Abstract
To learn the evolutionary trajectories of caste differentiation in eusocial species is a major goal of sociobiology. We present an explanatory framework for caste evolution in the eusocial wasp genus Polistes (Vespidae), which is a model system for insect eusocial evolution. We hypothesize that Polistes worker and gyne castes stem from two developmental pathways that characterized the bivoltine life cycle of a solitary ancestor. Through individual-based simulations, we show that our mechanistic framework can reproduce colony-level characteristics of Polistes and, thereby, that social castes can emerge from solitary regulatory pathways. Our explanatory framework illustrates, by specific example, a changed perspective for understanding insect social evolution.
The essence of eusociality in Polistes wasps is the differentiation of female offspring into two castes: nonreproductive females that work in their natal colony and reproductive gynes that found colonies in the next nesting cycle (1). Differentiation of Polistes offspring into workers and gynes has been proposed to rely on physiological events that are triggered after adults emerge from pupation (2). A more widely held view, however, is that caste in social wasps is determined, or at least predisposed, during larval development (3). Larval nourishment is believed to play a role in caste differentiation (1, 3), but the nature of this mechanism has not been determined. More importantly, no hypothesis addresses how a nutritional cue would translate into specific traits that characterize the two castes.
Regulatory machineries that control sequential shifts between phases in the life cycles of solitary insects may have been co-opted during social evolution (4). From this perspective, caste evolution can be envisioned as a remodeling process that uses solitary control circuits to build social phenotypes. The regulatory layout of a presocial form would thus become the ground plan that underlies the evolutionary design of its social descendants. One such layout may be the bivoltine life cycle, an adaptation to seasonal environments that may be tropical wet/dry as well as temperate warm/cold. Bivoltine life cycles have a first generation that undergoes uninterrupted development and reproduction, followed by a second generation that enters prepupal or adult diapause, passes the unfavorable season, and reproduces the following year. (The prepupa resides inside the pupal cocoon and is a quiescent state of the last larval instar.) The biology of wasps in the vespid subfamily Eumeninae can have major explanatory implications for the evolutionary trajectories of sociality in Vespidae (5). Bivoltinism occurs commonly in solitary eumenines (6), and the eumenine-like solitary ancestor of the eusocial vespid Polistes may have been bivoltine. With this initial assumption, we hypothesize that the regulatory circuits that separated the two generational trajectories in the ancestor were co-opted to build a social life-history pattern in which the worker brood of Polistes corresponds to the first generation (G1) (Fig. 1) and the gyne brood corresponds to the second (G2) (Fig. 1).
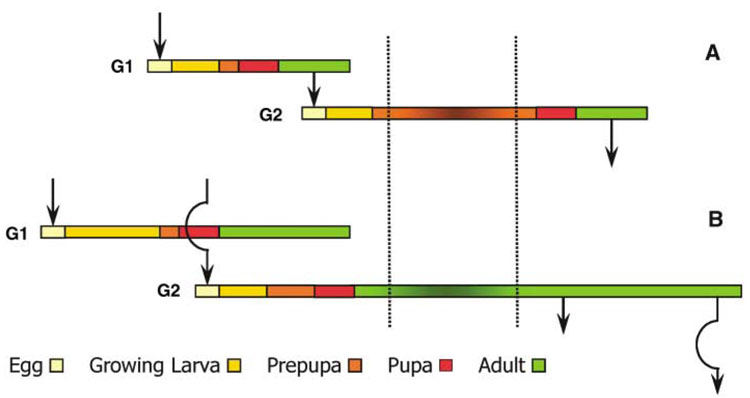
Fig. 1.
Life cycles of (A) a bivoltine solitary wasp and (B) Polistes in a seasonal environment. Eggs of the first brood (G1) are laid by adults from the second generation of the preceding favorable season (G2). In the solitary wasp, G1 females complete development, emerge as adults, and produce the G2 generation. These individuals then pass the unfavorable season, indicated by shading between the dotted vertical lines, in prepupal diapause (35). Three principal changes can convert this solitary life cycle into a social life strategy. One change is that diapause is passed as an adult rather than as a prepupa, which is known to occur in some bivoltine solitary wasps in family Sphecidae (35). A second change is that the life cycle is partially rather than discretely bivoltine. In partial bivoltinism, adults that lay eggs of the first generation also lay some eggs of the second generation. This strategy is common and may favor evolution of eusociality (6). The third change is that offspring of the first generation do not reproduce but instead undertake brood care at their natal nest; thus, no arrow connects the G1 and G2 broods in the Polistes diagram (B).
