Abstract
Estrogen-nonresponsive estrogen receptor-α (ERα) knock-in (ENERKI) mice were generated to distinguish between ligand-induced and ligand-independent ER-α actions in vivo. These mice have a mutation [glycine 525 to leucine (G525L)] in the ligand-binding domain of ERα, which significantly reduces ERα interaction with and response to endogenous estrogens, whereas not affecting growth factor activation of ligand-independent pathways. ENERKI mice had hypoplastic uterine tissues and rudimentary mammary gland ductal trees. Females were infertile due to anovulation, and their ovaries contained hemorrhagic cystic follicles because of chronically elevated levels of LH. The ENERKI phenotype confirmed that ligand-induced activation of ERα is crucial in the female reproductive tract and mammary gland development. Growth factor treatments induced uterine epithelial proliferation in ovariectomized ENERKI females, directly demonstrating that ERα ligand-independent pathways were active. In addition, the synthetic ERα selective agonist propyl pyrazole triol (PPT) and ER agonist diethylstilbestrol (DES) were still able to activate ligand-induced G525L ERα pathways in vitro. PPT treatments initiated at puberty stimulated ENERKI uterine development, whereas neonatal treatments were needed to restore mammary gland ductal elongation, indicating that neonatal ligand-induced ERα activation may prime mammary ducts to become more responsive to estrogens in adult tissues. This is a useful model for in vivo evaluation of ligand-induced ERα pathways and temporal patterns of response. DES did not stimulate an ENERKI uterotrophic response. Because ERβ may modulate ERα activation and have an antiproliferative function in the uterus, we hypothesize that ENERKI animals were particularly sensitive to DES-induced inhibition of ERα due to up-regulated uterine ERβ levels.
ESTROGEN RECEPTORS (ERs) play a crucial role in the growth, development, and differentiation of both male and female reproductive tissues and nonreproductive tissues like the bone, brain, and cardiovascular systems (1,2). The ER is a ligand-inducible transcription factor and member of the nuclear receptor superfamily. In classical ligand-induced signaling, estrogen binds to the ER C-terminal activation function (AF)-2 domain, inducing conformational changes that cause ER to dimerize and bind estrogen response elements (EREs) upstream of target genes. The transcriptional activity of these target genes is dependent on coactivator and basal transcription factor recruitment. ER transcriptional activity can also be stimulated by growth factors like IGF-I or epidermal growth factor (EGF) (3,4). During ligand-independent signaling, IGF-I or EGF signaling stimulates kinases to phosphorylate the N-terminal AF-1 domain of ER. Specifically, the MAPK pathway is activated by growth factors, and ERK 1/2 phosphorylates serine 118 of ER (3,5). AF-1 transcriptional activity is dependent on phosphorylation and recruitment of coactivators, which may be unique to AF-1 or identical to those that bind AF-2 (6).
The two ER subtypes, ERα and ERβ, have distinct tissue expression patterns in the mouse female reproductive tract. ERα is predominantly expressed in the uterus, vagina, mammary gland, and thecal cells of the ovary, whereas ERβ is principally expressed in the granulosa cells of the ovary (7,8). The particular roles of ERα and ERβ have been studied by disrupting one or both of the receptors in ERα knockout (αERKO), ERβ knockout (βERKO), and ERα/ERβ knockout (αβERKO) mice. Although αERKO females are infertile and anovulatory, βERKO mice primarily have impaired ovulation (4,8). αERKOs also have hypoplastic uterine and vaginal tissues, and mammary glands with only rudimentary ductal trees. The phenotypes of αERKO and αβERKO females are similar in all reproductive tissues but the ovary. When both ERα and ERβ are inactivated, adult ovarian follicles differentiate into structures that resemble the seminiferous tubules of the testes (9,10). These studies have provided a great deal of information about the role of ERα and ERβ in mouse development. However, because ER knockout mice have a deletion of the entire ER gene, it has been difficult to define the roles of the AF-1 and AF-2 domains.
The goal of this study was to distinguish ligand-induced and ligand-independent ERα actions in vivo. We developed a knock-in mouse model with a mutation in ERα [glycine 525 to leucine (G525L)] that permits exogenous regulation of its ligand-induced signaling pathways, whereas not affecting ligand-independent signaling. In these estrogen-nonresponsive ERα knock-in (ENERKI) mice, the ligand-binding pocket mutation significantly reduces ERα interaction with and response to endogenous estrogens. These studies confirm that ligand-induced activation of ERα is critical in the development of female reproductive tissues. In addition, the ERα selective agonist propyl pyrazole triol (PPT), a nonsteroidal synthetic compound, was still able to stimulate G525L ERα transcriptional activation in vivo. Therefore, ERα signaling pathways can be regulated in these mice through PPT administration or withdrawal. This is the first model for in vivo modulation of ligand-induced ERα pathways.
Materials and Methods
Cell culture and luciferase assays
Human endometrial adenocarcinoma Ishikawa cells were routinely cultured in phenol red-free DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. For transient transfections, cells were seeded in 48-well plates at a density of 3.5 × 104 cells per well. The next day, cells were transfected with Effectene (QIAGEN, Inc., Valencia, CA) according to the manufacturer’s instructions in phenol red-free DMEM supplemented with 10% charcoal-stripped fetal bovine serum. Several plasmids were kind gifts from colleagues. pMOR-mERα (full length mouse ERα) was a generous gift from Dr. Malcolm Parker (Imperial College London, London, UK). pMOR-mERα was mutated to pMOR-mERα G525L (residue 525 was mutated from glycine to leucine, codon GGC to CTC) by site-directed PCR mutagenesis. p3xERE-luc was a kind gift from Dr. Donald McDonnell (Duke University, Durham, NC). pCMV-βgal was obtained from Invitrogen Corp. (Carlsbad, CA). Transfections contained 25 ng G525L ERα plasmid, 100 ng 3xERE-luc reporter, and 25 ng β-galactosidase expression plasmid per well. Cells were treated 24 h after transfections with serial dilutions of vehicle, 17β-estradiol (E2) (Sigma-Aldrich, St. Louis, MO), diethylstilbestrol (DES) (Sigma-Aldrich), PPT (a kind gift from Dr. John Katzenellenbogen, University of Illinois, Urbana, IL), or genistein (Sigma-Aldrich) in fresh media. Cells were harvested and assayed for luciferase and β-galactosidase activity 24 h after treatments. Luciferase activity was normalized for transfection efficiency using β-galactosidase as an internal control and reported as a fold induction over vehicle activity. All results are representative of at least three independent experiments and represent the average ± sem of triplicate samples.
