Abstract
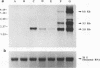
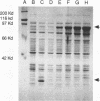
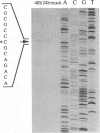
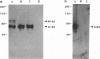
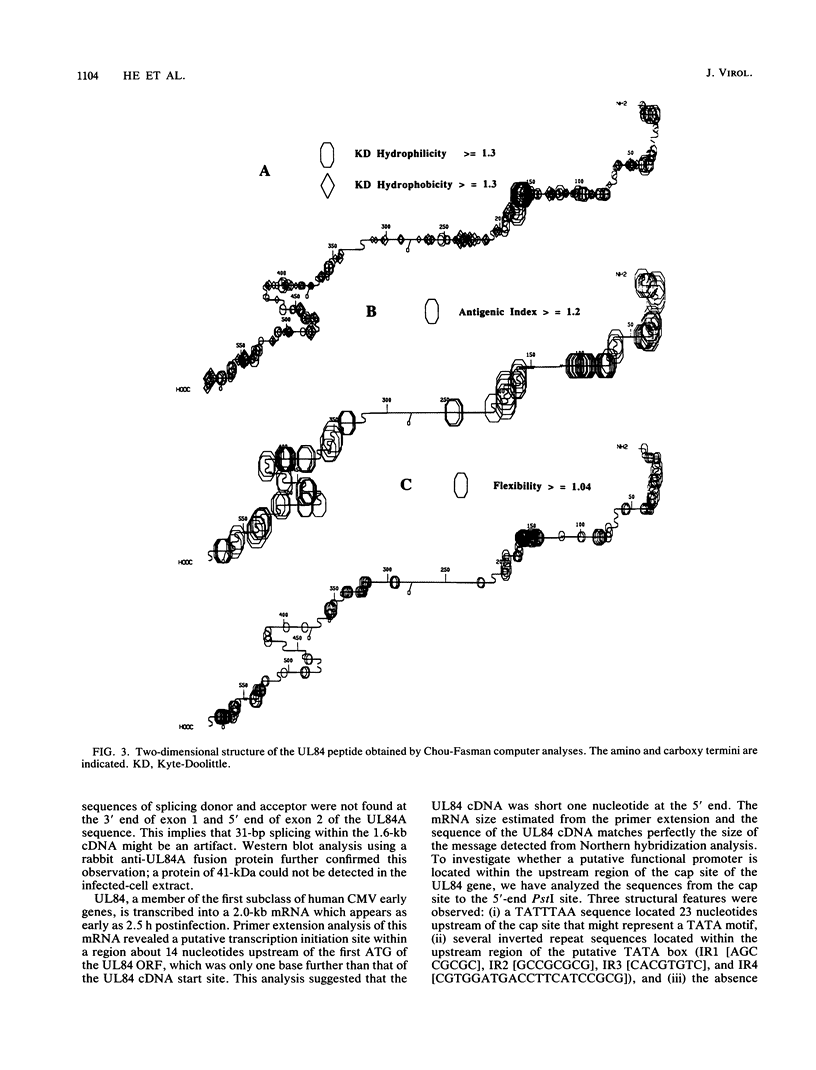
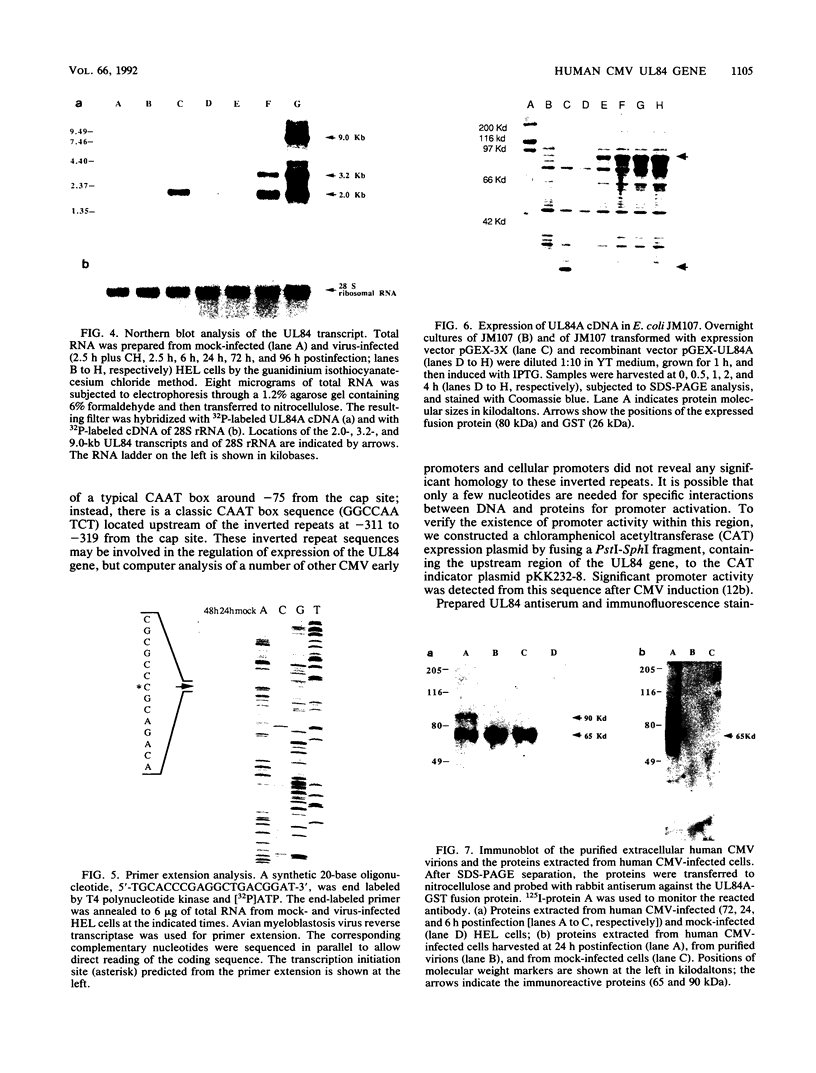
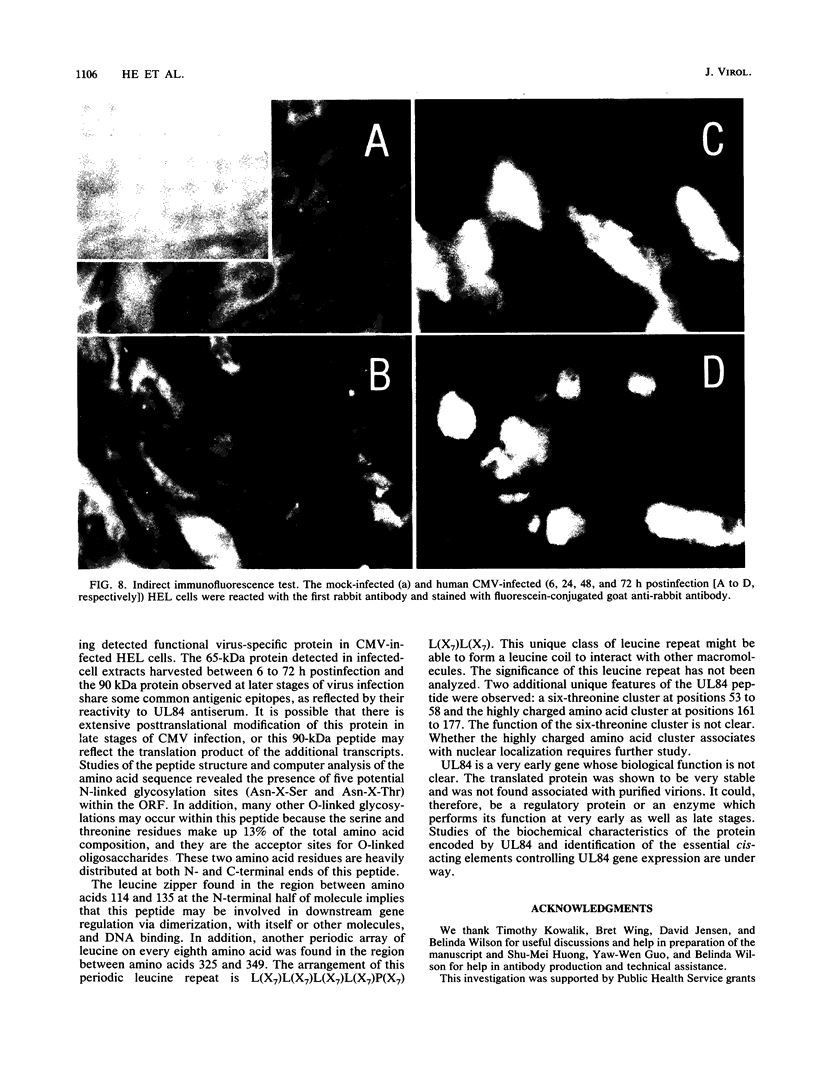
The DNA sequence and transcription pattern of human cytomegalovirus early gene UL84 were analyzed. This gene was mapped within a 2.6-kb PstI fragment located between 0.534 and 0.545 map unit of the large unique segment of the human cytomegalovirus genome, which is adjacent to the pp65 and pp71 genes. A 2.0-kb mRNA was transcribed from this region in the same leftward direction as the mRNAs of the pp65 and pp71 genes. The message was first detected at 2.5 h postinfection and reached a maximal level between 72 and 96 h postinfection. The nucleotide sequences of the 2.6-kb PstI genomic DNA fragment and the cDNA derived from this region were determined. The resulting data revealed a polyadenylation signal (AATAAA) located 14 nucleotides upstream from the poly(A) tail of the cDNA and a 1,761-bp open reading frame capable of encoding a 65-kDa polypeptide. A potential leucine zipper was found in the N-terminal half of the peptide molecule between amino acids 114 and 135. In addition, a different periodic leucine repeat with leucine at every eighth position was found between amino acids 325 and 373. The transcriptional initiation site of this early gene was determined by primer extension analysis. A putative TATA box (TATTTAA) located 24 bp upstream of the cap site and several inverted repeats were found in the region further upstream of the TATA box. To test whether the open reading frame of this cDNA encodes a virus-specific protein, the cDNA was overexpressed in Escherichia coli as a fusion protein used to generate antibodies in rabbits. A protein with a molecular size of 65 kDa was detected in the infected-cell extracts harvested at 6 to 72 h postinfection, but not in purified virions, using immunoblot analysis. Both nuclear and cytoplasmic fluorescences were found at late stages of virus infection. From the results obtained, we postulate that UL84 may be a stable, virus-specific, nonstructural protein capable of forming a homo- or heterodimeric molecule.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Chang C. P., Malone C. L., Stinski M. F. A human cytomegalovirus early gene has three inducible promoters that are regulated differentially at various times after infection. J Virol. 1989 Jan;63(1):281–290. doi: 10.1128/jvi.63.1.281-290.