Abstract
Organophosphorus (OP) pesticides poison more than 3,000,000 people every year in the developing world, mostly through intentional self-poisoning. Advances in medical therapy for OP poisoning have lagged, and current treatment is not highly effective with mortality of up to 40% in even the most advanced Western medical facilities. Administration of a broadly active bacterial OP hydrolase to patients in order to hydrolyze OPs in circulation might allow current therapies to be more effective. The objective of this work was to evaluate the efficacy of a new recombinant bacterial OP hydrolase (OpdA), cloned from Agrobacterium radiobacter, in rat models of two chemically distinct but highly toxic and rapidly acting OP pesticides: dichlorvos and parathion. Without OpdA treatment, median time to death in rats poisoned with 3 × LD50 of dichlorvos or parathion was 6 minutes and 25.5 minutes, respectively. Administration of a single dose of OpdA immediately after dichlorvos resulted in 100% survival at 24 hours, with no additional antidotal therapy. After parathion poisoning, OpdA alone caused only a delay to death. However, an additional two doses of OpdA resulted in 62.5% survival at 24 hours after parathion poisoning. In combination with pralidoxime therapy, a single dose of OpdA increased survival to 75% after parathion poisoning. Our results demonstrate that OpdA is able to improve survival after poisoning by two chemically distinct and highly toxic OP pesticides.
Keywords: Organophosphorus (OP), hydrolase, acetylcholinesterase (AChE), pralidoxime (2-PAM)
1. Introduction
Occupational exposure and intentional self-poisoning with organophosphorus (OP) pesticides are major global health problems (Jeyaratnam, 1990; Van der Hoek et al., 1998). The World Health Organization estimates that as many as 3,000,000 people per year are poisoned by pesticides; many are due to OP pesticides, resulting in around 200,000 deaths (Jeyaratnam). Although the greatest burden is borne by the developing world (Buckley et al., 2004; Eddleston and Phillips, 2004), it is also an important cause of fatal self-poisoning in developed countries (Bruyndonckx et al., 2002). Highly toxic and widely available OPs such as parathion also pose a threat to municipal water supplies from intentional or unintentional contamination.
OPs inhibit acetylcholinesterase (AChE, EC 3.1.1.7), resulting in overstimulation at cholinergic synapses. Clinical management of moderate and severe poisoning is difficult, requiring prolonged intensive care and use of large doses of atropine, oxime cholinesterase reactivators, and benzodiazepines (Eddleston et al., 2007). However, these therapies are insufficient, not always available in the developing world, and do not prevent the post-poisoning neurocognitive dysfunction that is common with severe poisonings (Dunn and Sidell, 1989). Overall mortality after OP poisoning in the developing world is as high as 25%, and in the most sophisticated Western hospitals mortality is as high as 40% (Eyer et al., 2003). This overall difference in mortality between the developed and developing world is due to the vast numbers of poisoned patients in the agricultural areas of the developing world, the majority of whom are not critically ill. However, OP pesticide poisoning is uncommon in the developed world, and patients who ingest OP pesticides for self-harm typically have substantial ingestions and are more likely to be critically ill.
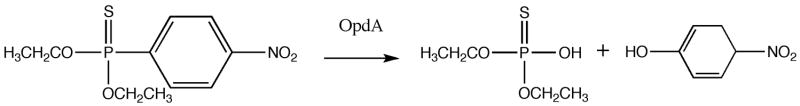
OpdA is a bacterial enzyme capable of hydrolyzing a wide variety of OP pesticides in vitro (Fig. 1) (Yang et al., 2003). The addition of an OP-degrading enzyme should improve the clinical results obtained with standard therapies by decreasing the concentration of OP pesticides in circulation. Clinical use of an enzyme with a broad range of substrates would be useful in the event of poisoning with many OPs, even when the identity of the pesticide is unknown.
Figure 1.