Generation of ENERKI animals
A targeting vector containing exons 8 and 9 of mouse ERα, with an added XbaI site in exon 9 and a C-terminal 6xHis-tag epitope tag, was obtained from Korach and colleagues (11). The G525L mutation was introduced into exon 9 (codon GGC to CTC) by site-directed PCR mutagenesis. The ACN cassette from pACN was cloned into a BamHI site downstream of the exon 9 stop codon (12). All regions of the targeting construct derived from PCR were completely sequenced. The linearized 11.5-kb targeting construct was electroporated into RW4 embryonic stem (ES) cells, and G418-resistant colonies were isolated and expanded. Clones were screened by Southern blot analysis, and two of 120 contained the correctly targeted ERα allele. The clones were injected into C57BL/6 blastocysts to generate chimeric mice in the Transgenic Mouse/Embryonic Stem Cell Facility (The University of Chicago, Chicago, IL). Male chimeras were bred to C57BL/6 females to establish germline transmission, and the resulting heterozygous mice were interbred. Animals were genotyped by Southern blot or PCR analysis, and self-excision of the ACN cassette in the mutant allele was confirmed. Animals were genotyped by PCR with DNA isolated from tail samples using primers flanking the one remaining loxP site (5′-GTATGGGCAATGACTGTGACTCGG-3 and 5′-AGAGGTCTTGGGTGGCTGTGG-3′). PCR amplification was performed in a buffer containing 1× TaKaRa LA Taq buffer (Fisher Scientific, Hanover Park, IL), 3 mm MgCl2, 0.4 mm deoxynucleotide triphosphates, 0.2 μm of each primer, and 5 U TaKaRa LA Taq polymerase (Fisher Scientific) for 500 ng genomic DNA. After 35 cycles (20 sec at 98 C, 8 min at 68 C), the resulting wild-type (WT) allele, ENERKI mutant allele with the ACN cassette excised, and mutant allele with the ACN cassette present, produced products of 497, 544, and 4246 bp, respectively. All procedures involving animals were conducted in accordance with the policies of the Institutional Animal Care and Use Committee at the University of Chicago. Animals were group housed in a barrier facility with 14-h light, 10-h dark cycles, and provided food and water ad libitum.
Selection of positive clones
Genomic DNA purified from ES cells or tail samples was digested with XmnI overnight and separated on a 0.7% agarose gel at 20 V. DNA was transferred to a Zeta-Probe GT Genomic Tested Blotting Membrane (Bio-Rad Laboratories, Inc., Hercules, CA), according to the manufacturer’s directions. DNA was attached to membrane by cross-linking. The membrane was blocked in ExpressHyb Hybridization Solution (BD Biosciences, Palo Alto, CA) for 1 h at 68 C. An 850-bp 5′ external probe to the targeting construct was labeled with the Rediprime II Random Prime Labeling System (Amersham Biosciences, Piscataway, NJ) and purified with a MicroSpin G-50 column (Amersham Biosciences). The denatured probed was added at 106 cpm/ml hybridization buffer and incubated overnight at 68 C. The blots were washed at 65 C with prewarmed 2× standard sodium citrate/1% sodium dodecyl sulfate for 30 min, and then with 0.2× standard sodium citrate/1% sodium dodecyl sulfate for 30 min. Blots were then visualized by a Phosphoimager (Storm 860; Molecular Dynamics, Sunnyvale, CA). PCR amplification of the 3′ end of the targeting construct was performed with primers in the targeting construct (5′-GTATGGGCAATGACTGTGACTCGG-3′) and external to the 3′ end of the targeting construct (5′-GTAACCGTGGGGAGGGGCATA-3′). Amplification was performed in a buffer containing 1× TaKaRa LA Taq buffer, 3 mm MgCl2, 0.4 mm deoxynucleotide triphosphates, 0.2 μm of each primer, and 5 U TaKaRa LA Taq polymerase for 1500 ng genomic DNA. After 35 cycles (20 sec at 98 C, 8 min at 68 C), the resulting WT and ES mutant alleles produced products of 3.5 and 7.0 kb, respectively.
Sample collection and histology
Female mice were euthanized by CO2 inhalation, followed by cervical dislocation at 6, 12, or 20 wk of age. The fat pads, uterus, vagina, ovaries, and mammary glands were dissected out, weighed, and fixed overnight in 10% neutral buffered formalin. Tissues were embedded in paraffin and sectioned at the Human Tissue Research Center (The University of Chicago, Chicago, IL). Standard hematoxylin and eosin (H&E) staining was then performed.
Tissue protein extractions and Western blot analyses
For protein extractions, flash-frozen tissues were pulverized on dry ice into a fine powder using a pulverizer (Cole Parmer Instrument Co., Vernon Hills, IL). Powdered samples were incubated in extraction buffer [20 mm Tris, 100 mm NaCl, 0.8 mm EDTA, and 0.05% NP-40 (Ipegal)] supplemented with 0.04 mm dithiothreitol and 0.008% protease inhibitor cocktail (Sigma-Aldrich) on ice for 1 h and vortexed every 15 min. Samples were spun at 3500 rpm for 30 min at 4 C. The supernatants were mixed with 2× sample buffer and boiled. For Western blot analyses, 20-μg protein samples were separated on SDS-PAGE gels and transferred to Hybond-enhanced chemiluminescence nitrocellulose membranes (Amersham Biosciences). ERα was detected with a 1:1000 dilution of polyclonal rabbit antibody (clone MC20; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Total protein was detected with a 1:1000 dilution of polyclonal rabbit pan-actin antibody (Cell Signaling Technology, Inc., Danvers, MA). A 1:2000 dilution of goat-antirabbit IgG horseradish peroxidase-labeled secondary antibody was applied (Santa Cruz Biotechnology). The immunocomplexes were visualized using the SuperSignal West Pico kit (Pierce, Rockford, IL) as described by the manufacturer.
Serum gonadotropin and steroid hormone assays
Blood was collected by cardiac puncture from 12-wk-old euthanized animals. Coagulation was prevented by treating blood with heparin. Blood was centrifuged and plasma frozen at −80 C for later use. Serum RIAs were performed by Brigitte Mann at Northwestern University (Chicago, IL). Serum LH and FSH RIAs were performed using iodinated standards (rLH-RP3, rFSH-RP2) and antisera (anti-rLH-S11, anti-rFSH-S11) from the National Institute of Diabetes and Digestive and Kidney Diseases. Serum E2 RIA was performed using a double antibody kit (Diagnostic Products Corp., Los Angeles, CA) according to the manufacturer’s directions. Serum testosterone RIA was performed with the double antibody kit (MP Biomedicals, Solon, OH) according to the manufacturer’s directions.
Mammary gland whole mounts
Mammary gland whole mounts were performed as previously described (13). Briefly, inguinal glands 4 and 5 were excised, placed on slides, and fixed in 25% glacial acetic acid and 75% ethanol for 1–3 h at room temperature. Slides were washed with 70% ethanol for 15 min, rinsed under running water for 5 min, and incubated in carmine alum solution overnight at room temperature. The next morning, tissues were dehydrated sequentially for 20–30 min each in 70, 90, and 100% ethanol, and cleared in xylene for 1 h. Coverslips were mounted with Permount (Fisher Scientific).
