10.1093/nar/14.15.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers C. C., Eastman E. M., O'Callaghan D. J. Sequence analysis of a glycoprotein D gene homolog within the unique short segment of the EHV-1 genome. Virology. 1991 Jan;180(1):175–184. doi: 10.1016/0042-6822(91)90021-3. [DOI] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., Caughman G. B., O'Callaghan D. J., Staczek J. Regulation of equine herpesvirus type 1 gene expression: characterization of immediate early, early, and late transcription. Virology. 1987 May;158(1):79–87. doi: 10.1016/0042-6822(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., O'Callaghan D. J., Staczek J. Characterization and mapping of equine herpesvirus type 1 immediate early, early, and late transcripts. Virus Res. 1987 Sep;8(3):233–244. doi: 10.1016/0168-1702(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Grundy F. J., Baumann R. P., O'Callaghan D. J. DNA sequence and comparative analyses of the equine herpesvirus type 1 immediate early gene. Virology. 1989 Sep;172(1):223–236. doi: 10.1016/0042-6822(89)90124-4. [DOI] [PubMed] [Google Scholar]
- Harty R. N., Colle C. F., Grundy F. J., O'Callaghan D. J. Mapping the termini and intron of the spliced immediate-early transcript of equine herpesvirus 1. J Virol. 1989 Dec;63(12):5101–5110. doi: 10.1128/jvi.63.12.5101-5110.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty R. N., O'Callaghan D. J. An early gene maps within and is 3' coterminal with the immediate-early gene of equine herpesvirus 1. J Virol. 1991 Jul;65(7):3829–3838. doi: 10.1128/jvi.65.7.3829-3838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. E., Robinson R. A., Dauenhauer S. A., Atherton S. S., Hayward G. S., O'Callaghan D. J. Structure of the genome of equine herpesvirus type 1. Virology. 1981 Nov;115(1):97–114. doi: 10.1016/0042-6822(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Holden V. R., Yalamanchili R. R., Harty R. N., O'Callaghan D. J. ICP22 homolog of equine herpesvirus 1: expression from early and late promoters. J Virol. 1992 Feb;66(2):664–673. doi: 10.1128/jvi.66.2.664-673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. L., Szabocsik J. M., Randall C. C., Gentry G. A. Equine abortion (herpes) virus-specific RNA. Virology. 1971 Aug;45(2):381–389. doi: 10.1016/0042-6822(71)90339-4. [DOI] [PubMed] [Google Scholar]
- Inchauspe G., Nagpal S., Ostrove J. M. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology. 1989 Dec;173(2):700–709. doi: 10.1016/0042-6822(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Inchauspe G., Ostrove J. M. Differential regulation by varicella-zoster virus (VZV) and herpes simplex virus type-1 trans-activating genes. Virology. 1989 Dec;173(2):710–714. doi: 10.1016/0042-6822(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Brauer D. H. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 1986 Feb 25;14(4):1727–1745. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987 Mar;61(3):858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D. J., Cheevers W. P., Gentry G. A., Randall C. C. Kinetics of cellular and viral DNA synthesis in equine abortion (herpes) virus infection of L-M cells. Virology. 1968 Sep;36(1):104–114. doi: 10.1016/0042-6822(68)90120-7. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985 Mar;53(3):751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Expression of recombinant genes containing herpes simplex virus delayed-early and immediate-early regulatory regions and trans activation by herpesvirus infection. J Virol. 1984 Nov;52(2):522–531. doi: 10.1128/jvi.52.2.522-531.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J Virol. 1985 Dec;56(3):723–733. doi: 10.1128/jvi.56.3.723-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson T., Everett R. D. Mutational dissection of the HSV-1 immediate-early protein Vmw175 involved in transcriptional transactivation and repression. Virology. 1988 Sep;166(1):186–196. doi: 10.1016/0042-6822(88)90160-2. [DOI] [PubMed] [Google Scholar]
