Abstract
The presence of a perforation is known to significantly compromise the outcome of endodontic treatment. One potential use of regenerative endodontic therapy may be the repair of root canal perforations. In addition to nutrients and systemic in-situ interactions, the three main components believed to be essential for tissue regeneration are: stem cells, scaffold, and growth factors. This study investigated the role of each component of the tissue engineering triad in the organization and differentiation of Dental Pulp Stem Cells (DPSCs) in a simulated furcal perforation site using a mouse model. Collagen served as the scaffold and dentin matrix protein 1 (DMP1) was the growth factor. Materials were placed in simulated perforation sites in dentin slices. MTA was the control repair material. At six weeks, the animals were sacrificed and the perforation sites were evaluated by light microscopy and histological staining. Organization of newly derived pulp tissue was seen in the group containing the triad of DPSCs, a collagen scaffold, and DMP1. The other four groups did not demonstrate any apparent tissue organization. Under the conditions of the present study, it may be concluded that the triad of DPSCs, a collagen scaffold, and DMP1 can induce an organized matrix formation similar to that of pulpal tissue, which may lead to hard tissue formation.
Keywords: regenerative endodontics, tissue engineering, human pulpal stem cells, dentin matrix protein, perforation repair
INTRODUCTION
Recent progress in tissue engineering technology has led to a growing interest in the development of regenerative endodontic procedures (1-4). Regeneration of the pulp-dentin complex could allow for natural replacement of damaged or missing tooth structures. Perforation repair is a potential clinical application of regenerative endodontics. The presence of a preoperative perforation has been identified as one of the most significant negative predictors of treatment outcome for orthograde retreatment root canal therapy (5). Variables affecting the long term prognosis of perforations include: location of the defect in relation to the crestal bone, length of the root trunk, accessibility for repair, size of the defect, presence or absence of periodontal communication to the defect, time lapse between the perforation and repair, sealing ability of the restorative repair material, and other factors such as the technical skill of the clinician and the patient’s oral hygiene (6). An ideal perforation repair material should possess good sealing ability, be easy to manipulate and place, be compatible with the surrounding tissues and promote tissue regeneration in the area of the perforation. Although many materials have been used over the years, none of the currently available materials fulfill all of the ideal requirements. Mineral trioxide aggregate (MTA) is the current material of choice for perforation repair and has demonstrated good potential for clinical success (7,8). Even though MTA possesses excellent sealing ability and biocompatibility (7-10), placement of MTA (or any restorative material) prevents regeneration of dentin-like hard tissue in the perforation site. MTA does not degrade to allow for replacement with natural tissues.
In addition to nutrients and systemic in situ interactions, the three key ingredients required for tissue regeneration are: stem cells, an appropriate scaffold material, and growth factors. Dental Pulp Stem Cells (HPSCs) are classified as postnatal, multipotent stem cells (1). That is, they are committed but still retain the capacity for transformation into a number of other cell types, such as adipocytes or chondrocytes. DPSCs are very similar to bone marrow stem cells but may have more limited potential for developing into cells other than those normally found in the pulp-dentin complex (11). Postnatal DPSCs provide the most potential for endodontic tissue regeneration (1, 12, 13). A scaffold provides the framework for cell growth and differentiation at a local site. The scaffold may be implanted alone or in combination with cells and growth factors. When implanted, a scaffold allows for cell migration and organization. Ideally, a scaffold should be porous (to allow for placement of cells and growth factors), biocompatible with the host tissues, the correct shape and form to allow for replacement of the lost tissues, and biodegradable, leaving no toxic by-products. Natural scaffolds (e.g., collagen) offer good biocompatibility and bioactivity, whereas synthetic scaffolds [polylactic acid (PLA) or polyglycolic acid (PGA)], offer more control over the degradation rate and mechanical properties (14). Collagen is the major component in extracellular matrices, and provides great tensile strength in tissues. As a scaffold, collagen allows for easy placement of cells and growth factors and allows for replacement with natural tissues after undergoing degradation (15, 16). Growth factors are substances governing the pattern of tissue development and, in particular, the positions of the various specialized cell types within a tissue. Growth factors spread from a localized source and form a concentration gradient across developing tissues. In the formation of teeth, bone morphogenetic proteins (BMPs) dictate when initiation, morphogenesis, cytodifferentiation, and matrix secretion will occur. Without the BMP family of growth factors, the enamel knot would not be formed, and teeth would be unlikely to develop (17).