Growth factor treatments
Mice were maintained on a soy-free diet (2919; Charles River Laboratories, Wilmington, MA) for all growth factor experiments. Alzet micro-osmotic pumps (model 10003D; DURECT Corp., Cupertino, CA) were filled with 100 μl 5.0 μg/μl long R3 IGF-I, a potent synthetic IGF analog with low affinity for IGF binding proteins (Cell Sciences, Canton, MA), dissolved in 0.1 m acetic acid. Pumps were incubated in 1× PBS at 37 C for 4–6 h and implanted into the peritoneal cavity of ovariectomized mice according to the manufacturer’s directions. Control mice were injected ip with vehicle or 1 μg E2 dissolved in 100 μl sesame oil. Each treatment group contained three to four mice. Mice were euthanized 16–24 h after treatments, and uteri were fixed as previously described.
ER ligand treatments
Mice were maintained on a soy-free diet (2919) for all injection experiments. Compounds were prepared in a vehicle of 2% ethanol, 10% Cremophor EL (Sigma-Aldrich), and 88% 1× PBS at defined concentrations, so that the treatment volume was 0.01 ml/g body weight. Four to five animals were used for each treatment group. For uterotrophic assays, immature 18- to 21-d-old WT or ENERKI female mice were sc injected with 0–100,000 μg/kg E2, DES, PPT, or diarylpropionitrile (DPN) (Tocris Bioscience, Ellisville, MO) for 3 d. Uterine wet weights were measured on the fourth day, 24 h after the last injection. For short-term uterotrophic assays, adult ovariectomized WT or αERKO female mice were ip injected with vehicle or 10,000 μg/kg PPT. Uterine wet weights were measured 24 h later. αERKO mice, with no detectable ERα transcript levels due to exon 3 deletion, were generated by and obtained from K.S.K. For long-term injections, female ENERKI mice were sc injected with vehicle or 10,000 μg/kg PPT. In the first group, mice were injected every fourth day from 4 d to 8 wk of age. In the second group, mice were injected every fourth day from 3.5–8 wk of age. Mice were then euthanized, and reproductive tissues were excised for analysis.
Quantitative real-time PCR (RT-PCR)
Total RNA was prepared with TRIzol reagent according to the manufacturer’s directions (Invitrogen). One microgram of RNA was treated with DNase I (Invitrogen) before being reverse transcribed using the Superscript III First-Strand Synthesis System (Invitrogen). Random hexamers were used to prime the cDNA synthesis reaction. The resultant cDNA products were diluted to 100 μl, and 5 μl cDNA was used in RT-PCR using the QuantiTect SYBR Green PCR kit (QIAGEN). RT-PCR was performed with QuantiTect primers for ribosomal protein L13A (RPL13A) and ERβ (QIAGEN). The reactions were performed using the ABI 7300 Real-Time PCR System (Applied Biosystems) for 45 cycles (95 C for 15 sec, 55 C for 30 sec, 72 C for 40 sec) after an initial 15-min incubation at 95 C. RNA levels were determined for ERβ and RPL13A by comparison with standard curves generated from reference RNA (Stratagene, La Jolla, CA). ERβ expression was then normalized to the reference gene RPL13A, and the relative expression was determined by normalizing to the WT control. The reported results represent the average ± sem of triplicate samples and are representative of two independent experiments.
Statistical analysis
All reported values represent the mean ± sem. Differences were considered significant at P < 0.05 using factorial ANOVA with appropriate post hoc tests (SigmaStat 3.5; Systat Software, Inc., San Jose, CA).
Results
Features of the G525L mutation
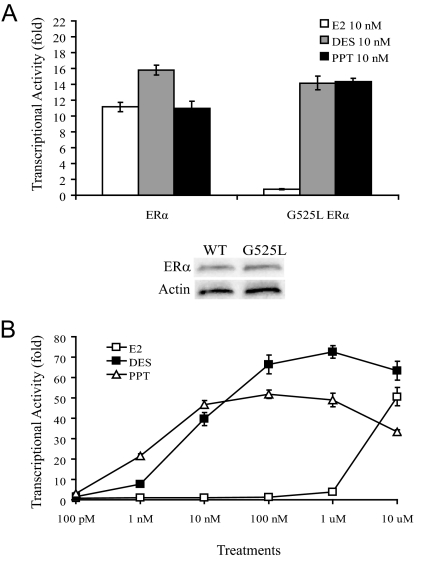
The G525L mutation in the ligand-binding domain of mouse ERα is predicted to interfere with the D-ring of E2 and greatly decrease its binding affinity (data not shown). However, this mutation is not predicted to interfere with the binding of several synthetic nonsteroidal compounds, such as DES or PPT. These molecules, unlike the more rigid and planar E2, are flexible and able to rotate around single bonds to bind the mutant ligand-binding domain. WT mouse ERα transcription was activated in the presence of low concentrations of E2, DES, or PPT (Fig. 1A). However, G525L ERα transcription was only stimulated by low concentrations of DES or PPT, and not E2. Western blots confirmed there were equal amounts of ERα and G525L ERα in these experiments, indicating that the G525L mutation did not affect ERα protein stability in vitro (Fig. 1B). G525L ERα transcription was only stimulated by a very high concentration of 10 μm E2 (Fig. 1B). Transcriptional activity of the endogenous estrogens estriol and estrone, along with other estrogen metabolites, had a similar response as E2 (data not shown). EGF treatments stimulated similar transcriptional activation of ERα and G525L ERα, confirming functional G525L ERα ligand-independent signaling in vitro (data not shown).
Figure 1.
Ligand-induced transcriptional activity of ERα and G525L ERα. A, Ishikawa cells were transfected with mouse ERα or G525L ERα and 3xERE-luc plasmids, and treated with E2, DES, or PPT (top panel). For E2 treatments, G525L ERα transcriptional activity is significantly different from ERα activity (P < 0.001). Western blots confirmed that there were equal levels of ERα and G525L ERα in these experiments, indicating that the G525L mutation does not affect ERα protein stability (bottom panel). B, A dose curve shows that G525L ERα transcription was only stimulated by low concentrations of DES or PPT, and only a very high concentration of E2.