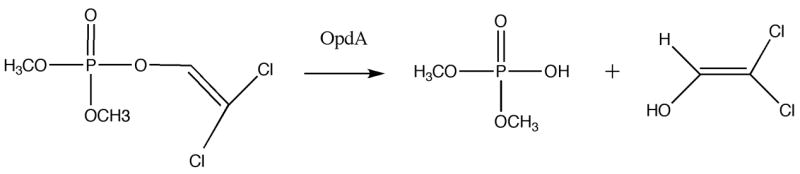
Hydrolysis of dichlorvos (A) and parathion (B) by OpdA.
We sought to determine the in vivo efficacy of OpdA in rat models of two chemically distinct and highly toxic OP pesticides: dichlorvos and parathion. Demonstration of OpdA’s effectiveness should provide the impetus for further development of this enzyme for eventual use in humans.
2. Methods
All animals were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 85–23, Revised 1985). The Institutional Animal Care and Use Committee of the University of Massachusetts Medical School approved the study protocol.
Subjects
Male wistar rats weighing 250 ± 50 g were obtained from Charles River Laboratories (Wilmington, Massachusetts, USA). Animals were housed in pairs, maintained on 12:12 light:dark cycle and provided food and water ad libitum except for 2 hours prior to experimentation.
Expression of highly active OpdA
OpdA is a metaloenzyme that is catalytically active with a variety of metal ions (Zn2+, Mn2+, Co2+ or Cd2+) with highest activity toward organophosphate insecticides when Co2+ is present in the active site (Jackson et al., 2006; Yang et al., 2003). Therefore highly active OpdA was prepared by expressing the enzyme in the presence of cobalt as follows. OpdA discovery and cloning are described elsewhere (Horne et al., 2002). The OpdA gene in the plasmid pCy76-opdA (Yang et al., 2003) was transformed into electrocompetent E. coli DH5α cells. A 20 ml starter culture in Luria–Bertani medium supplemented with 50 μg/ml ampicillin (LB Amp) was inoculated then incubated at 30 °C for 8 h. This inoculum was used to inoculate 1L of Terrific broth medium (24 g/l of yeast extract, 12 g/l of tryptone, 100 mM K2HPO4/KH2PO4, pH 7.0, 0.4% glycerol, and 50 μg/ml ampicillin) supplemented with 1 mM CoCl2. After 30hrs at 37 °C, cells were harvested and resuspended in 50 mM HEPES buffer (pH 8.0). Cells were lysed using a French Press, and cell debris removed by centrifugation at 30000 g for 30 min. The soluble fraction was passed through a DEAE Fractogel column that does not bind OpdA. Following 12-hr dialysis against 50 mM HEPES (pH 7.0) the protein solution was loaded on to a Sulphopropyl-Sepharose column. Protein was using a linear gradient from 0 to 1 M NaCl, with OpdA eluting at around 150 mM NaCl. SDS/PAGE analysis of the eluted OpdA indicated a purity of greater than 95%. The protein was stored in 50 mM HEPES (pH 7.0)/150 mM NaCl at 4 °C until required.
Ethyl parathion and dichlorvos activity assays with purified OpdA
Ethyl parathion OpdA assays were conducted in duplicate in assay buffer (50 mM HEPES, 10 % methanol, 1 mM CoCl2, pH 7.0) with an ethyl parathion range of 0 – 100 μM and 2 nM of purified OpdA. The initial rates of the reactions were determined by measuring the change in absorbance at 412 nM over time, until 10 % of the substrate had been converted. The rate of conversion was calculated from a standard curve of 0 – 10 μM nitrophenol (the ethyl parathion hydrolysis product) measured at 412 nM (using a Molecular Devices SpectraMax 190). The kinetic parameters for the hydrolysis of ethyl parathion by OpdA were estimated using hyperbolic regression (using the Hyper32.exe enzyme kinetic analysis software; a freeware package). The kcat of OpdA with parathion was calculated to be 1,500 per second, and the Km was estimated at 1 μM.
Dichlorvos assays were conducted in duplicate in assay buffer with 0–1 mM dichlorvos and 19 nM OpdA. The hydrolysis products were detected by mass spectrometry (MS) using an Agilent G1969 LC/MS TOF after filtration through a liquid chromatography (LC) guard column at a flow rate of 1 ml.min−1 and 80 % acetonitrile, 0.002 % formic acid. A 5μl sample was analyzed by MS, with the fragmentor set at 120V. The mass spectral fragmentation patterns of authentic dichlorvos standards (Sigma-Aldrich, Castle Hill, New South Wales, Australia) were used to validate the identity of the substrates. The assay reactions were assayed at 3-minute intervals for 12 minutes at each substrate concentration. The kinetic parameters for this reaction were estimated using hyperbolic regression (as above) giving a Km of 183 μM and kcat of 149 per second.