Dentin matrix protein 1 (DMP1) is one of the non-collagenous extracellular matrix proteins (along with dentin sialoprotein [DSP] and dentin phosphoprotein [DPP]). It is a growth factor which is primarily found in dentin and bone, and has been implicated in the regulation of mineralization (18). Like the BMP family, DMP1 has been shown to be crucial in the formation of teeth. In a recent study using a rat model, DMP1 was shown to act as a growth factor on undifferentiated mesenchymal cells present in the pulp-dentin complex (19). These differentiated cells had the potential to regenerate dentin-like tissue, which was confirmed by the presence of a collagenous matrix, odontoblast specific markers (DSP and DPP) and calcified deposits. The collagenous matrix that is seen when dentin-like tissue is formed mimics the structure of primary dentin with ordered type 1 collagen fibers perpendicular to the odontoblast layer, and non-collagenous proteins (DSP, DPP, and DMP1) (19). The fibers provide strength to the mineralized dentin and the non-collagenous proteins are considered to have mineralization-regulatory capacities (20). The mineralized portion (calcified deposits) of the dentin matrix comes from odontoblast-like cells (post-mitotic cells which are differentiated from DPSCs).
Gronthos et al. showed that it was possible to induce dentin formation from DPSCs placed subcutaneously in mice (21). In a more recent study, Goncalves et al. demonstrated that subcutaneous placement of human pulpal tissue in immunodeficient mice promoted vascularization of the pulpal tissue and subsequent angiogenesis in the area (22). In 2003, Nakashima demonstrated the importance of growth factors (in particular, BMP) in tissue engineering (23). Narayanan et al. showed that DMP1 was a particularly important regulator in the biomineralization of teeth and bone (24). From the same lab in 2004, DMP1 was found to be more specific for binding to type 1 collagen, and that mineralization did not occur unless DMP1 was bound (25). By seeding DMP1 into a collagen scaffold and performing direct pulp cap procedures in a rat model, Almushayt et al, were able to induce the differentiation of DPSCs into odontoblasts and demonstrate dentin-like hard tissue formation (19).
The objective of this study was to measure the effectiveness of DPSCs, a collagen scaffold, and a growth factor (DMP1) in the in-vivo generation of dental pulp-like tissue following subcutaneous transplantation in mice.
MATERIALS & METHODS
DMP1 was expressed and purified according to published methods (26). Purity of the DMP1 was confirmed using SDS-PAGE electrophoresis. Twenty, freshly extracted human third molar teeth were collected from the University of of Illinois outpatient oral surgery clinic. The teeth were cleaned in 6.0% NaOC1 and stored in 1.0% NaOCl. All molars were decoronated at the CEJ using a grinding disc (#6918B, Brasseler USA) and any excess coronal pulp tissue was removed. The teeth were then shaved down to the level of the floor of the pulp chamber, both apically and coronally, using the grinding disc mentioned previously to create a 2.5 mm thick dentin slice. Utilizing a #557 bur (Brasseler USA), one perforation was made in each tooth through the entire thickness of the dentin, approximately in the center of the furcation. The perforation was as wide as the #557 bur (1.0 mm). Loupes (4.5x magnification) were used to assure consistency of placement and size. These dentin slices were then sterilized in a steam autoclave (250°F for 30 minutes) and stored in sterile packaging. The prepared slices were randomly divided into five groups (four in each group): The DPSCs (a kind gift from Dr. Songtao Shi - University of Southern California) were plated at a density of 1.5 × 104/ml and cultured in Dulbecco’s modified Eagle’s medium/F-12 (Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) fetal bovine serum (Cellgro, Mediatech, Inc., Manassas, VA), 100 units/ml penicillin/streptomycin, and 2.5 units/ml fungizone. The cells were incubated at 37°C in 5% CO2. About 5 × 106 of DPSCs at passage 5 were mixed with 1.0 × 2.0 mm2 of Collagraft Bone Graft Matrix Strip (Zimmer, Warsaw, IN), incubated at 37°C for 0.5 h, and then transplanted into the dentin slices for subcutaneous implantation on the dorsal surfaces of immunodeficient mice (NU/NU Mice Strain, Charles River Laboratories, Wilmington, MA) in experimental groups 4 and 5 (as described below). The mice weighed approximately 27 g and were anesthetized with 0.07ml xylazine and 0.27ml ketamine. All procedures were performed in accordance with specifications of an approved small animal protocol (UIC No. 06-039). Each sample was placed subcutaneously on the dorsal side of the mouse, using a straight-line incision, with a pocket to the contralateral side (Figure 1). Three to four staples were used to reapproximate the incision sites. An ear punch was used to designate which mice had received which samples. All experimentation was completed using sterile instruments and an aseptic handling technique, inside a flow hood to avoid contamination.
