Abstract
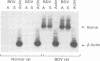
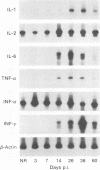
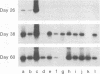
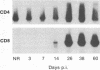
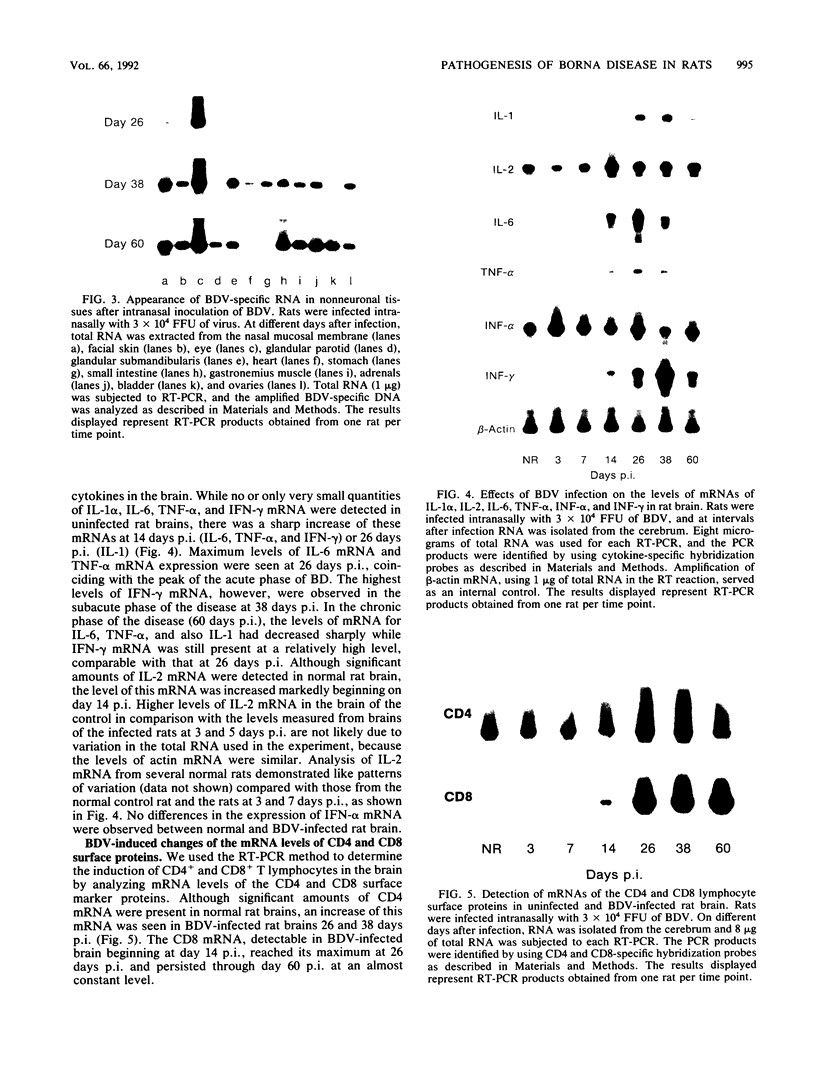
We have used the reverse transcriptase-polymerase chain reaction technique to gain insight into the pathogenesis of encephalitis caused by Borna disease virus (BDV). RNA specific for BDV was first detected in the olfactory bulb of intranasally infected rats at 6 days postinfection (p.i.). At 14 days p.i., high levels of BDV RNA were found in all brain regions, and at 26 days p.i., BDV-specific RNA was also present in the eye, nasal mucosa, and facial skin. In the chronic phase of the disease, BDV RNA was identified in many peripheral organs but not in blood. Analysis of brain tissue for the presence of cytokine mRNAs revealed that the mRNA levels of interleukin-6 (IL-6), tumor necrosis factor alpha, and IL-1 alpha had increased sharply at 14 and 26 days p.i. These cytokine mRNAs reached maximum levels at the peak of inflammatory reactions and decreased drastically in the chronic phase of the disease. Although IL-2 mRNA was also found in normal brain, it was markedly increased in BDV-infected brain at 14 days p.i. Expression of gamma interferon (IFN-gamma) mRNA, which was not observed in normal rat brain, was detected at 14 days p.i. and reached a maximum level at 38 days p.i. IL-2 and IFN-gamma mRNA expression correlated with expression of CD4 and CD8 mRNAs, indicating that both CD4+ and CD8+ T lymphocytes are induced in the early stages of BDV infection. Since IFN-gamma and CD8 mRNA levels were still highly elevated in the chronic phase of Borna disease, it is likely that CD8+ T lymphocytes act to reduce inflammation and to ameliorate neurological signs during the chronic phase of infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Duchala C. S., Griffin J. W., Kincaid A. L., Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987 Nov;61(11):3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Moench T. R., Lipkin W. I. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol. 1991 May;50(3):205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- Deschl U., Stitz L., Herzog S., Frese K., Rott R. Determination of immune cells and expression of major histocompatibility complex class II antigen in encephalitic lesions of experimental Borna disease. Acta Neuropathol. 1990;81(1):41–50. doi: 10.1007/BF00662636. [DOI] [PubMed] [Google Scholar]
- Dittrich W., Bode L., Ludwig H., Kao M., Schneider K. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol Psychiatry. 1989 Dec;26(8):818–828. doi: 10.1016/0006-3223(89)90122-4. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Dunlop M. B., Parish C. R., Zinkernagel R. M. Inflammatory process in murine lymphocytic choriomeningitis is maximal in H-2K or H-2D compatible interactions. J Immunol. 1976 Jul;117(1):187–190. [PubMed] [Google Scholar]
- Erickson J. D., Trojanowski J. Q., Eiden L. E. Regional distribution and partial molecular characterization of CD4-related mRNA in human brain and peripheral tissues. Brain Res Mol Brain Res. 1991 Apr;10(1):23–31. doi: 10.1016/0169-328x(91)90052-y. [DOI] [PubMed] [Google Scholar]