Early-emerging Polistes offspring work as G1 females in a condition of reproductive readiness but in a context that curtails their reproductive contribution to the G2 brood (Fig. 2). Worker behavior in Polistes is context-dependent expression of maternal care behaviors (1), and when the females begin to forage and engage in nest construction, they do so as workers at the natal nest. The reproductive potential of Polistes G1 females is nonetheless readily apparent, as “laying workers” (7), replacement queens (8), and satellite nest foundresses (9, 10) all come from the early brood. Thus, at the conceptual level, our approach characterizes Polistes offspring workers as reproductives but with their reproduction in context-dependent suppression. Newly emerged gynes may similarly be characterized as non-reproductives. Gynes do not forage or reproduce their first year, and gynes show no ovarian development or nest building behavior when isolated under favorable conditions in which G1 wasps (that normally would be workers) initiate reproductive maturation and nest construction (11, 12). These data suggest that gynes emerge in a state of reproductive diapause, which is physiological arrest rather than behavioral quiescence. Developmental and adult traits that distinguish gynes from workers suggest that gynes derive from a G2 phenotype (Fig. 2).
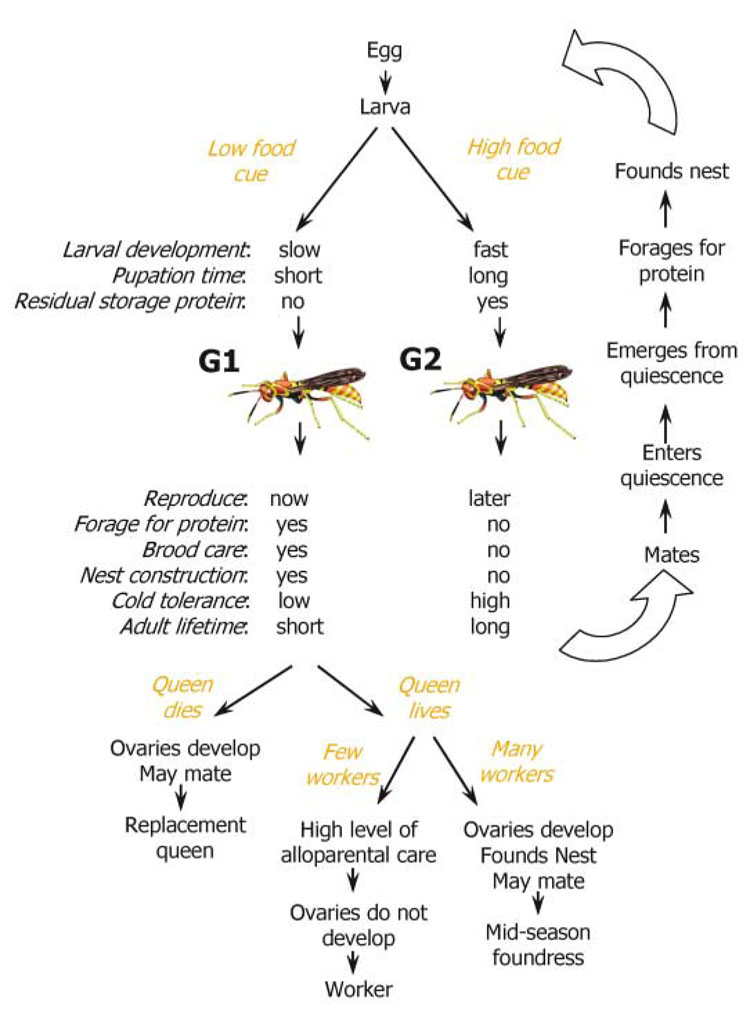
Fig. 2.