Generation of ENERKI mice
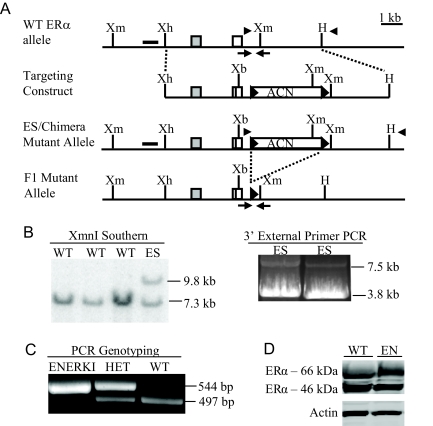
An 11.5-kb targeting construct containing exons 8 and 9 of mouse ERα with an extra XbaI site in exon 9, and C-terminal 18 bp 6xHis-tag epitope, was engineered to facilitate homologous recombination into the mouse genome (11) (Fig. 2A). The G525L mutation was introduced into exon 9 by site-directed mutagenesis. The targeting construct also contained a self-excising neomycin cassette (ACN cassette) (12). After electroporation of the targeting construct into ES cells, followed by G418 drug selection, 120 surviving colonies were screened by Southern blot analysis with a 5′ probe external to the targeting construct. Two clones gave the expected sized bands of 7.3 kb for the WT allele and 9.8 kb for the targeted allele with an XmnI digestion (Fig. 2B). PCR with a 3′ external primer confirmed that homologous recombination had occurred. PCR analysis of exon 9 established the presence of the G525L mutation, extra XbaI site, and 6xHis-tag epitope (data not shown). Both clones, 42 and 71, were injected into mouse blastocysts, and chimeras were generated. Male chimeras from both clones were mated to WT C57BL/6 females to establish germline transmission, and the resulting heterozygous were interbred. Southern blot (data not shown) and PCR (Fig. 2C) genotyping confirmed the removal of the ACN cassette in the F2 generation. The presence of the mutated allele was established by sequencing PCR products from homozygous genomic DNA. Western blot analyses indicated that there were comparable levels of ERα among the WT and ENERKI uterine tissues (Fig. 2D). The 46-kDa ERα splicing variant, which lacks the first 173 amino acids of full-length ERα, is probably not active in ENERKI animals because it lacks the AF-1 domain (14). WT values ranged widely because ERα levels fluctuate throughout the estrous cycle (15). Uterine ENERKI G525L ERα protein levels were also successfully measured by 6xHis-tag immunoblotting (data not shown), indicating that the 6xHisTag did not affect G525L ERα protein expression. ENERKI animals generated from both clones 42 and 71 exhibited identical phenotypes at 12 wk of age. All results in this manuscript are from animals derived from clone 42.
Figure 2.
Targeting strategy for G525L ERα knock-in mutation. A, Schematic illustration of the targeting strategy used to introduce the mutation. Diagrams show the WT ERα locus, targeting construct, ES-targeted mutant allele, and F1 mutant allele after ACN cassette self-excision. The targeting construct contained ERα exons 8 (gray box) and 9 (white box), the G525L mutation (black bar in exon 9), an extra XbaI site, and an 18 bp 6xHis-tag epitope. The ACN cassette was flanked at the 5′ and 3′ ends by loxP sites (black arrowheads). Restriction enzyme sites shown include XmnI (Xm), XhoI (Xh), HindIII (H), and XbaI (Xb). B, Screening for target insertion by homologous recombination. ES cell DNA was digested with XmnI and Southern blotting was performed with a 5′ external probe (black rectangle in panel A), resulting in WT and ES allele products of 7.3 and 9.8 kb, respectively (left panel). PCR amplification with primers in the targeting construct and external to the 3′ end of the targeting construct (black arrowheads in A) resulted in WT and ES allele products of 3.5 and 7.0 kb, respectively (right panel). There was less 7.5-kb ES mutant targeted product than 3.8-kb WT product because it is very difficult to amplify long genomic sequences. The integrity of both products was confirmed by sequencing. C, DNA isolated from tail snips was analyzed by PCR using primers surrounding the remaining loxP site in the F1 mutant allele (black arrows in Fig. 2A). The WT and F1 mutant allele generate a 497- and 544-bp PCR product, respectively. HET, Heterozygous. D, Uterine ERα levels from 12-wk-old representative WT and ENERKI (EN) mice. Uterine extracts were analyzed by Western blotting using ERα or actin antibodies. Both full-length ERα (66 kDa) and a splicing variant (46 kDa) lacking the first 173 amino acids were present (14). ERα levels were equal among all genotypes.
ENERKI females gain excess adipose tissue
Female ENERKI mice were significantly larger than their WT and heterozygous littermates from 12 wk of age onwards (Table 1). Body weights were increased by 19 and 33%, respectively, in ENERKI 12- and 20-wk-old females. ENERKI gonadal and mammary fat pad weights were 35 and 43% larger, respectively, than their WT and heterozygous littermates at 20 wk of age (Table 1).
Table 1.
Female body, gonadal fat pad, and mammary gland weights
| Mice | Body weight (g) | Gonadal fat pad (% body weight) | Mammary gland (% body weight) |
|---|---|---|---|
| 6 wk | |||
| WT (n = 8) | 17.9 ± 0.5 | 1.3 ± 0.2 | 0.8 ± 0.1 |
| ENERKI (n = 9) | 18.9 ± 1.0 | 1.3 ± 0.2 | 0.9 ± 0.1 |
| 12 wk | |||
| WT (n = 10) | 21.4 ± 0.9 | 2.4 ± 0.3 | 1.1 ± 0.1 |
| ENERKI (n = 10) | 25.4 ± 1.1a | 2.1 ± 0.2 | 1.1 ± 0.1 |
| 20 wk | |||
| WT (n = 8) | 26.5 ± 1.5 | 3.5 ± 0.6 | 1.5 ± 0.2 |
| ENERKI (n = 10) | 35.5 ± 2.5a | 5.3 ± 0.7 | 2.1 ± 0.2b |
Data are expressed as mean (±sem).
The ENERKI value is significantly different from the WT value (P < 0.01).
The ENERKI value is significantly different from the WT value (P < 0.05).
ENERKI uterine tissues are immature and hypoplastic
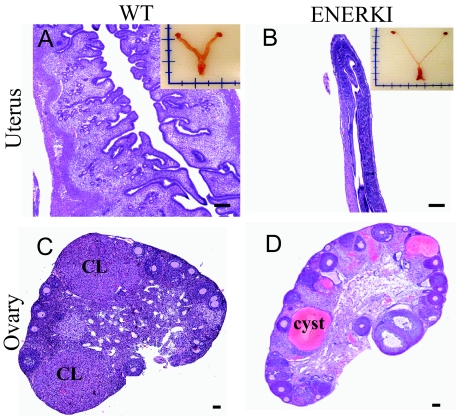
Female reproductive tract organs from WT and ENERKI mice were collected from 12-wk-old mice, and analyzed for morphological and histological indication of function and development. ENERKI uteri were significantly smaller than their WT littermates (compare Fig. 3, A and B). ENERKI uterine tissues possessed luminal and glandular epithelium, evidence of vascularization, and endometrial stroma. However, they were also severely hypoplastic and completely lacked an edematous stroma (Fig. 3B). ENERKI vaginal tissues were also severely hypoplastic and lacked cornification (data not shown).
Figure 3.
Reproductive tract histology of 12-wk-old representative mice. Uterine (top) and ovarian (bottom) tissue H&E staining from WT (left) and ENERKI (right) mice. WT uteri (A) developed normally, but ENERKI tissues (B) did not exhibit an increase in uterine wet weight, and were immature and hypoplastic. Insets depict reproductive tract morphology. WT ovaries (C) contained many corpora lutea and healthy follicles, whereas ENERKI ovaries (D) had no corpora lutea (CL), and the majority contained large hemorrhagic cysts. Bars, 100 μm.
