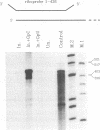
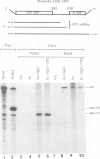
Abstract
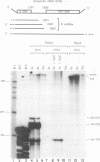
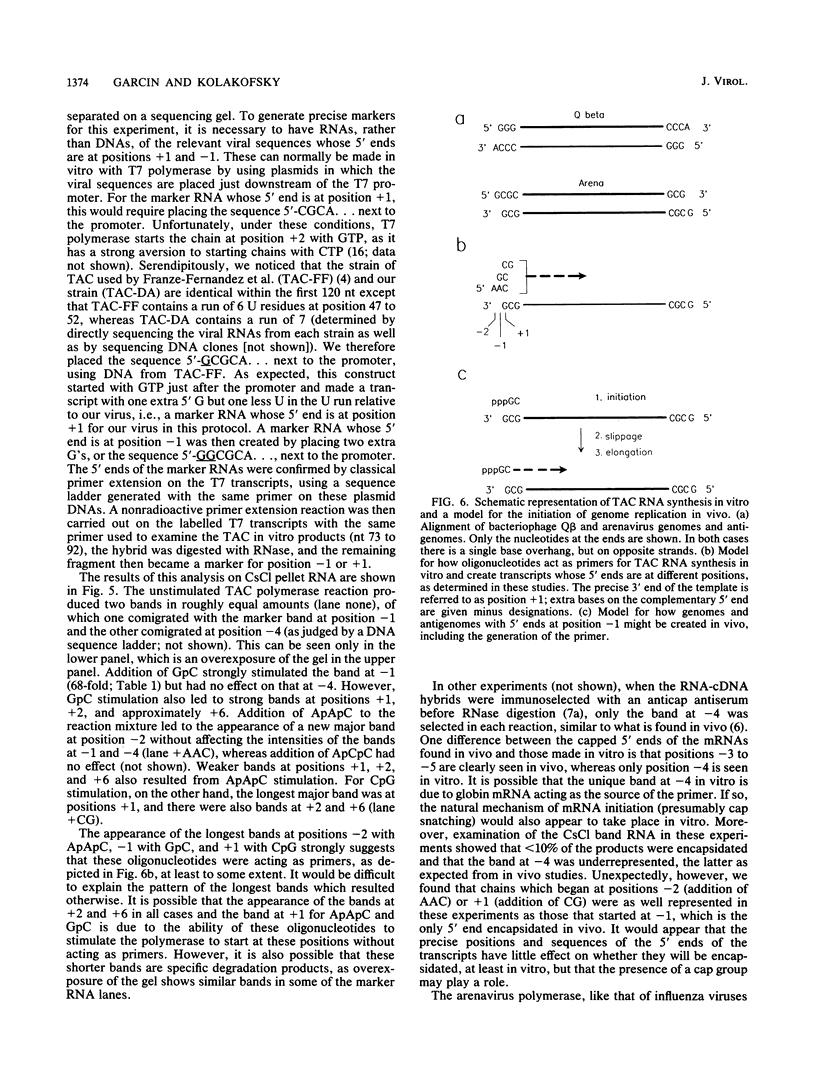
A Tacaribe virus in vitro system for RNA synthesis was established and found in large part to faithfully reproduce RNA synthesis in vivo. Similar to influenza virus and bunyavirus in vitro systems, this system was also highly dependent on added oligonucleotides. Of the eight tested, only three were active, in the order GpC greater than CpG greater than ApApC. Determination of the 5' ends of the transcripts suggested that the oligonucleotides were acting as primers. In particular, whereas stimulation with CpG (complementary to positions +1 and +2 of the template) led to RNAs whose 5' ends were at position +1 as expected, GpC stimulation led to transcripts whose 5' ends were at position -1 rather than at position +2, as GpC is complementary to positions +2 and +3 of the template. This finding suggests a model for the initiation of genome replication in which pppGpC is first made on the template at positions +2 and +3 but slips backwards on the template so that the 5' end is at position -1 before elongation can continue.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Auperin D. D. Arenavirus gene structure and organization. Curr Top Microbiol Immunol. 1987;133:5–17. doi: 10.1007/978-3-642-71683-6_2. [DOI] [PubMed] [Google Scholar]
- Downey K. M., Jurmark B. S., So A. G. Determination of nucleotide sequences at promoter regions by the use of dinucleotides. Biochemistry. 1971 Dec 21;10(26):4970–4975. doi: 10.1021/bi00802a021. [DOI] [PubMed] [Google Scholar]
- Franze-Fernández M. T., Zetina C., Iapalucci S., Lucero M. A., Bouissou C., López R., Rey O., Daheli M., Cohen G. N., Zakin M. M. Molecular structure and early events in the replication of Tacaribe arenavirus S RNA. Virus Res. 1987 Jun;7(4):309–324. doi: 10.1016/0168-1702(87)90045-1. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace F. V., Southern P. J. Detection of virus-specific RNA-dependent RNA polymerase activity in extracts from cells infected with lymphocytic choriomeningitis virus: in vitro synthesis of full-length viral RNA species. J Virol. 1989 May;63(5):1938–1944. doi: 10.1128/jvi.63.5.1938-1944.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D., Kolakofsky D. A novel mechanism for the initiation of Tacaribe arenavirus genome replication. J Virol. 1990 Dec;64(12):6196–6203. doi: 10.1128/jvi.64.12.6196-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Hacker D., Rochat S., Kolakofsky D. Anti-mRNAs in La Crosse bunyavirus-infected cells. J Virol. 1990 Oct;64(10):5051–5057. doi: 10.1128/jvi.64.10.5051-5057.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iapalucci S., López N., Franze-Fernández M. T. The 3' end termini of the Tacaribe arenavirus subgenomic RNAs. Virology. 1991 May;182(1):269–278. doi: 10.1016/0042-6822(91)90670-7. [DOI] [PubMed] [Google Scholar]