Poisoning models
Animals were briefly anesthetized with isoflurane while a 24-gauge intravenous lateral tail vein catheter was placed. Immediately upon awakening, 3 times the oral LD50 for parathion (LD50=6 mg/kg) (Sigma-Aldrich, St. Louis, Missouri, USA) or dichlorvos (LD50=50 mg/kg) (Sigma-Aldrich, St. Louis, Missouri, USA) suspended in peanut oil was given by gavage feeding tube in a volume of 1.5 mL/kg. Three times the LD50 of the pesticides were used in order to mimic severe human poisoning and to assure that all, or nearly all, control animals would die, thereby decreasing the number of animals needed to demonstrate statistical significance. All injections were given via a 24-gauge catheter (Surflo catheter, Terumo Corporation, Somerset, New Jersey, USA) through a lateral tail vein in volume of 0.5 mL. Nitrile gloves and chemical safety goggles were worn when working with the OP pesticides or handling animals after poisoning. Consumable supplies were soaked in a dilute OpdA solution in order to hydrolyze any residual pesticide before disposal in a biohazards container for collection by the institutional Environmental Health and Safety department. Animal carcasses were burned in an incinerator equipped with an afterburner and scrubber.
Data Analysis
Blinded outcomes of interest were survival to 4 and 24 hours. Grouped survival data were compared using a two-tailed Fisher’s exact test. For 80% power to detect a 50% reduction in mortality, assuming an alpha of 0.05, 8 animals per group were required. For all analyses, a P value of less than 0.05 was considered significant. All statistical analyses were performed with GraphPad Prism software version 4 for Mac (GraphPad Software, Inc., San Diego, California, USA).
3. Results
Safety of repeated doses of OpdA in rats
Since OpdA had not previously been administered to animals, we first examined whether the hydrolase would elicit severe allergic reactions in the rat at OpdA doses likely to be needed in efficacy studies. 0.5 mg of OpdA (a 10-fold excess of OpdA estimated for effective hydrolysis of dichlorvos based on extrapolations from in vitro kinetic data) was injected into a tail vein of four rats once a week for four weeks. Rats were continuously observed for 4 hours after each injection, then regularly for 24 hours. No evidence of an allergic reaction was observed in any rat, and all exhibited normal behavior and normal weight gain over the 4-week period. With no evidence of an allergic reaction, we proceeded to test the efficacy of OpdA against dichlorvos and parathion.
Dichlorvos and parathion oral poisoning models
To mimic human poisoning, we developed oral poisoning models for parathion and dichlorvos in the Wistar rat. To assure severe poisoning and at least a lethality of 90% in controls, all rats received 3 × LD50 parathion (LD50~ 6mg/kg) or dichlorvos (LD50~50 mg/kg) via gavage feeding tube. The LD50s for dichlorvos and parathion were determined from exhaustive literature reviews. All rats that received dichlorvos developed signs of cholinergic toxicity (muscle fasciculations and tremors; gait disturbances; salivation; and urination) within 3 minutes and died by 12 minutes (median time to death 6 minutes), while all rats that received parathion developed cholinergic signs within 20 minutes and died by 35 minutes (median time to death 25.5 minutes) (Fig 2). This rapid onset of poisoning is consistent with clinical experience of human poisoning with these pesticides (Eyer et al., 2003; Peng et al., 2004).
Figure 2. Acute mortality after poisoning with 3 × LD50 of dichlorvos or parathion.