Figure 1.
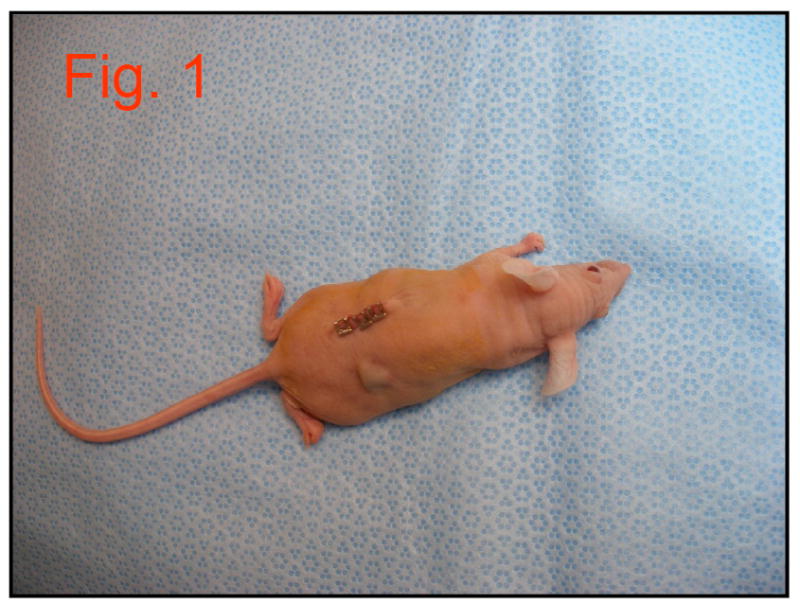
Image of mouse with subcutaneous implant placed contralateral to incision site.
Group1
MTA only: Grey MTA (ProRoot® MTA – DENTSPLY Tulsa Dental, Johnson City, TN) was mixed according to the manufacturer’s directions and placed to completely fill the simulated perforation site.
Group 2
Collagen scaffold only: Collagen scaffold material (Type I collagen and ceramic powder - Zimmer, Warsaw, IN) was cut to match the dimensions of the perforation, moistened with PBS, and placed in the simulated dentin perforation site.
Group 3
Collagen scaffold impregnated with DMP1: Lyophilized DMP1 recombinant protein was dissolved in cell culture medium. The DMP1 concentration was 1.2 mg/ml in a volume of 100 μl medium for each scaffold. The collagen scaffold was placed in cell culture medium containing DMP1 protein at 37°C for 30 minutes so that the scaffold could be impregnated with the DMP1 protein and then placed in the simulated dentin perforation site.