- Iapalucci S., López N., Rey O., Zakin M. M., Cohen G. N., Franze-Fernández M. T. The 5' region of Tacaribe virus L RNA encodes a protein with a potential metal binding domain. Virology. 1989 Nov;173(1):357–361. doi: 10.1016/0042-6822(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Jacques J. P., Kolakofsky D. Pseudo-templated transcription in prokaryotic and eukaryotic organisms. Genes Dev. 1991 May;5(5):707–713. doi: 10.1101/gad.5.5.707. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Hacker D. Bunyavirus RNA synthesis: genome transcription and replication. Curr Top Microbiol Immunol. 1991;169:143–159. doi: 10.1007/978-3-642-76018-1_5. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–149. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The gene structure and replication of influenza virus. Annu Rev Biochem. 1983;52:467–506. doi: 10.1146/annurev.bi.52.070183.002343. [DOI] [PubMed] [Google Scholar]
- McGeoch D., Kitron N. Influenza virion RNA-dependent RNA polymerase: stimulation by guanosine and related compounds. J Virol. 1975 Apr;15(4):686–695. doi: 10.1128/jvi.15.4.686-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Holloway B., Kolakofsky D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J Virol. 1984 Oct;52(1):215–222. doi: 10.1128/jvi.52.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Segments of influenza virus complementary RNA synthesized in vitro. J Virol. 1978 Feb;25(2):579–586. doi: 10.1128/jvi.25.2.579-586.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Raju L., Hacker D., Garcin D., Compans R., Kolakofsky D. Nontemplated bases at the 5' ends of Tacaribe virus mRNAs. Virology. 1990 Jan;174(1):53–59. doi: 10.1016/0042-6822(90)90053-t. [DOI] [PubMed] [Google Scholar]
- Salvato M. S., Shimomaye E. M. The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology. 1989 Nov;173(1):1–10. doi: 10.1016/0042-6822(89)90216-x. [DOI] [PubMed] [Google Scholar]
- Vidal S., Curran J., Kolakofsky D. A stuttering model for paramyxovirus P mRNA editing. EMBO J. 1990 Jun;9(6):2017–2022. doi: 10.1002/j.1460-2075.1990.tb08330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Weissmann C. The 3'-termini of bacteriophage Q-beta plus and minus strands. J Mol Biol. 1970 Jul 28;51(2):215–224. doi: 10.1016/0022-2836(70)90138-5. [DOI] [PubMed] [Google Scholar]