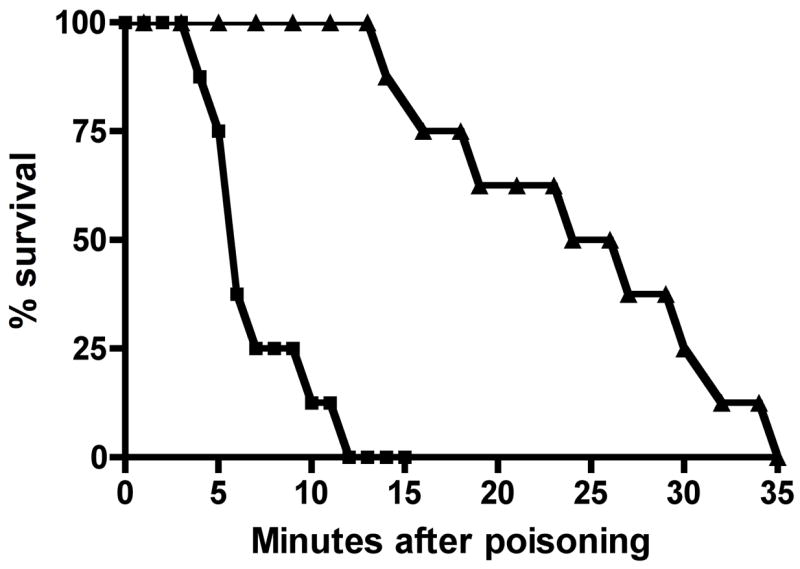
Survival after poisoning with dichlorvos (■) or parathion (▲) alone. Rats were given 0.5 mL of saline placebo IV concomitantly with poisoning by 3 × LD50 dichlorvos or parathion via gavage.
Efficacy of OpdA versus dichlorvos and parathion
Because of the difference in speed of poisoning onset, we gave a single dose of 0.15 mg/kg OpdA (or an equal volume of 0.9% normal saline) intravenously immediately after giving dichlorvos and immediately or ten minutes after giving parathion. We chose 0.15 mg/kg of OpdA in order to give a margin of error based upon an estimated minimum dose of 0.05 mg/kg OpdA derived from in vitro data with dichlorvos, while remaining within the range of larger OpdA doses given in the preliminary safety dosing studies. All dichlorvos-poisoned rats that received OpdA survived to 4 hours and 24 hours (p = 0.0002 vs placebo). Delaying OpdA administration to 3 minutes after dichlorvos administration resulted in complete loss of OpdA efficacy.
A single dose of OpdA either immediately or 10 minutes after poisoning had no survival effect on parathion-poisoned rats, with all dead at 4 hours. However, repeat doses of 0.15 mg/kg OpdA at 45 minutes and 90 minutes after poisoning improved survival: all animals survived to 4 hours after this repeated treatment with OpdA (p = 0.0002 vs placebo). Twenty-four hours after poisoning 5/8 animals were alive (OpdA vs placebo group, p = 0.026).
OpdA demonstrates prolonged efficacy against dichlrovos and parathion
The prolonged in vivo enzymatic activity of an OP hydrolase is essential if it is to be used effectively against OPs, like parathion, that partition out of the blood and into adipose tissue and other physiologic compartments. To test the duration of OpdA’s effect versus parathion and dichlorvos, 250 g male Wistar rats were given 1.5 mg/kg iv OpdA, followed by 3 × LD50 oral dichlorvos 30, 60, or 90 minutes later. All rats survived to 24 hours and no animals exhibited any signs of OP toxicity (p = 0.0002 vs placebo). When these experiments were repeated using 3 × LD50 parathion, again all animals survived to 24 hours (p = 0.0002 vs placebo). Lastly, a cohort of 4 rats was given 1.5 mg/kg of OpdA 180 minutes before parathion poisoning. Again, all rats survived to 24 hours. Therefore, OpdA maintains clinically relevant enzymatic activity in vivo for several hours.