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. P., Vesole D. H., Nelson J., Oldstone M. B., Stinski M. F. Identification and expression of a human cytomegalovirus early glycoprotein. J Virol. 1989 Aug;63(8):3330–3337. doi: 10.1128/jvi.63.8.3330-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Huang E. S. Nucleotide sequence of a human cytomegalovirus DNA fragment encoding a 67-kilodalton phosphorylated viral protein. J Virol. 1985 Oct;56(1):7–11. doi: 10.1128/jvi.56.1.7-11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Mar E. C., Wu Y. M., Huang E. S. Mapping and expression of a human cytomegalovirus major viral protein. J Virol. 1984 Oct;52(1):129–135. doi: 10.1128/jvi.52.1.129-135.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depto A. S., Stenberg R. M. Regulated expression of the human cytomegalovirus pp65 gene: octamer sequence in the promoter is required for activation by viral gene products. J Virol. 1989 Mar;63(3):1232–1238. doi: 10.1128/jvi.63.3.1232-1238.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Multiple sequence-specific transcription factors modulate cytomegalovirus enhancer activity in vitro. Mol Cell Biol. 1988 Apr;8(4):1809–1811. doi: 10.1128/mcb.8.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Specific interactions between transcription factors and the promoter-regulatory region of the human cytomegalovirus major immediate-early gene. J Virol. 1988 Mar;62(3):1076–1079. doi: 10.1128/jvi.62.3.1076-1079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway P. J., Wilkinson G. W. Nucleotide sequence of the most abundantly transcribed early gene of human cytomegalovirus strain AD169. Virus Res. 1987 Feb;7(1):17–31. doi: 10.1016/0168-1702(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Hennighausen L., Fleckenstein B. Nuclear factor 1 interacts with five DNA elements in the promoter region of the human cytomegalovirus major immediate early gene. EMBO J. 1986 Jun;5(6):1367–1371. doi: 10.1002/j.1460-2075.1986.tb04368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Monick M. M., Liu B., Stinski M. F. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. J Virol. 1989 Jul;63(7):3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Kouzarides T., Mach M., Scholl B. C., Plachter B., Traupe B., Preddie E., Satchwell S. C., Fleckenstein B., Barrell B. G. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J Virol. 