Group 4
Collagen scaffold impregnated with DPCSs and DMP1: Lyophilized DMP1 recombinant protein was dissolved in cell culture medium. The DMP1 concentration was 1.2 mg/ml in a volume of 100 μl medium for each scaffold. The collagen scaffold was placed in cell culture medium containing DMP1 protein at 37°C for 30 minutes so that the scaffold could be impregnated with the DMP1 protein. DPSCs were trypsinized and the cell number determined using trypan blue stain before pelleting. A pellet containing 5 × 106 of DPSCs was resuspended in the culture medium containing the scaffold and DMP1 and then placed in the simulated dentin perforation site.
Group 5
Collagen scaffold impregnated with DPSCs: The collagen scaffold was placed in cell culture medium at 37°C for 30 minutes. DPSCs were trypsinized and the cell number determined using trypan blue stain before pelleting. A pellet containing 5 × 106 of DPSCs was resuspended in the culture medium containing the scaffold and then placed in the simulated dentin perforation site.
The mice were sacrificed at six weeks using carbon dioxide in an asphyxia tank. Samples were removed and excess tissue was trimmed from the surgical sites. The samples were immediately placed into 10% formalin. The dentin slices were placed in 4% paraformaldehyde solution buffered with PBS (pH 7.4) overnight and then washed with water. The blocks were decalcified in EDTA for 10 days, embedded in paraffin, and sectioned (5.0 to 7.0 μm thick) for histological examination. The samples were stained with hematoxylin and eosin (H&E), safranin-O, von Kossa, and Mason’s Trichrome.
RESULTS
Group 1
MTA did not stain well and appears as its original grey color (Figure 2). A few red blood cells were seen at the periphery of the perforation site.
Figure 2.
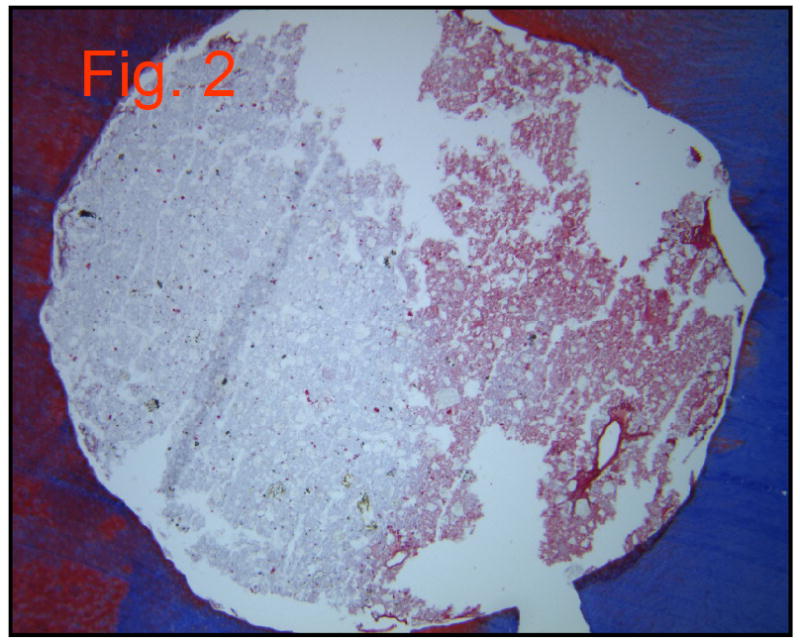
Group 1: MTA (10×10)
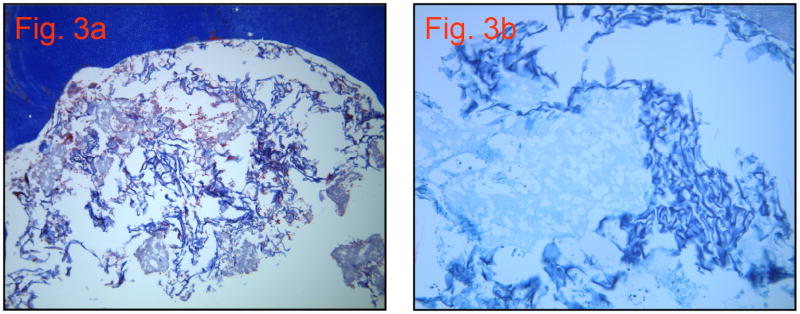
Group 2
Mason’s Trichome stained the collagen scaffold blue. The scaffold is in the process of degrading (based on visualization using H&E and Mason’s Trichrome staining), yet no replacement tissues can be seen. A few scattered red blood cells and nucleated cells are present (Figures 3a and 3b).