- Fontana A., Frei K., Bodmer S., Hofer E. Immune-mediated encephalitis: on the role of antigen-presenting cells in brain tissue. Immunol Rev. 1987 Dec;100:185–201. doi: 10.1111/j.1600-065X.1987.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Gorman S. D., Tourvieille B., Parnes J. R. Structure of the mouse gene encoding CD4 and an unusual transcript in brain. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7644–7648. doi: 10.1073/pnas.84.21.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I., Atkinson J. H. Neurogenic and psychogenic behavioral correlates of HIV infection. Res Publ Assoc Res Nerv Ment Dis. 1990;68:291–304. [PubMed] [Google Scholar]
- Harris C. A., Derbin K. S., Hunte-McDonough B., Krauss M. R., Chen K. T., Smith D. M., Epstein L. B. Manganese superoxide dismutase is induced by IFN-gamma in multiple cell types. Synergistic induction by IFN-gamma and tumor necrosis factor or IL-1. J Immunol. 1991 Jul 1;147(1):149–154. [PubMed] [Google Scholar]
- Heyes M. P., Brew B. J., Martin A., Price R. W., Salazar A. M., Sidtis J. J., Yergey J. A., Mouradian M. M., Sadler A. E., Keilp J. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991 Feb;29(2):202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Hirano N., Kao M., Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus-infected rats. J Gen Virol. 1983 Jul;64(Pt 7):1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Baemayr J., Weil M., Merrill J. E. Lymphokines and immunoregulatory molecules in subacute sclerosing panencephalitis. Clin Immunol Immunopathol. 1991 Mar;58(3):331–342. doi: 10.1016/0090-1229(91)90124-s. [DOI] [PubMed] [Google Scholar]
- Holodniy M., Katzenstein D. A., Sengupta S., Wang A. M., Casipit C., Schwartz D. H., Konrad M., Groves E., Merigan T. C. Detection and quantification of human immunodeficiency virus RNA in patient serum by use of the polymerase chain reaction. J Infect Dis. 1991 Apr;163(4):862–866. doi: 10.1093/infdis/163.4.862. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Wright S. C. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990 Nov;4(14):3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- Lipkin W. I., Travis G. H., Carbone K. M., Wilson M. C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M., Kuraishi Y., Satoh M. Effects of kainic acid on messenger RNA levels of IL-1 beta, IL-6, TNF alpha and LIF in the rat brain. Biochem Biophys Res Commun. 1991 Apr 30;176(2):593–598. doi: 10.1016/s0006-291x(05)80225-6. [DOI] [PubMed] [Google Scholar]
- Mintz M., Rapaport R., Oleske J. M., Connor E. M., Koenigsberger M. R., Denny T., Epstein L. G. Elevated serum levels of tumor necrosis factor are associated with progressive encephalopathy in children with acquired immunodeficiency syndrome. Am J Dis Child. 1989 Jul;143(7):771–774. doi: 10.1001/archpedi.1989.02150190021012. [DOI] [PubMed] [Google Scholar]
- Morales J. A., Herzog S., Kompter C., Frese K., Rott R. Axonal transport of Borna disease virus along olfactory pathways in spontaneously and experimentally infected rats. Med Microbiol Immunol. 1988;177(2):51–68. doi: 10.1007/BF00189527. [DOI] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Behavioral disease in rats caused by immunopathological responses to persistent borna virus in the brain. Science. 1983 Jun 24;220(4604):1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983 Aug;148(2):305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Price R. W., Brew B. J., Rosenblum M. The AIDS dementia complex and HIV-1 brain infection: a pathogenetic model of virus-immune interaction. Res Publ Assoc Res Nerv Ment Dis. 1990;68:269–290. [PubMed] [Google Scholar]