OpdA improves efficacy of pralidoxime versus parathion
High OP concentrations are thought to limit the effectiveness of oxime cholinesterase reactivators in OP poisoning (Eddleston et al., 2005; Sogorb et al., 2004). To address this issue we treated parathion-poisoned rats with either pralidoxime chloride (2-PAM) alone, or 2-PAM with OpdA. Rats received a bolus dose of 30 mg/kg 2-PAM ten minutes after parathion administration, followed by a continuous IV infusion of 30 mg/kg/hour (standard 2-PAM doses used in previous OP rat studies) for 24 hours. 2-PAM treatment alone resulted in 4 of 10 animals surviving to 4 hours and 1 of 10 animals surviving to 24 hours (24-hour 2-PAM infusion vs placebo, p = 1.00). After a single IV dose of 0.15 mg/kg OpdA administered concomitantly with the 2-PAM bolus and infusion, 8 of 8 animals survived to 4 hours, and 6 of 8 animals survived to 24 hours (p = 0.007 compared to single dose OpdA alone; p = 0.013 compared to 2-PAM alone).
4. Discussion
Since their first description in 1946 (Mazur, 1946), several OP degrading enzymes have been isolated. These enzymes include phosphotriesterase (PTE, aka OPH) (Lewis et al., 1988), paraoxonase (PON) (Ortigoza-Ferado et al., 1984), DFPase (Ahmad and Forgash, 1976), sarinase (Adie, 1956), and OpdA. OpDA is a particularly good candidate enzyme for clinical applications, partly because it is a very efficient hydrolase, performing at near diffusion-limited rates towards its favored substrates in vitro. In addition, and unlike some of the other OP-degrading enzymes, OpdA is active against a wide range of phosphotriester OP pesticides, including substrates with a phosphoryl sulfur (rather than an oxygen) and substrates with both methoxy and ethoxy groups. One consequence of the breadth of OpdA’s substrate range is that treatments based on OpdA have a high probability of success against OP poisoning even when the exact identity of the OP pesticide is unknown. Additionally, phosphoryl sulfur containing OP pesticides (such as parathion) can, in principle, be hydrolyzed before they are activated by P450s, so long as they are available to the enzyme in circulation.
As a test for broad-spectrum hydrolytic activity, we sought to test the efficacy and safety of OpdA in two very different rat models of severe oral OP poisoning. We chose parathion and dichlorvos because their different chemical structures result in variable requirements for bioactivation and differences in fat solubility (Table 1). However, both pesticides are highly toxic, fast acting, widely used globally; parathion and dichlorvos are the two most commonly used OPs in Chinese suicides, where an estimated 170,000 deaths occur annually from pesticide poisoning (Eddleston and Phillips, 2004; Phillips et al., 2002), and therefore highly clinically relevant.
Table 1.
Physicochemical properties of the studied pesticides
| OP pesticide | Chemistry | WHO toxicity class | Rat oral LD50 mg/kg* | Human mortality | Fat solubility (Kow logP)† | ||
|---|---|---|---|---|---|---|---|
| Dichlorvos | Aliphatic | Dimethyl | Oxon | Ib | 25–100 | 34% | Low (1.43) |
| Parathion | Aromatic | Diethyl | Thion | Ia | 2–13 | 40% | High (3.83) |
Values vary according to source: Crop Protection Handbook 2003, 2nd WHO Classification of Pesticide Toxicity.
National Toxicology Program, Forest Stewardship Council Pesticides Policy 2007.
We found no evidence of allergic reactions to this bacterial hydrolase in rats. Clinical use of another microbial enzyme, streptokinase, has shown that repeated administration is safe and effective within 5 days or after one year (Fears et al., 1992). In the acute OP pesticide-poisoning scenario, it is unlikely that OpdA would be required after the first few hours or days. Therefore, theoretical concerns regarding prolonged re-exposure to a bacterial OP hydrolase such as OpdA are likely not applicable.
OpdA was surprisingly effective against dichlorvos and parathion, two quite different OP insecticides. In vitro enzymatic studies had previously suggested that OpdA would be poorly effective against dimethylated oxon OPs, such as dichlorvos (Horne et al., 2002). The Kcat of OpdA towards dichlorvos is 149 ± 26 sec−1 at room temperature, compared to ~1500 sec−1 towards parathion (Table 2). The similar clinical responses observed after dichlorvos and parathion poisoning suggest that in vitro catalytic activities above a certain threshold may not be important in determining the clinical effectiveness of an OP hydrolase.
Table 2.