ENERKI females are anovulatory
The ovaries of both genotypes displayed follicles ranging from primordial to developed antral follicles (Fig. 3, C and D). The ovaries also had no developmental abnormalities in the ovarian surface epithelium or the oocytes. Ovaries from ENERKI animals in general had a higher number of antral follicles, with the majority containing pyknotic nuclei consistent with atresia (data not shown). The higher number of developed antral follicles is likely due to the lack of corpora lutea formation (Fig. 3D). These data show an anovulatory ovarian phenotype consistent with their likely infertility. Theca cells that respond to LH by proliferating and producing androgens became hyperplastic in the ENERKI female mice (data not shown). The lack of negative feedback by estrogen at the level of the hypothalamic-pituitary axis would result in chronic stimulation of these cells. LH levels were, in fact, significantly higher in the ENERKI females (Table 2). Unique to the ENERKI animals was the presence of hemorrhagic cysts, apparent in 89% of the analyzed animals, which appeared to develop from atretic antral follicles (Fig. 3D). Finally, the appearance of granulosa cells not encapsulated into follicles in ENERKI animals was detected in all of the ovaries analyzed but did not progress at the 12-wk age to granulosa cell tumors.
Table 2.
Female serum hormone levels
| Mice | LH (ng/ml) | FSH (ng/ml) | E2 (pg/ml) | T (ng/ml) |
|---|---|---|---|---|
| WT (n = 6–14) | 0.31 ± 0.05 | 3.80 ± 0.36 | 9.74 ± 2.11 | 0.02 ± 0.00 |
| ENERKI (n = 8–14) | 3.72 ± 0.42a | 3.43 ± 0.22 | 38.22 ± 7.19a | 0.88 ± 0.22a |
Data are expressed as mean (±sem). T, Testosterone.
The ENERKI value is significantly different from the WT values (P < 0.01).
Serum gonadotropin and steroid hormone levels
ENERKI female serum LH levels were elevated 12-fold over WT levels due to the lack of estrogen negative feedback in the brain (Table 2). ENERKI E2 and testosterone levels were 3.5- and 8-fold higher than WT levels, respectively. FSH levels were not significantly different (Table 2).
Mammary glands of ENERKI females do not develop beyond rudimentary ducts
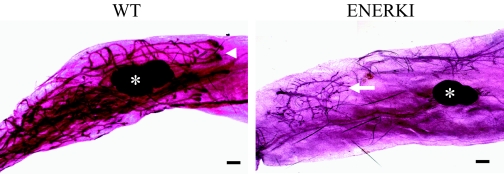
Mammary gland whole mounts were analyzed for evidence of ductal proliferation and differentiation. In 6-wk-old WT mice, ductal trees extended past the lymph node and had enlarged terminal end buds (Fig. 4). In 12-wk-old WT mice, ducts filled the entire mammary fat pad, and had extensive branching and budding (data not shown). ENERKI mammary glands never developed beyond a rudimentary epithelial ductal tree (Fig. 4).
Figure 4.
Mammary gland whole mounts from 6-wk-old representative mice. WT (left) ductal trees extended to the lymph node (white asterisk) and had enlarged terminal end buds (white arrowhead), whereas ENERKI (right) mammary glands only had a rudimentary epithelial ductal tree (white arrow). Bars, 400 μm.
IGF-I treatments induce robust WT and irregular ENERKI uterine epithelial proliferation
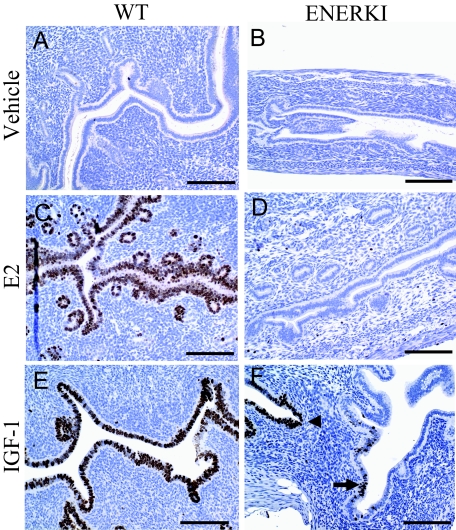
Uterine epithelial proliferation was induced in ovariectomized WT, but not αERKO, mice treated with IGF-I, indicating that this response is due to IGF-I activation of ERα (16). To demonstrate directly that ligand-independent ERα pathways are functional in ENERKI animals, ovariectomized 12-wk-old female mice were treated with vehicle, E2, or IGF-I. Uterine tissues were removed during the peak DNA synthesis period, 16–24 h later, and epithelial proliferation was measured by immunohistochemical analysis of Ki67. Vehicle treatments did not stimulate increased uterine proliferation in either WT or ENERKI animals (Fig. 5, A and B). As expected, E2 treatments increased proliferation in the luminal and glandular epithelium of WT animals, but not ENERKI females (Fig. 5, C and D). IGF-I treatments induced proliferation in both genotypes, demonstrating that ENERKI ligand-independent ERα pathways are most likely functional (Fig. 5, E and F). However, Ki67 staining was patchy in the ENERKI uterine tissues and much less robust compared with that of WT tissues.
Figure 5.
Uterine Ki67 immunohistochemistry after vehicle, E2, or IGF-I treatments of representative mice. Uterine tissues were removed 16–24 h after vehicle, E2, or IGF-I treatments in 12-wk-old ovariectomized WT (left) or ENERKI (right) females and analyzed for proliferation via Ki67 immunohistochemistry. WT and ENERKI animals treated with vehicle (A and B) had no uterine Ki67 staining. WT, but not ENERKI, animals treated with E2 (C and D) exhibited uterine glandular and luminal epithelial Ki67 staining. Both WT and ENERKI animals treated with IGF-I (E and F) exhibited epithelial uterine Ki67 staining. WT proliferation was robust (E), whereas ENERKI proliferation was patchy, with a marginal response in some regions (black arrow) and a rare strong response in a few areas (black arrowhead) (F). Bars, 100 μm.
PPT treatment elicits a uterotrophic response in immature ENERKI females
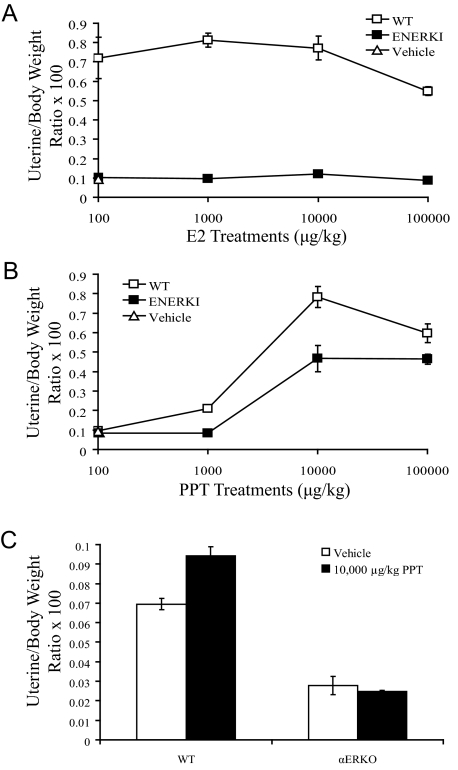
Immature 18- to 21-d-old mice were treated sc with various doses of E2, DES, or PPT for 3 d, and uterine wet weights were measured 24 h after the last treatment. As anticipated, E2 significantly increased uterine wet weights of WT mice. Although 100 μg/kg E2 saturated the WT response, even 100,000 μg/kg E2 did not increase ENERKI uterine wet weights (Fig. 6A). Treatments of 10,000 μg/kg PPT significantly increased uterine wet weights of both WT and ENERKI mice (Fig. 6B). To confirm that PPT is an ERα selective agonist in vivo, adult ovariectomized WT and αERKO females were also treated with PPT. As expected, PPT significantly increased the uterine wet weights of WT, but not αERKO, females (Fig. 6C). WT animals treated with PPT, unlike αERKO females, also exhibited increased uterine epithelial proliferation and altered uterine transcript levels of ERα target genes (data not shown).