Figure 3.
a. Group 2: Collagen scaffold only (10×20)
b. Group 2: Mason’s Trichrome Stain with higher magnification (10×40)
Group 3
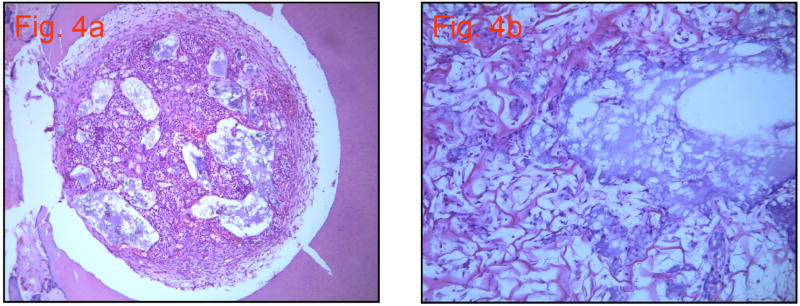
This group showed large numbers of viable cells, as well as some peripheral collagen matrix formation, but matrix deposition was not observed in the central perforation site (Figure 4a). The collagen scaffold was beginning to degrade, but some cells partially filled in the voids. A magnified view of the unfilled voids shows debris and a lack of cellular organization (Figure 4b).
Figure 4.
a. Group 3: Collagen scaffold and DMP1. H&E stain (10×10) (note: the gap between the sample and dentin seen in this and other images is a processing artifact)
b. Group 3: H & E stain with higher magnification. (10×40)
Group 4
This group demonstrated several signs of tissue regeneration. As the collagen scaffold was degrading, cells presumed to be fibroblasts (based on morphology) and endothelial cells were beginning to lay down a new matrix (Figure 5a). Blood vessels (Figure 5b) and collagen matrix can be visualized throughout the tissue in the perforation site. Outer portions of the perforation site showed cellular proliferation, indicating the progression of tissue regeneration in this area (Figures 5a, 5b, and 5c).
Figure 5.
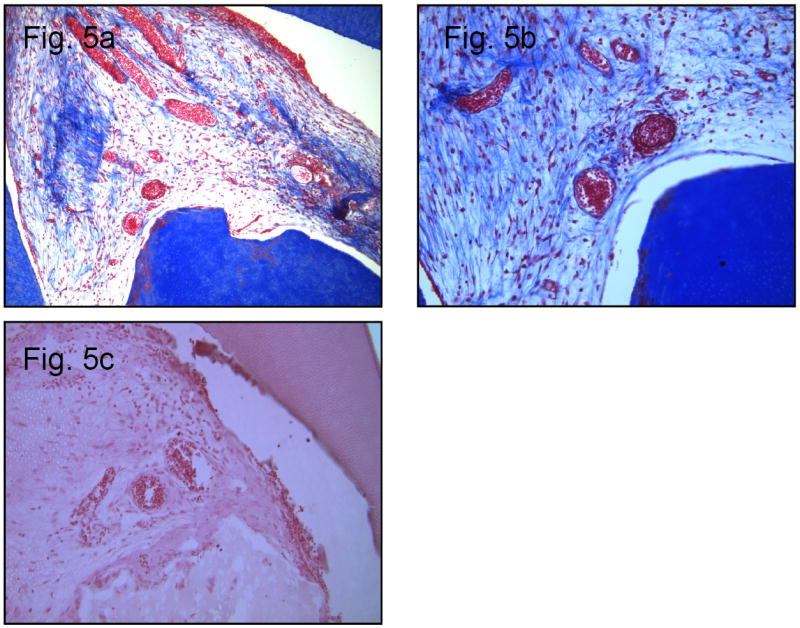
a. Group 4: Collagen scaffold, HPSCs and DMP1. Mason’s Trichrome Stain (10×10)
b. Group 4: Higher magnification of Figure 5a. (10×40)
c. Group 4: H & E stain, note blood vessels present. (10×40)
Group 5
This group appeared very similar to group 2 (collagen scaffold alone). Some red blood cells are visible, a few nucleated cells, and the break-down remnants of the collagen scaffold (Figure 6). No new matrix appeared, nor did there appear to be any cellular infiltrate.