The Polistes life cycle incorporates fundamental elements of the bivoltine ground plan. Larvae respond during development to a food cue and diverge onto one of two trajectories. Scanty provisioning leads to the G1 pathway, which is signaled by slow larval development (due to low nutrient inflow), short pupation time (14), and no storage protein residuum in emerging adults (13). More abundant provisioning leads to more rapid larval development, longer pupation time (14, 20), and residual storage protein in emerging G2 adults (13). G1 females have a “reproduce now” phenotype, and they forage for protein, care for the brood, and construct nests. The expression of these behaviors is conditional, as indicated by branching points in the G1 sequence. If the queen is lost, a G1 female can develop her ovaries, mate if males are present, and become a replacement queen. If a queen is present but the number of workers is low, a G1 female will alloparentally express maternal behaviors (i.e., nest construction, nest defense, brood care, and foraging) as a worker at her natal nest. Finally, if a queen is present and the number of workers is high, a G1 female may depart the natal nest and found a satellite nest in midseason. Because the cold tolerance of G1 females is low, they do not survive quiescence, and lifetimes are short. In contrast, G2 females have a “reproduce later” phenotype. They express no maternal behaviors the first year, but after emerging from quiescence, they break reproductive diapause and shift to the reproduce now phenotype.
This explanation identifies a developmental machinery that can channel individuals into two castes. Specifically, caste differentiation could rely on regulatory circuits that, in the bivoltine ancestor, controlled the conditional prepupal diapause pathway that separated the two generational trajectories (Fig. 1A). This prediction is supported by experimental evidence. The differentiation appears to be largely determined before emergence (3, 13), and traits indicative of G1 and G2 phenotypes are apparent from early in life (Fig. 2). Further, the pupation time of gynes, measured from cocoon spinning to adult emergence, is longer than the pupation time of workers (14). Gynes also contain high levels of hexameric storage protein at the end of pupal development (13). These are traits normally associated with prepupal diapause (15). It is also worth noting that the high level of storage protein in Polistes gynes is not an obligatory trait in Vespidae, being absent from gynes of Vespinae (13). This suggests that the presence of hexamerin in Polistes is the signature of a specific differentiation machinery.
In solitary insects, diapause is under innate regulatory control and is characterized by specific hormonal signatures and patterns of gene expression (15). A social co-option of the corresponding regulatory circuits would imply that a cue stemming from the social condition now controls a modified machinery that induces a set of diapause characteristics during development and early adult life rather than triggering a state of developmental arrest. Nutrition is one factor that governs diapause initiation in solitary insects (16–18), and differential larval nutrition is a common means to induce caste-specific developmental programs in social taxa (2). It is also the most prevalent hypothesis for caste differentiation in Polistes (1, 3, 13, 14).
To explore the validity of the assumption that a diapause switch triggered by larval nutrition is the basis of caste differentiation in Polistes, we examined the demographic implications by use of individual-based modeling (Fig. 3) (19). Each resulting pattern can be matched to examples from nature. Moderate food levels (Fig. 3A) produce dynamics that are typical for Polistes in seasonal environments: A peak of workers precedes a peak of gynes (20). Specifically, workers are individuals channeled into a G1 pathway, whereas gynes follow a G2 trajectory. Variation in nutrient inflow can translate into two distinct phenomena known in Polistes: a minor peak of early gynes (21) and a minor peak of late workers (Fig. 3B) (22). Higher food levels lead to early termination of brood rearing (Fig. 3C) (23), whereas very low food levels lead to few workers and almost no gynes (Fig. 3D) (24).
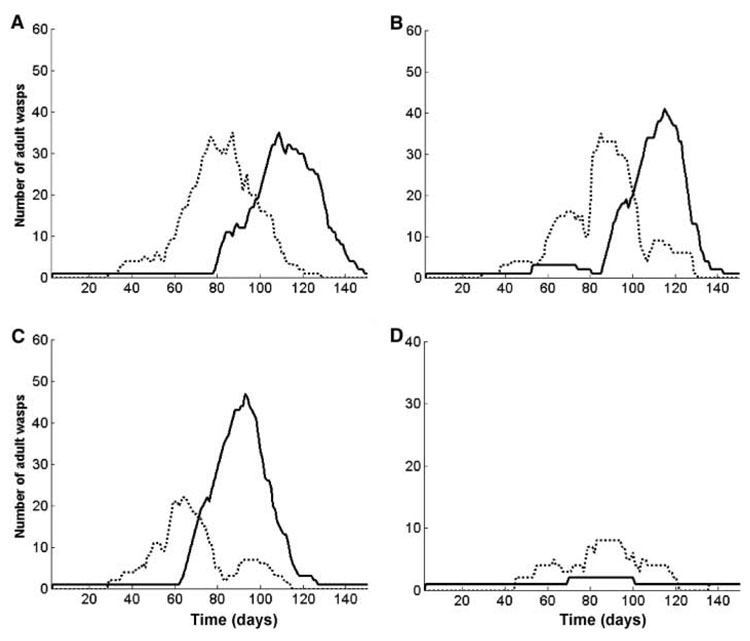
Fig. 3.