Figure 6.
Uterotrophic activity of E2 and PPT. A and B, Immature WT or ENERKI female mice were sc injected with the indicated doses of vehicle, E2 (A), or PPT (B) for 3 consecutive days. Uterine wet weight was measured on the fourth day. Values represent mean ± sem with three to five animals per group. WT values are significantly different from ENERKI values at all E2 concentrations (P < 0.001) and at 1000–100,000 μg/kg PPT (P < 0.04). C, Adult ovariectomized WT or αERKO female mice were ip injected with vehicle or 10,000 μg/kg PPT. Uterine wet weight was measured 24 h later. Values represent mean ± sem with three to five animals per group. Vehicle and PPT WT values are significantly different (P < 0.01).
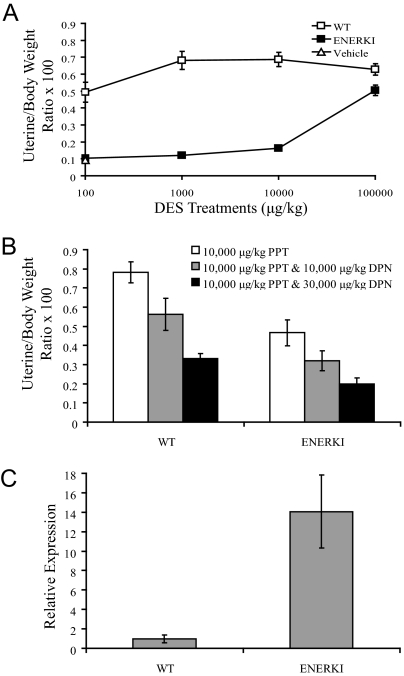
Treatments of 100 μg/kg DES caused a significant uterotrophic response in WT animals (Fig. 7A). Surprisingly, ENERKI mice only exhibited a significant increase in uterine wet weight when treated with a very high dose of 100,000 μg/kg DES. Because DES can activate both ERα and ERβ, unlike PPT, which is an ERα selective agonist, we hypothesized that DES-activated ERβ may antagonize G525L ERα in the uterus. When WT and ENERKI females were treated simultaneously with both PPT and DPN, an ERβ selective agonist, ERβ activation did inhibit the uterotrophic response induced by PPT (Fig. 7B). We hypothesized that ENERKI animals may be particularly sensitive to DES-induced inhibition of ERα due to up-regulated uterine ERβ levels. ENERKI uterine ERβ transcript levels were, in fact, an average of 14 times higher than WT levels (Fig. 7C). Therefore, DES most likely did not work well in vivo due to high ENERKI ERβ levels.
Figure 7.
Uterotrophic activity of DES. A and B, Immature WT or ENERKI female mice were sc injected with the indicated doses of vehicle, DES (A), or PPT and DPN (B) for 3 consecutive days. Uterine wet weight was measured on the fourth day. Values represent mean ± sem with four to five animals per group. WT values are significantly different from ENERKI values at all DES concentrations (P < 0.03). The WT 10,000 μg/kg PPT value is significantly different from both the 10,000 μg/kg PPT and 10,000 μg/kg DPN, and 10,000 μg/kg PPT and 30,000 μg/kg DPN values (P < 0.02). The ENERKI 10,000 μg/kg PPT value is significantly different from the 10,000 μg/kg PPT and 30,000 μg/kg DPN value (P < 0.01). C, Relative expression levels of WT and ENERKI uterine ERβ transcript levels. ERβ expression levels were normalized to RPL13A expression, and the relative expression was determined by normalizing to the WT control. The reported results represent the average ± sem of triplicate samples. G525L ERβ transcript levels are significantly different from WT levels (P < 0.01).
PPT treatments induce uterine and mammary gland ductal growth in adult ENERKI females
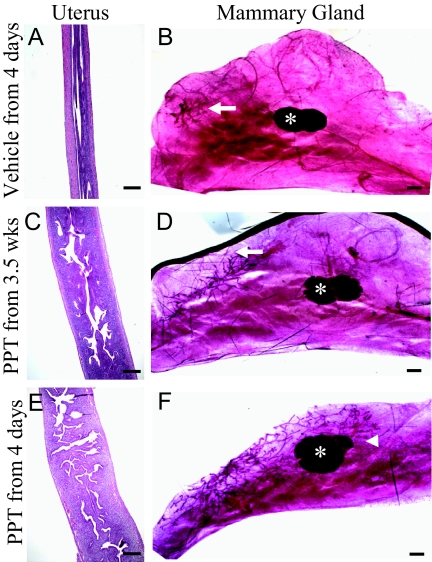
In an effort to induce normal female reproductive tract development, ENERKI females were sc injected over several weeks with vehicle or 10,000 μg/kg PPT. Injections were performed every fourth day to simulate the estrous cycle. As anticipated, mice treated with vehicle displayed no uterine or mammary gland development (Fig. 8, A and B). Mice injected with PPT from 3.5–8 wk of age exhibited an increase in uterine wet weight and development, but no mammary gland ductal growth (Fig. 8, C and D) However, mice injected with PPT from 4 d to 8 wk of age exhibited both uterine and mammary gland ductal development (Fig. 8, E and F). Therefore, neonatal PPT treatments were needed for adult mammary gland but not uterine development. The ovarian phenotype was not reversed with either treatment schedule at 10,000 or 100,000 μg/kg PPT (data not shown).
Figure 8.
Effect of long-term PPT treatments on ENERKI uterine and mammary gland tissues. Groups of four to five ENERKI females were injected sc with vehicle from 4 d of age (A and B), 10,000 μg/kg PPT from 3.5 wk of age (C and D), or 10,000 μg/kg PPT from 4 d of age (E and F) every fourth day until 8 wk of age. Uterine tissue H&E staining (A, C, and E) and mammary gland whole mounts (B, D, and F) were then performed. Bars, 100 μm (A, C, and E) and 400 μm (B, D, and F). Although uterine growth was stimulated with either PPT treatment schedule (C and E), mammary gland ductal development was only stimulated with neonatal treatments (F). In ENERKI animals treated with 10,000 μg/kg PPT from 4 d to 8 wk of age, mammary ductal trees extended past the lymph node (white asterisk) and had enlarged terminal end buds (white arrowhead) (F). Animals treated with vehicle or with PPT from 3.5 wk had a rudimentary ductal tree (white arrow) (B and D).