1987 May;61(5):1358–1367. doi: 10.1128/jvi.61.5.1358-1367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Scholl B. C., Traupe B., Fleckenstein B. The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties. J Gen Virol. 1987 May;68(Pt 5):1327–1337. doi: 10.1099/0022-1317-68-5-1327. [DOI] [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S. Human cytomegalovirus genome: partial denaturation map and organization of genome sequences. J Virol. 1977 Oct;24(1):261–276. doi: 10.1128/jvi.24.1.261-276.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher K. M., Rabert D. K., Spector D. H. Sequences in the human cytomegalovirus 2.7-kilobase RNA promoter which mediate its regulation as an early gene. J Virol. 1989 Dec;63(12):5334–5343. doi: 10.1128/jvi.63.12.5334-5343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher K. M., Spector D. H. The human cytomegalovirus 2.7-kilobase RNA promoter contains a functional binding site for the adenovirus major late transcription factor. J Virol. 1990 Sep;64(9):4189–4198. doi: 10.1128/jvi.64.9.4189-4198.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahijani R. S., Otteson E. W., Adlish J. D., St Jeor S. C. Characterization of a human cytomegalovirus 1.6-kilobase late mRNA and identification of its putative protein product. J Virol. 1991 Jan;65(1):373–381. doi: 10.1128/jvi.65.1.373-381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late-gene expression: differential use of three start sites in the transcriptional activation of ICP36 gene expression. J Virol. 1989 Apr;63(4):1783–1791. doi: 10.1128/jvi.63.4.1783-1791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner R., Meyer H., Mach M. Identification and characterization of a human cytomegalovirus gene coding for a membrane protein that is conserved among human herpesviruses. J Virol. 1989 Sep;63(9):3792–3800. doi: 10.1128/jvi.63.9.3792-3800.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach M., Stamminger T., Jahn G. Human cytomegalovirus: recent aspects from molecular biology. J Gen Virol. 1989 Dec;70(Pt 12):3117–3146. doi: 10.1099/0022-1317-70-12-3117. [DOI] [PubMed] [Google Scholar]
- Mach M., Utz U., Fleckenstein B. Mapping of the major glycoprotein gene of human cytomegalovirus. J Gen Virol. 1986 Jul;67(Pt 7):1461–1467. doi: 10.1099/0022-1317-67-7-1461. [DOI] [PubMed] [Google Scholar]