Enzymatic activity of OpdA vs OPs representative of different chemical classes
| Enzyme | Chemistry of OP | |||||||
|---|---|---|---|---|---|---|---|---|
| Ethyl-aromatic thion (parathion) | Methyl-aromatic thion (methyl parathion) | Methyl-aromatic oxon (methyl paraoxon) | Methyl-aliphatic (dichlorvos) | |||||
| Km | kcat | Km | kcat | Km | kcat | Km | kcat | |
| OpdA | 110 | 1500 | 100 | 1200 | 230 | 2500 | 183 | 149 |
Km is expressed in μM and kcat in sec−1.
Despite excellent efficacy against dichlorvos, a single dose of OpdA was not effective against parathion whether OpdA was given concomitantly with poisoning or 10 minutes later. Differences in pesticide toxicokinetics and need for bioactivation of the two OPs explain the differences in speed of poisoning onset and the effectiveness of OpdA: dichlorvos does not require bioactivation (because of its oxon group) and is therefore immediately toxic. In addition, dichlorvos is rapidly present at high concentrations in the blood due to its low distribution into fat, and is therefore available for hydrolysis by OpdA. Parathion, in contrast, requires bioactivation from a thion to oxon and distributes rapidly to fat. This latter characteristic decreases the amount available in the circulation for OpdA hydrolysis and provides a reservoir for subsequent leaching of parathion back into circulation. Three doses of OpdA at 45-minute intervals were needed to counter parathion returning from the fat into the blood.
2-PAM is considered by most researchers and clinicians to be an integral part of therapy after OP poisoning (Eddleston et al., 2007). However, a 2-PAM bolus followed by continuous 24-hour infusion was ineffective as a treatment for parathion poisoning. The addition of a single bolus dose of OpdA markedly increased the effectiveness of 2-PAM. This result suggests that early use of OpdA lowers the amount of pesticide that reaches the fat, improving 2-PAM’s ability to reactivate AChE. The combination of OpdA and 2-PAM may well improve outcomes for other fat soluble OPs that have a long half-life, such as fenthion (Eddleston et al., 2005), that are also associated with severe poisonings in the developing world.
With dichlorvos poisoning, OpdA was required immediately to have a beneficial effect, which will make it difficult for it be useful clinically for very fast acting pesticides. However, it is possible that OpdA may be effective at later time points in poisoning with lower dose of OP pesticides. OpdA may also be effective in poisoning with slowly activated and poorly fat-soluble OPs, such as dimethoate, for which there is more time for OpdA administration.
Given the profound human health burden caused by OP pesticide poisoning, and potential terrorist use of OP pesticides, these studies should provide the impetus for accelerated research and human trials of broad spectrum, low-cost therapeutic OP hydrolases such as OpdA.
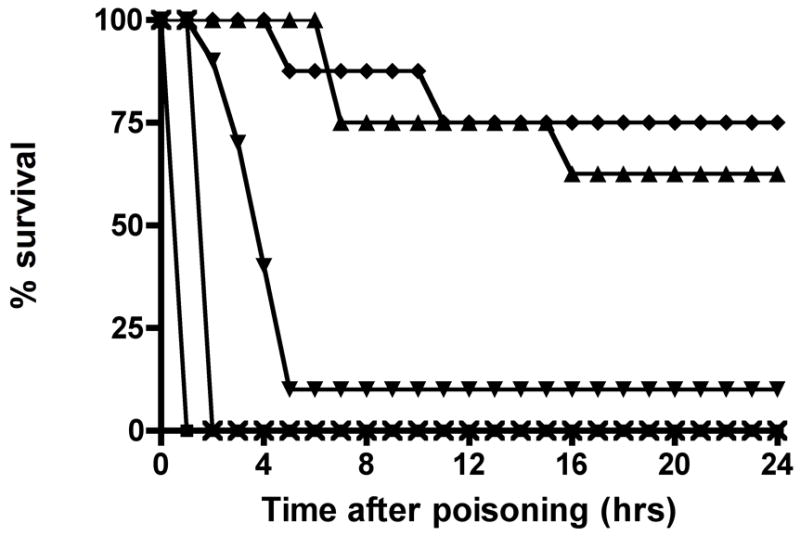
Figure 3. OpdA improves survival after poisoning with 3 × LD50 of A) dichlorvos, or B) parathion.