Discussion
In the present study, we report the initial characterization of ENERKI mice, which have a ligand-binding pocket G525L mutation that significantly reduces ERα interaction with and response to endogenous estrogens, but not to the ERα selective agonist PPT. ENERKI mice were used to examine the role of AF-1 domain ligand-independent ERα signaling in vivo. In addition, PPT treatments were used to modulate AF-2 domain ligand-induced ERα pathways.
This work supports the idea that ligand-induced ERα signaling is important in regulation of female adiposity. ENERKI mice exhibited a progressive increase in adiposity, which is partly due to excessive abdominal fat pad accumulation. This phenotype is similar to that of the αERKO females, which developed significantly larger stores of intraabdominal fat by 12 wk of age (17). αERKO animals had impaired glucose tolerance, insulin resistance, and increased leptin levels (17,18,19). αERKO females also had increased food intake and decreased satiation, whereas αERKO males expended less energy and had higher cholesterol levels (17,19,20). Whether the ENERKI phenotype is due to changes in regulation of metabolism, reduced physical activity, or increased appetite, remains to be determined.
The severe uterine and vaginal hypoplasia in the ENERKI females was also reported in the αERKO animals (8). This confirms that ERα ligand-induced signaling is critical for the normal development of these tissues. Interestingly, the uteri of nonclassical ERα knock-in (NERKI) mice are larger than αERKO uteri but smaller than WT uteri (21). NERKI mice have a mutation in ERα that disrupts DNA binding but leaves nonclassical signaling intact, so ERα can still alter gene transcription through protein-protein interactions with transcription factors like c-fos, activator protein-1, and specificity protein-1 (22). The NERKI phenotypes reported in this manuscript are from mice on an ERα-null background, with one NERKI allele and one αERKO allele. Heterozygous NERKI animals are not fertile and, therefore, cannot produce homozygous animals (21,22). The NERKI uterine phenotype indicates that nonclassical ERα pathways may contribute to uterine growth and development, perhaps via up-regulation of growth factors (22). Phenotypical evaluation of the ENERKI mice has also established that binding of ERα to its ligand is crucial for ovulation and prevention of hemorrhagic cyst formation. The most likely explanation for this physiology is a lack of estrogen negative feedback in the brain, allowing for elevated circulating levels of LH (8). This reasoning is consistent with the increased ENERKI E2 and testosterone levels and theca cell hypertrophy, which can be caused by chronic LH ovarian hyperstimulation. Large fluid or blood-filled cysts, characterized by the presence of a single layer of atretic granulosa cells, were present in the ENERKI animals. The cysts resemble those found in the αERKO, NERKI, and LH-β C-terminal-peptide overexpressing (LHβCTP) transgenic mice (22,23,24). These phenotypical similarities, along with the fact that the cystic phenotype was eliminated in αERKO mice by treatments with GnRH antagonists (25), suggest that the formation of cysts is due to the increase in LH levels. NERKI mice had lower serum LH levels than αERKO mice, suggesting that ERα-mediated negative feedback depends on both classical DNA binding and nonclassical protein interactions (26). However, αERKO, NERKI, and ENERKI mice did not develop corpora lutea, whereas the LHβCTP animals produced enlarged ovaries containing numerous corpora lutea (8,26,27). In addition, because the ENERKI females exhibited elevated LH levels, cystic ovaries, and heightened androgen production, they could be used to study human polycystic ovarian syndrome (27,28). The ovarian surface epithelium, which appeared normal in the ENERKI animals, is the source of the majority of gonadal tumors. However, the presence of disordered granulosa cells not encapsulated in the follicular structures in the ENERKI females is reminiscent of certain types of ovarian granulosa cell tumors. The older LHβCTP mice developed granulosa cell tumors, but this predisposition toward tumor formation was found to be strain specific (29). The 12-wk-old ENERKI animals had swirls of granulosa cells, but none of them presented as transformed tumors. This lack of granulosa cell tumors is consistent with that of comparably aged αERKO mice (8). The ENERKI mammary glands resembled those of a prepubertal female. There was no evidence of terminal end bud formation, ductal elongation, branching, or alveolar differentiation. This is the same phenotype reported in the αERKO animals. The overall similarity of the ENERKI and αERKO phenotype confirms that ERα ligand-induced, but not ligand-independent, signaling is critical in female reproductive tract and mammary gland development.
Recent studies demonstrate the existence of cross talk between uterine IGF-I and ERα signaling pathways (16). Epithelial proliferation was induced in ovariectomized WT but not αERKO mice treated with IGF-I. Therefore, ENERKI animals were treated with IGF-I in an effort to demonstrate directly that their ligand-independent G525L ERα pathways are functional. IGF-I treatment induced strong uniform uterine epithelial proliferation in ovariectomized WT mice. Although ovariectomized ENERKI mice treated with IGF-I exhibited patchy uterine epithelial proliferation, it was much less robust than that of WT mice. In addition, IGF-I doses twice as high as those used in the αERKO studies were needed to induce this response, so we cannot be completely sure that the increase in ENERKI uterine proliferation is due to ligand-independent ERα signaling (16). However, these results are not surprising considering that the AF-1 and AF-2 domains work synergistically or in a feed forward mechanism in many tissues, and ENERKI mice lack AF-2 activation (6). Both AF-1 and AF-2 signaling may be required for robust IGF-I-induced uterine proliferation in the uterus. Although we used ovariectomized animals to abolish circulating hormone levels during the experiments, AF-2 activation in the animals before their surgeries could be imperative for uterine AF-1 function, e.g. by up-regulating coactivators. Unfortunately, there are currently no additional ways to prove directly that ENERKI AF-1 signaling pathways are active. However, analysis of the ENERKI male phenotype provides indirect evidence of functional ligand-independent G525L ERα pathways in these animals. ENERKI males are subfertile and have a less severe phenotype (data not shown) than infertile αERKO males (8,23). ENERKI mice will be used to learn more about the mechanistic interactions and tissue-specific functions of the AF-1 and AF-2 domains in vivo.
ERα ligand-induced signaling can be regulated in ENERKI mice through PPT administration. As anticipated, PPT increased immature ENERKI uterine wet weights in a dose-dependent manner in standard uterotrophic assays. Recent studies have demonstrated that PPT is as efficacious as E2 in animals, but less potent (30). Therefore, it is not surprising that a relatively high PPT dose of 10,000 μg/kg, compared with 100 μg/kg E2 or DES, was needed to stimulate a uterotrophic response in the mice. Importantly, αERKO females did not exhibit increased uterine wet weights in response to PPT treatments. These results demonstrate that PPT is an ERα selective agonist in vivo and confirm that PPT induces a uterotrophic response in ENERKI females via G525L ERα activation.
Because ENERKI mice only exhibited a significant increase in uterine wet weight when treated with a high dose of 100,000 μg/kg DES, we hypothesized that DES-activated ERβ may antagonize G525L ERα in the uterus. There is evidence that ERβ may modulate ERα activation and have an antiproliferative function in the uterus (31). This was confirmed when WT and ENERKI females treated simultaneously with PPT and DPN exhibited a much lower uterotrophic response than animals treated with PPT alone. ENERKI uterine ERβ levels were an average of 14 times higher than WT levels, confirming that ENERKI animals may be particularly sensitive to DES-induced inhibition of ERα due to up-regulated uterine ERβ levels.