- Paterson T., Everett R. D. The regions of the herpes simplex virus type 1 immediate early protein Vmw175 required for site specific DNA binding closely correspond to those involved in transcriptional regulation. Nucleic Acids Res. 1988 Dec 9;16(23):11005–11025. doi: 10.1093/nar/16.23.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Knipe D. M. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985 May;5(5):957–963. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990 Apr;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. S., Boundy A., O'Hare P., Pizzorno M. C., Ciufo D. M., Hayward G. S. Direct correlation between a negative autoregulatory response element at the cap site of the herpes simplex virus type 1 IE175 (alpha 4) promoter and a specific binding site for the IE175 (ICP4) protein. J Virol. 1988 Nov;62(11):4307–4320. doi: 10.1128/jvi.62.11.4307-4320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. T., Caughman G. B., Gray W. L., Baumann R. P., Staczek J., O'Callaghan D. J. Analysis of the in vitro translation products of the equine herpesvirus type 1 immediate early mRNA. Virology. 1988 Oct;166(2):451–462. doi: 10.1016/0042-6822(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Robertson G. R., Whalley J. M. Evolution of the herpes thymidine kinase: identification and comparison of the equine herpesvirus 1 thymidine kinase gene reveals similarity to a cell-encoded thymidylate kinase. Nucleic Acids Res. 1988 Dec 9;16(23):11303–11317. doi: 10.1093/nar/16.23.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Ross L. J., Binns M. M. Properties and evolutionary relationships of the Marek's disease virus homologues of protein kinase, glycoprotein D and glycoprotein I of herpes simplex virus. J Gen Virol. 1991 Apr;72(Pt 4):939–947. doi: 10.1099/0022-1317-72-4-939. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Dauenhauer S. A., O'Callaghan D. J. Electron microscopic study of equine herpesvirus type 1 DNA. J Virol. 1982 Apr;42(1):297–300. doi: 10.1128/jvi.42.1.297-300.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulovich R. E., Leary K., Sandri-Goldin R. M. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988 Dec;62(12):4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stanley E., Metcalf D., Sobieszczuk P., Gough N. M., Dunn A. R. The structure and expression of the murine gene encoding granulocyte-macrophage colony stimulating factor: evidence for utilisation of alternative promoters. EMBO J. 1985 Oct;4(10):2569–2573. doi: 10.1002/j.1460-2075.1985.tb03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Knipe D. M. Herpes simplex virus alpha protein ICP27 can inhibit or augment viral gene transactivation. Virology. 1989 Jun;170(2):496–504. doi: 10.1016/0042-6822(89)90441-8. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980 May 29;285(5763):329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Davison A. J. Analysis of the genome of equine herpesvirus type 1: arrangement of cleavage sites for restriction endonucleases EcoRI, BglII and BamHI. J Gen Virol. 1981 Dec;57(Pt 2):307–323. doi: 10.1099/0022-1317-57-2-307. [DOI] [PubMed] [Google Scholar]
- Wu C. L., Wilcox K. W. Codons 262 to 490 from the herpes simplex virus ICP4 gene are sufficient to encode a sequence-specific DNA binding protein. Nucleic Acids Res. 1990 Feb 11;18(3):531–538. doi: 10.1093/nar/18.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. L., Wilcox K. W. The conserved DNA-binding domains encoded by the herpes simplex virus type 1 ICP4, pseudorabies virus IE180, and varicella-zoster virus ORF62 genes recognize similar sites in the corresponding promoters. J Virol. 1991 Mar;65(3):1149–1159. doi: 10.1128/jvi.65.3.1149-1159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili R. R., O'Callaghan D. J. Sequence and organization of the genomic termini of equine herpesvirus type 1. Virus Res. 1990 Feb;15(2):149–161. doi: 10.1016/0168-1702(90)90005-v. [DOI] [PubMed] [Google Scholar]