Output from four separate runs illustrates the dynamics of the individual-based model. Each simulation shows the number of workers from the G1 pathway (dotted line) and gynes of the G2 trajectory (solid line) present on a nest. The amount of food available to individuals that forage is the only variable changed between runs. (A) Moderate food levels generate a peak of workers followed by a peak of gynes. (B) Day-to-day random fluctuations in food generated by the model can result in early gynes, late workers, or both in the same run. (C) More food leads to an earlier worker peak and to earlier production of more gynes, and the food demands of those gynes cause a late peak of workers and early termination of brood rearing. (D) Very low food conditions result in few offspring, almost all of which are workers, and early termination of brood rearing.
Summary data illustrate how the model responds to changes in average food level (Fig. 4). The level indicated by arrows is of particular interest. Here, the number of gynes has the largest variance and spans the full range for the observations (0 to 60 gynes). At the same time, the number of days until colonies reach maximum gyne production is largest. These results describe colonies that vary greatly in gyne production, with many colonies producing few gynes and a few colonies producing many gynes. High variability among colonies, with a few colonies placing large numbers of gynes into quiescence late in the nesting season, exactly characterizes annually seasonal Polistes populations (1).
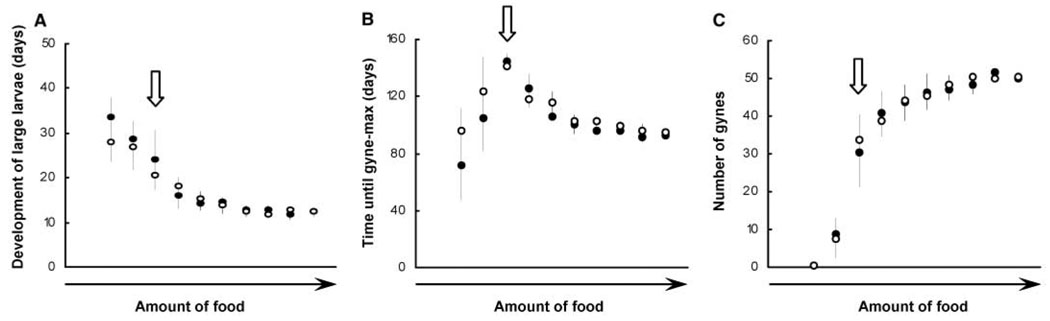
Fig. 4.
Summary statistics from two sets of simulations (n = 15 runs each) illustrate both the consistency of results and the model’s response to changes in average food level. Open and solid circles are the means of the two sets; vertical lines are standard errors. (A) The development time for large larvae is long and has a high standard error at low food levels, and both development time and standard error decrease with increasing food. In Polistes, larval developmental time is inversely correlated with the duration of the pupal stage (14), although total development time is longer for individuals that develop under low food conditions. (B) The time from the start of the simulation until the peak of gynes present on the nest has high variance at low levels and reaches a maximum at an intermediate food level. (C) The number of gynes produced increases with increasing food level. Arrows indicate the same food level, and in (B) and (C), also denote the simultaneous occurrence of the longest time until peak gyne production and the highest standard error in the number of gynes produced.
The fit of the model output to actual observations in nature, both in general patterns and particular variations, suggests that a nutrient-dependent switch mechanism during larval growth is sufficient to explain caste differentiation in Polistes. A correlative relationship between feeding and caste determination has long been suspected (1, 3), but a framework that combines a nutritional switch with a specific hypothesis on its origin has been missing. We hypothesize that Polistes workers and gynes derive from a regulatory ground plan: the bivoltine life cycle that was designed to produce a developmental bifurcation in an ancestral solitary form. Therefore, we not only provide a specific prediction about the developmental circuits that have been co-opted by social evolution to produce the two castes, but we also provide an explanation for the suite of traits associated with each phenotype (Fig. 2).