Figure 6.
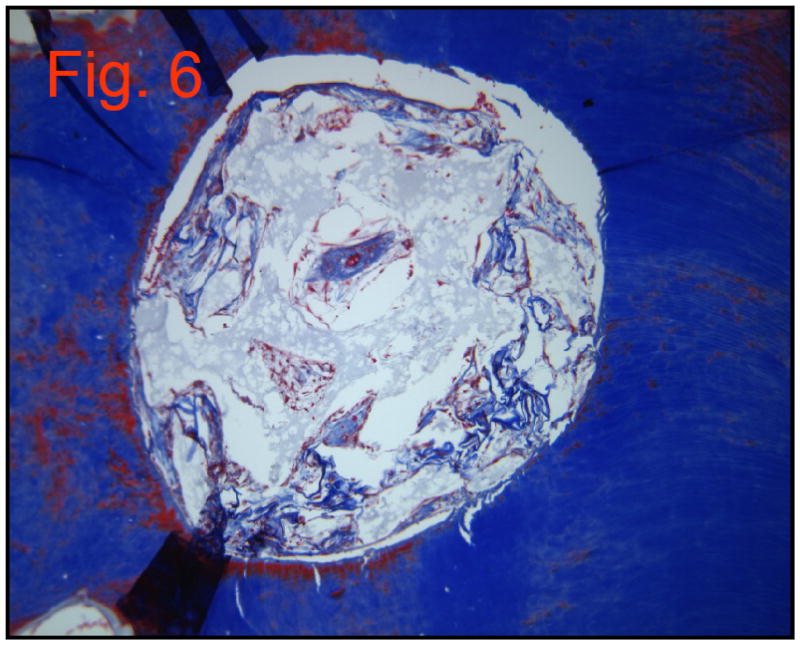
Group 5: Collagen scaffold and DPSCs. Mason’s Trichrome Stain (10×40)
DISCUSSION
Although other growth factors such as BMPs have been shown to induce differentiation of pulpal stem cells (27), DMP1 was selected for this study since it has been demonstrated that DMP1 could induce differentiation of stem cells into odontoblast-like cells and stimulate the formation of mineralized tissues (18,28,29). Mineral deposition was increased ten-fold in the presence of DMP1 (18). DMP1 is considered a nucleator for apatite deposition in vitro (18, 25) and is a regulator of hydroxyapatite deposition, in-vivo (19, 30). To the best of our knowledge, we are the first laboratory to successfully extract and purify DMP1 for use in dental tissue regeneration (26). DMP1 is not commercially available at this time.
MTA has been shown to be an excellent perforation repair material; however, it does not degrade and allow for natural tissue ingrowth to replace it. In fact, the ability of MTA to resist degradation in vivo is generally considered to be one of its positive attributes. On the other hand, a collagen scaffold will naturally degrade and allow for tissue regeneration. A collagen scaffold was selected instead of other scaffold types because of its ease of placement and similarity to the most common collagen component of normal human dental pulp, type I collagen.
In this study, immunodeficient mice were necessary to prevent possible immunogenic reactions to the DPSCs or other components. The harvesting and use of autologous cells is one possible solution to the problem of immunocompatibility in higher mammals and humans but was not practical for this study. A mouse model was used because a simulated environment where the dentin slices could be placed in close proximity to connective tissue and blood vessels was desired.