- Martinez J., Lahijani R. S., St Jeor S. C. Analysis of a region of the human cytomegalovirus (AD169) genome coding for a 25-kilodalton virion protein. J Virol. 1989 Jan;63(1):233–241. doi: 10.1128/jvi.63.1.233-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., St Jeor S. C. Molecular cloning and analysis of three cDNA clones homologous to human cytomegalovirus RNAs present during late infection. J Virol. 1986 Nov;60(2):531–538. doi: 10.1128/jvi.60.2.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Bankier A. T., Landini M. P., Brown C. M., Barrell B. G., Rüger B., Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988 Jul;62(7):2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Pereira L., Michael N. Precise localization of genes on large animal virus genomes: use of lambda gt11 and monoclonal antibodies to map the gene for a cytomegalovirus protein family. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1266–1270. doi: 10.1073/pnas.82.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak B., Gmeiner A., Sarnow P., Levine A. J., Fleckenstein B. Physical mapping of human cytomegalovirus genes: identification of DNA sequences coding for a virion phosphoprotein of 71 kDa and a viral 65-kDa polypeptide. Virology. 1984 Apr 15;134(1):91–102. doi: 10.1016/0042-6822(84)90275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande H., Baak S. W., Riggs A. D., Clark B. R., Shively J. E., Zaia J. A. Cloning and physical mapping of a gene fragment coding for a 64-kilodalton major late antigen of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4965–4969. doi: 10.1073/pnas.81.15.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987 Feb;61(2):446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambucetti L. C., Cherrington J. M., Wilkinson G. W., Mocarski E. S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989 Dec 20;8(13):4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S. I., Rabert D. K., Spector D. H. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J Virol. 1988 Sep;62(9):3463–3473. doi: 10.1128/jvi.62.9.3463-3473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S. I., Spector D. H. 2.2-kilobase class of early transcripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J Virol. 1986 Feb;57(2):591–602. doi: 10.1128/jvi.57.2.591-602.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Stinski M. F. Autoregulation of the human cytomegalovirus major immediate-early gene. J Virol. 1985 Dec;56(3):676–682. doi: 10.1128/jvi.56.3.676-682.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Witte P. R., Stinski M. F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985 Dec;56(3):665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Roehr T. J. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985 Aug;55(2):431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]