The transition from bivoltinism to eusociality may have occurred after the bivoltine ancestor dispersed into a favorable environment that enabled uninterrupted development of G2 females. Evolution of eusociality, however, would additionally require that G1 wasps remained as alloparental caregivers at the natal nest. Trophallactic transfer of amino acid–rich saliva (25) from larvae to adults may have been the key evolutionary invention that induced reproductively tuned females to remain at their natal nest (26). Indeed, experimental disruption of adult feeding on saliva causes social wasp colonies to fail (24, 27). However, although larval saliva as an amino acid source for vitellogenesis would tie G1 individuals to the nest, the costs of foraging and nest building would constrain them from oogenesis (28). This scenario implies that castes in Polistes evolved from interactions between adults and larvae rather than from interactions among group-living adults, as hypothesized by West-Eberhard (29).
The bivoltine ground plan hypothesis has considerable explanatory power to reinterpret natural history and experimental data on Polistes. Foremost among these is that Polistes dichotomizes offspring into two behavioral categories, workers and gynes, despite an absence of morphological differences between them. These now can be seen as G1 and G2 females whose behavioral tuning reflects underlying bivoltine phenotypes. Early gynes (21) and late workers (22) show that G1 and G2 phenotype expression in Polistes is cued to colony conditions, which typically change in a seasonal pattern, rather than to seasonal environmental variation itself. Individual- and colony-level responses to nutrition manipulations (14, 24), as well as our simulation results, support this explanation. Our framework can also be meaningfully applied to Polistes species that are social parasites of other Polistes species. The social parasites emerge from quiescence after host nesting is initiated, and they invade nests when host offspring begin to emerge. Host workers then rear all of the parasites’ offspring as gynes (30). This suggests that the G1 generation has been deleted from the inquiline life cycle.
Specific and testable predictions derive from our framework. If the differentiation of G1 and G2 females is driven by a co-opted diapause switch, we would expect the prolonged times spent by gynes in cocoons (14) to be predominantly due to a longer prepupal stage. During this phase, gynes will show increased production rates of hexameric storage proteins, higher levels of accumulation compared to workers, and hormonal signatures of diapause. Further, the G1 and G2 signatures should be residual in primitively social polistine wasps living in nonseasonal tropics. This is strongly suggested by evidence from Ropalidia marginata, in which about half of female offspring will build nests when isolated at emergence and half will not, even though colony cycles are indeterminate and asynchronous (31). We further expect this form of differentiation to be absent in species that originated in a nonseasonal environment, as potentially exemplified by “facultative eusociality” in Stenogastrinae (32). Halictine bees may represent a separate taxon where sociality evolved from bivoltine ancestry. A prerequisite for sociality in this group is that the species is bi- or multivoltine (33), and the halictine literature is rich with descriptions suggestive of G1 and G2 phenotypes (34).
The bivoltine ground plan hypothesis of caste evolution in Polistes inaugurates a changed perspective on the evolution and maintenance of sociality in insects. It shifts emphasis away from altruism, away from costs and benefits, and away from conflict and cooperation. It states that evolutionary trajectories of sociality are best understood as having been shaped by regulatory circuits present in solitary ancestral forms. It calls for a mechanistic approach to caste evolution, and it illustrates, by specific example, that social evolution in insects can be fully—and finally—understood.