In group 1 (MTA only), the MTA was the only material visible in all of the samples. No cells, collagen matrix, blood vessels or calcified tissues were present. Because our experiment was only six weeks in duration, no calcified tissues were expected although hard tissue formation around MTA has been reported after longer time periods (31). In group 2, (collagen scaffold only), only degrading collagen was observed. Mason’s Trichrome stain stained the degrading scaffold blue and all other areas were clear. Von Kossa’s stain did not demonstrate the presence of any calcified tissues. In groups 3 and 5, one component of the tissue engineering triad was missing (DPSCs and DMP1, respectively). Although both groups showed possible initial stages of tissue organization, they lacked any organization substantial enough to support further tissue growth. However, in group 3, there was a large infiltration of nucleated cells. The precise identity and origin of these cells is unknown, although they are presumed to be fibroblasts. Group 3 demonstrated a greater number of nucleated cells than all other groups except group 4. In group 4, all three components of the tissue engineering triad (scaffold, DPSCs, and growth factor) were present. This group had clear tissue organization. Mason’s Trichrome stained several areas, demonstrating new collagen matrix formation. Parts of the collagen scaffold could be seen, but the bulk of the scaffold had degraded and fibroblast produced collagen had taken its place. Von Kossa staining did not demonstrate any hard tissue formation, but this was not expected at six weeks. It is interesting to note that there was a clear formation of blood vessels, specific to the area of the perforation site. It is known that angiogenesis is a precursor to tissue repair. Angiogenesis allows vital growth factors and nutrients to reach the damaged site. In a study by Roberts-Clark and Smith, it was concluded that the dentin matrix contains angiogenic growth factors and that their release from the matrix after injury could make an important contribution to the overall reparative response of the pulp-dentin (32). It is evident that tissue regeneration was in its initial stages in experimental group 4. In the absence of DMP1, we were unable to demonstrate any kind of tissue organization or cellular differentiation; thus, DMP1 appears to induce the cytodifferentiation of undifferentiated pulp cells to matrix-synthesizing cells.
This study confirmed the potential use of the group 4 model (triad of DPSCs, collagen scaffold, and DMP1) for exploring mechanisms of tissue regeneration with the ultimate goal of hard tissue formation in a perforation site. Although translation of this regenerative technology to endodontic practice will require extensive additional animal testing, development of in situ models, and clinical trials, we are optimistic that this or similar technology will eventually provide a clinical tool for perforation repair by regeneration of dentin.
Acknowledgments
This research was supported in part by a Research Grant from the American Association of Endodontists Foundation and NIH grant DE 11657.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray PE, García Godoy F, Hargreaves KM. Regenerative endodontics: a review of the current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Murray PE, García Godoy F. The outlook for implants and endodontics: a review of the tissue engineering strategies to create replacement teeth for patients. Dent Clin North Am. 2006;50:299–315. doi: 10.1016/j.cden.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Baum BJ, Mooney DJ. The impact of tissue engineering on dentistry. J Am Dent Assoc. 2000;131:309–18. doi: 10.14219/jada.archive.2000.0174. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–8. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 5.Farzaneh M, Abitol S, Friedman S. Treatment outcome in endodontics: the Toronto study. Phases I and II: Orthograde retreatment. J Endod. 2004;30:627–33. doi: 10.1097/01.don.0000129958.12388.82. [DOI] [PubMed] [Google Scholar]
- 6.Jew RC, Weine FS, Keene JJ, Jr, Smulson MH. A histologic evaluation of periodontal tissues adjacent to root perforations filled with cavit. Oral Surg Oral Med Oral Pathol. 1984;54:124–35. doi: 10.1016/0030-4220(82)90427-3. [DOI] [PubMed] [Google Scholar]