Long-term PPT treatments were performed in an attempt to induce normal ENERKI female reproductive tract development. These injections were originally begun at the initiation of puberty, around 3.5 wk of age, and concluded in adult animals at 8 wk of age. Although PPT treatments every fourth day stimulated uterine development, mammary gland ductal growth did not occur. We hypothesized that hormonal priming might be required for mammary gland ductal development. When ENERKI females were treated every fourth day with PPT from 4 d to 8 wk of age, mammary gland ductal elongation occurred. Therefore, neonatal ligand-induced ERα activation may prime the ducts, e.g. by up-regulation of coactivators, to become more responsive to estrogen in adult tissues. Interestingly, preliminary experiments indicated that 10,000 μg/kg PPT injected everyday from 3.5–8 wk of age also stimulated mammary ductal elongation (data not shown). Therefore, neonatal ligand-induced ERα activation may not be required for normal adult mammary gland ductal development. It will be interesting to determine which phenotype occurs with the most physiologically relevant amounts of PPT.
In summary, we report the first description of the consequence of the loss of ligand-induced ERα signaling in vivo. The phenotype of the ENERKI females confirms the importance of ligand-induced ERα signaling in the female reproductive tract. Ligand-independent ERα activation may be important in tissues where estrogen levels are low, like in the male reproductive tract or in nonreproductive tissues (6,32). Therefore, studies are currently underway to examine the ENERKI male phenotype and several nonreproductive tissues. In addition, ENERKI ligand-induced ERα signaling can be regulated in vivo through PPT administration. This is the first model, to our knowledge, of in vivo modulation of ligand-induced ERα pathways. The mice generated in this study will be valuable for determining the expression and function of ERα in development and tumorigenesis.
Acknowledgments
We thank Linda Degenstein for her work in the Transgenic Mouse/Embryonic Stem Cell Facility. We are indebted to John Katzenellenbogen for a generous supply of propyl pyrazole triol. We are grateful to Akira Imamoto, Jon Levine, Sally Radovick, and Andrew Wolfe for helpful discussions and advice. We appreciate the excellent technical skills of Jennifer Brace, Brigitte Mann, and Jim Radek. We also value the contributions of Deborah Swope, who is no longer with us.
Footnotes
This work was supported in part by Department of Defense Breast Cancer Program predoctoral traineeship W81XWH-04-1-0347 (to K.W.S.), National Cancer Institute Grant CA89089 (to G.L.G.), Department of Defense Grant DAMD17-01-1-0199 (to S.L.S. and G.L.G.), and National Institutes of Health Grant HD044464 R01 (to T.K.W.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 13, 2008
Abbreviations: AF, Activation function; DES, diethylstilbestrol; DPN, diarylpropionitrile; EGF, epidermal growth factor; E2, 17β-estradiol; ENERKI, estrogen-nonresponsive estrogen receptor-α knock-in; ER, estrogen receptor; ERE, estrogen response element; αERKO, estrogen receptor-α knockout; βERKO, estrogen receptor-β knockout; αβERKO, estrogen receptor-α/estrogen receptor-β knockout; ES, embryonic stem; G525L, glycine 525 to leucine; H&E, hematoxylin and eosin; LHβCTP, LH-β C-terminal-peptide overexpressing; NERKI, nonclassical estrogen receptor-α knock-in; PPT, propyl pyrazole triol; RT-PCR, real-time PCR; WT, wild type.
References
- Klinge CM 2000 Estrogen receptor interaction with co-activators and co-repressors. Steroids 65:227–251 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S 2001 Perspective: nonreproductive sites of action of reproductive hormones. Endocrinology 142:2200–2204 [DOI] [PubMed] [Google Scholar]
- Coleman KM, Smith CL 2001 Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci 6:D1379–D1391 [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Kenneth KS 2002 Estrogen receptors: structure, mechanisms and function. Rev Endocr Metab Disord 3:193–200 [DOI] [PubMed] [Google Scholar]
- Driggers PH, Segars JH 2002 Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab 13:422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Inoue S 2000 Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun 270:1–10 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev [Erratum (1999) 20:459] 20:358–417 [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS 1999 Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science 286:2328–2331 [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Swope DL, Castranio T, Harrell JC, Mishina Y, Korach KS 2002 AF-2 knock-in mutation of estrogen receptor α: cre-loxP excision of a PGK-neo cassette from the 3′ UTR. Genesis 32:99–101 [DOI] [PubMed] [Google Scholar]
- Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR 1999 Targeting genes for self-excision in the germ line. Genes Dev 13:1524–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, Korach KS 2000 Induction of mammary gland development in estrogen receptor-α knockout mice. Endocrinology 141:2982–2994 [DOI] [PubMed] [Google Scholar]
- Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F 2000 Identification of a new isoform of the human estrogen receptor-alpha (hER-α) that is encoded by distinct transcripts and that is able to repress hER-α activation function 1. EMBO J 19:4688–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman MD, Schachter BS, Karelus K, Combatsiaris EP, Garcia T, Nelson JF 1992 Up-regulation of the uterine estrogen receptor and its messenger ribonucleic acid during the mouse estrous cycle: the role of estradiol. Endocrinology 130:1923–1930 [DOI] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS 2002 Requirement of estrogen receptor-α in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem 277:8531–8537 [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS 2000 Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson J-A, Efendic S, Khan A 2006 Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49:588–597 [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Hellberg NH, Parini P, Vidal O, Bohlooly M, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson J-A 2000 Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem Biophys Res Commun 278:640–645 [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S 2001 Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142:4751–4757 [DOI] [PubMed] [Google Scholar]
- O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL 2006 Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J Biol Chem 281:26683–26692 [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL 2002 An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH 1995 Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci USA 92:1322–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS 1999 Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-α knockout mouse. Endocrinology 140:5855–5865 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulli SB, Huhtaniemi I 2005 What have gonadotrophin overexpressing transgenic mice taught us about gonadal function? Reproduction 130:283–291 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Sanford R, Nyska A, Nilson JH, Korach KS 2004 Formation of cystic ovarian follicles associated with elevated luteinizing hormone requires estrogen receptor-β. Endocrinology 145:4693–4702 [DOI] [PubMed] [Google Scholar]
- Keri RA, Lozada KL, Abdul-Karim FW, Nadeau JH, Nilson JH 2000 Luteinizing hormone induction of ovarian tumors: oligogenic differences between mouse strains dictates tumor disposition. Proc Natl Acad Sci USA 97:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS 2002 Characterization of the biological roles of estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology 143:4172–4177 [DOI] [PubMed] [Google Scholar]
- Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, Warner M, Gustafsson J 2000 Estrogen receptor (ER)β, a modulator of ERα in the uterus. Proc Natl Acad Sci USA 97:5936–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, Lowik C, Maggi A 2003 In vivo imaging of transcriptionally active estrogen receptors. Nat Med 9:82–86 [DOI] [PubMed] [Google Scholar]