- Quagliarello V. J., Wispelwey B., Long W. J., Jr, Scheld W. M. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Invest. 1991 Apr;87(4):1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richt J. A., Stitz L., Wekerle H., Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989 Sep 1;170(3):1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richt J., Stitz L., Deschl U., Frese K., Rott R. Borna disease virus-induced meningoencephalomyelitis caused by a virus-specific CD4+ T cell-mediated immune reaction. J Gen Virol. 1990 Nov;71(Pt 11):2565–2573. doi: 10.1099/0022-1317-71-11-2565. [DOI] [PubMed] [Google Scholar]
- Rott R., Herzog S., Fleischer B., Winokur A., Amsterdam J., Dyson W., Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985 May 10;228(4700):755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- Schijns V. E., Van der Neut R., Haagmans B. L., Bar D. R., Schellekens H., Horzinek M. C. Tumour necrosis factor-alpha, interferon-gamma and interferon-beta exert antiviral activity in nervous tissue cells. J Gen Virol. 1991 Apr;72(Pt 4):809–815. doi: 10.1099/0022-1317-72-4-809. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Mössner R., Schwender S., ter Meulen V. Major histocompatibility complex-expressing nonhematopoietic astroglial cells prime only CD8+ T lymphocytes: astroglial cells as perpetuators but not initiators of CD4+ T cell responses in the central nervous system. J Exp Med. 1991 May 1;173(5):1235–1246. doi: 10.1084/jem.173.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar V., Dietzschold B., Koprowski H. Direct entry of rabies virus into the central nervous system without prior local replication. J Virol. 1991 May;65(5):2736–2738. doi: 10.1128/jvi.65.5.2736-2738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz L., Schilken D., Frese K. Atypical dissemination of the highly neurotropic Borna disease virus during persistent infection in cyclosporine A-treated, immunosuppressed rats. J Virol. 1991 Jan;65(1):457–460. doi: 10.1128/jvi.65.1.457-460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W., Connick J. H. Quinolinic acid and other kynurenines in the central nervous system. Neuroscience. 1985 Jul;15(3):597–617. doi: 10.1016/0306-4522(85)90063-6. [DOI] [PubMed] [Google Scholar]
- VandeWoude S., Richt J. A., Zink M. C., Rott R., Narayan O., Clements J. E. A borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990 Nov 30;250(4985):1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- Wolinsky J. S. Subacute sclerosing panencephalitis, progressive rubella panencephalitis, and multifocal leukoencephalopathy. Res Publ Assoc Res Nerv Ment Dis. 1990;68:259–268. [PubMed] [Google Scholar]
- de la Torre J. C., Carbone K. M., Lipkin W. I. Molecular characterization of the Borna disease agent. Virology. 1990 Dec;179(2):853–856. doi: 10.1016/0042-6822(90)90154-j. [DOI] [PubMed] [Google Scholar]