- 7.Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004;30:80–3. doi: 10.1097/00004770-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 9.Ford TR, Torabinejad M, McKendry DJ, Hong CU, Kariyamasam SP. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:756–63. doi: 10.1016/s1079-2104(05)80313-0. [DOI] [PubMed] [Google Scholar]
- 10.Fischer EJ, Arens DE, Miller CH. Bacterial leakage of mineral trioxide aggregate as compared with zinc-free amalgam, intermediate restorative material and Super-EBA as a root-end filling material. J Endod. 1998;24:176–9. doi: 10.1016/S0099-2399(98)80178-7. [DOI] [PubMed] [Google Scholar]
- 11.Krebsbach PH, Robey PG. Dental and skeletal stem cells: Potential cellular therapeutics for craniofacial regeneration. J Dent Educ. 2002;66:766–773. [PubMed] [Google Scholar]
- 12.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima M, Iohara K, Ishikawa M, Ito M, Tomokiyo A, Tanaka T, Akamine A. Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11) Hum Gene Ther. 2004;15:1045–53. doi: 10.1089/hum.2004.15.1045. [DOI] [PubMed] [Google Scholar]
- 14.Sharma B, Elisseeff JH. Engineering structurally organized cartilage and bone tissues. Annals of Biomed Eng. 2004;32:148–159. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 15.Feng Z, Yamato M, Akutsu, Nakamura T, Okano T, Umezu M. Investigation on the mechanical properties of contracted collagen gels as a scaffold for tissue engineering. Artificial Organs. 2003;27:84–90. doi: 10.1046/j.1525-1594.2003.07187.x. [DOI] [PubMed] [Google Scholar]
- 16.Thibodeau B, Teixira F, Yamauchi M, Caplan D, Trope M. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33:680–9. doi: 10.1016/j.joen.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima M. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine & Growth Factor Reviews. 2005;16:369–376. doi: 10.1016/j.cytogfr.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 18.He J, Dahl T, Veis A, George A. Dentin matrix protein 1 initiates hydroxyapatite formation in-vitro. Connect Tissue Res. 2003;44(Suppl 1):240–5. [PubMed] [Google Scholar]
- 19.Almushayt A, Narayanan K, Zaki A, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006;13:611–20. doi: 10.1038/sj.gt.3302687. [DOI] [PubMed] [Google Scholar]
- 20.Smith AJ, Tobias RS, Cassidy N, Bégue-Kirn C, Ruch JV, Lesot H. Influence of substrate nature and immobilization of implanted dentin matrix componentsduring induction of reparative dentinogenesis. Connect Tissue Res. 1995;321:291–295. doi: 10.3109/03008209509013736. [DOI] [PubMed] [Google Scholar]
- 21.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in-vitro and in-vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncalves SB, Dong Z, Bramante CM, Holland GR, Smith AJ, Nor JE. Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod. 2007;33:811–814. doi: 10.1016/j.joen.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima M, Reddi AH. The application of BMPs to dental tissue engineering. Nat Biotech. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. Dual function roles of Dentin Matrix Protein 1. J Biol Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 25.He G, George A. Dentin Matrix Protein 1 immobilized on type 1 collagen fibrils facilitates apatite deposition in-vitro. J Biol Chem. 2004;279:11649–11656. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan R, Chen B, Gorski JP, George A. Recombinant expression and characterization of dentin matrix protein 1. Connect Tiss Res. 1999;40:251–258. doi: 10.3109/03008209909000703. [DOI] [PubMed] [Google Scholar]
- 27.Hao J, Zou B, Narayanan K, George A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone. 2004;34:921–932. doi: 10.1016/j.bone.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Hao J, Narayanan K, Ramachandran A, He G, Almushayt A, Evans C, George A. Odontoblast cells immortalized by telomerase produce mineralized dentin-like tissue both in-vitro and in-vivo. J Biol Chem. 2002;277:19976–81. doi: 10.1074/jbc.M112223200. [DOI] [PubMed] [Google Scholar]
- 29.Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. PNAS. 2001;98:4516–21. doi: 10.1073/pnas.081075198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesot H. Collagen type I trimer synthesis by cultured embryonic mouse molars. Eur J Biochem. 1981;3:541–546. doi: 10.1111/j.1432-1033.1981.tb05370.x. [DOI] [PubMed] [Google Scholar]
- 31.Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod. 2004;30:95–9. doi: 10.1097/00004770-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Archives of Oral Biology. 2000;45:1013–1016. doi: 10.1016/s0003-9969(00)00075-3. [DOI] [PubMed] [Google Scholar]