Footnotes
Supporting Online Material www.sciencemag.org/cgi/content/full/308/5719/264/DC1
Materials and Methods
References and Notes
References and Notes
- 1.Hunt JH. In: The Social Biology of Wasps. Ross KG, Matthews RW, editors. Ithaca, NY: Cornell Univ. Press; 1991. pp. 426–450. [Google Scholar]
- 2.Wheeler DE. Am. Nat. 1986;128:13. [Google Scholar]
- 3.O’Donnell S. Annu. Rev. Entomol. 1998;43:323. doi: 10.1146/annurev.ento.43.1.323. [DOI] [PubMed] [Google Scholar]
- 4.Amdam GV, Norberg K, Fondrk MK, Page RE., Jr Proc. Natl. Acad. Sci. U.S.A. 2004;101:11350. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan DP. In: The Social Biology of Wasps. Ross KG, Matthews RW, editors. Ithaca, NY: Cornell Univ. Press; 1991. pp. 33–73. [Google Scholar]
- 6.Seger J. Nature. 1983;301:59. [Google Scholar]
- 7.Suzuki T. Ethol. Ecol. Evol. 1998;10:347. [Google Scholar]
- 8.Strassmann JE, Meyer DC. Anim. Behav. 1983;31:431. [Google Scholar]
- 9.Strassmann JE. Behav. Ecol. Sociobiol. 1981;8:55. [Google Scholar]
- 10.Page RE, Jr, Post DC, Metcalf RA. Am. Nat. 1989;134:731. [Google Scholar]
- 11.Bohm MK. J. Insect Physiol. 1972;18:1875. [Google Scholar]
- 12.Mead F, Gabouriaut D, Habersetzer C. Insectes Soc. 1995;42:385. [Google Scholar]
- 13.Hunt JH, Buck NA, Wheeler DE. J. Insect Physiol. 2003;49:785. doi: 10.1016/s0022-1910(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 14.Karsai I, Hunt JH. Environ. Entomol. 2002;31:99. [Google Scholar]
- 15.Godlewski J, Kludkiewicz B, Grzelak K, Cymborowski B. J. Insect Physiol. 2001;47:759. doi: 10.1016/s0022-1910(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 16.Boyne JV, Rock GC. J. Entomol. Sci. 1986;21:301. [Google Scholar]
- 17.Steinberg S, Podoler H, Applebaum SW. Entomol.Exp. Appl. 1992;62:269. [Google Scholar]
- 18.Munyiri FN, Shintani Y, Ishikawa Y. J. Insect Physiol. 2004;50:295. doi: 10.1016/j.jinsphys.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Materials and methods are available as supporting material on Science Online.
- 20.West Eberhard MJ. Misc. Pub. Mus. Zool. Univ. Mich. 1969;140:1. [Google Scholar]
- 21.Reeve HK, Peters JM, Nonacs P, Starks PT. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13737. doi: 10.1073/pnas.95.23.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dapporto L, Palagi E, Turillazzi S. Ann. Zool Fenn. in press. [Google Scholar]
- 23.Strassmann JE. J. Kans. Entomol. Soc. 1989;62:353. [Google Scholar]
- 24.Hunt JH, Dove MA. Ecol. Entomol. 2002;27:467. [Google Scholar]
- 25.Hunt JH, Baker I, Baker HG. Evolution. 1982;36:1318. doi: 10.1111/j.1558-5646.1982.tb05501.x. [DOI] [PubMed] [Google Scholar]
- 26.Roubaud E. Ann. Sci. Nat. 10e sér. Zool. 1916;1:1. [Google Scholar]
- 27.Ishay J, Ikan R. Anim. Behav. 1968;16:289. doi: 10.1016/0003-3472(68)90013-4. [DOI] [PubMed] [Google Scholar]
- 28.Marchal P. C. R. Soc. Biol. (Paris) 1897;1897:556. [Google Scholar]
- 29.West-Eberhard MJ. J. Kans. Entomol. Soc. 1978;51:832. [Google Scholar]
- 30.Cervo R, Dani FR. In: Natural History and Evolution of Paper-Wasps. Turillazzi S, West-Eberhard MJ, editors. Oxford: Oxford Univ. Press; 1996. pp. 98–112. [Google Scholar]
- 31.Gadagkar R. The Social Biology of Ropalidia marginata: Toward Understanding the Evolution of Eusociality. Cambridge, MA,: Harvard Univ. Press; 2001. [Google Scholar]
- 32.Field J, Shreeves G, Sumner S. Behav. Ecol. Sociobiol. Vol. 45. 1999. p. 378. [Google Scholar]
- 33.Eickwort GC. In: Social Insects. Hermann HR, editor. vol. II. New York: Academic Press; 1981. pp. 199–280. [Google Scholar]
- 34.Michener CD. In: Social Insects: An Evolutionary Approach to Castes and Reproduction. Engels W, editor. Berlin: Springer-Verlag; 1990. pp. 77–121. [DOI] [PubMed] [Google Scholar]
- 35.O’Neill KM. Solitary Wasps: Behavior and Natural History. Ithaca, NY: Cornell Univ. Press; 2001. [Google Scholar]
- 36.We thank R. E. Page Jr. for spirited discussion of this topic and K. Hartfelder, J. M. Herbers, K. G. Ross, C. K. Starr, and four anonymous reviewers for helpful comments on the manuscript. Supported by the Wissenschaftskolleg zu Berlin (J.H.H. and G.V.A.); the Norwegian Research Council Project 157851/432(G.V.A.) and National Instituto on Aging grant no. P01 AG 22500 (G.V.A.)