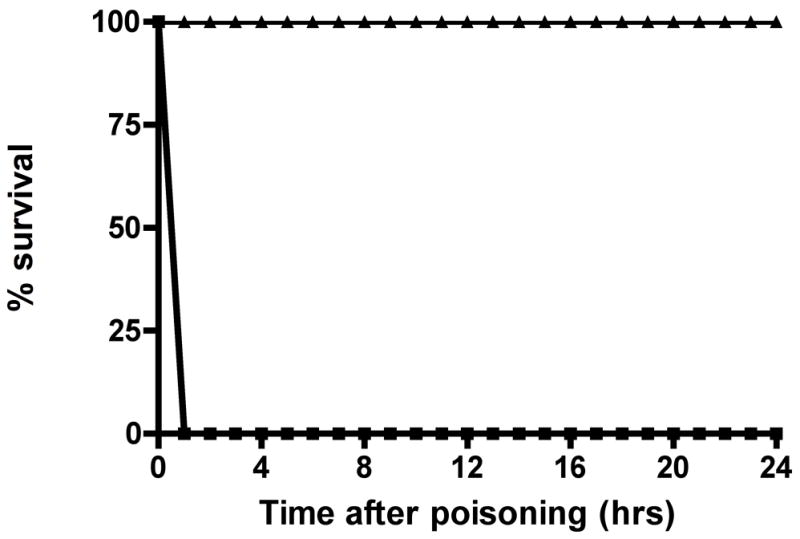
A. 24-hour survival after poisoning with dichlorvos. Rats were given 0.15 mg/kg of OpdA or the same volume of saline placebo concomitantly with poisoning by 3 × LD50 dichlorvos via gavage. ■ = dichlorvos plus saline placebo; ▲ = dichlorvos plus single dose 0.15 mg/kg OpdA. Each group contained 8 rats.
B. 24-hour survival after poisoning with parathion. Rats were given OpdA 0.15 mg/kg or placebo, with or without 2-PAM, after poisoning with 3 × LD50 parathion via gavage. ■ = parathion plus saline placebo; x = parathion plus single dose 0.15 mg/kg OpdA; ▲ = parathion plus 3 doses 0.15 mg/kg OpdA; ▼ = parathion plus 2-PAM bolus and infusion of 30 mg/kg/hr for 24 hours; ♦ = parathion plus single dose 0.15 mg/kg OpdA plus 2-PAM bolus and infusion of 30 mg/kg/hr for 24 hours. Each group contained 8 rats with the exception of the 24-hour 2-PAM alone group, which contained 10 rats.
Acknowledgments
Support for this research was provided by NIEHS grant K08 ES012897, the Emergency Medicine Foundation, and the Orphan Medical/Jazz Pharmaceuticals research award from the American College of Medical Toxicology. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tara D. Sutherland, Email: tara.sutherland@csiro.au.
Chip Gresham, Email: chipgresham@yahoo.com.
John Oakeshott, Email: john.oakeshott.csiro.au.
Colin Scott, Email: colin.scott@csiro.au.
Michael Eddleston, Email: eddlestonm@yahoo.com.
References
- Adie PA. The purification of sarinase from bovine plasma. Can J Biochem Physiol. 1956;34:1091–1094. [PubMed] [Google Scholar]
- Ahmad S, Forgash AJ. Nonoxidative enzymes in the metabolism of insecticides. Annals of clinical biochemistry. 1976;13:141–164. [PubMed] [Google Scholar]
- Bruyndonckx RB, Meulemans AI, Sabbe MB, Kumar AA, Delooz HH. Fatal intentional poisoning cases admitted to the University Hospitals of Leuven, Belgium from 1993 to 1996. Eur J Emerg Med. 2002;9:238–243. doi: 10.1097/00063110-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Buckley NA, Roberts D, Eddleston M. Overcoming apathy in research on organophosphate poisoning. Bmj. 2004;329:1231–1233. doi: 10.1136/bmj.329.7476.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MA, Sidell FR. Progress in medical defense against nerve agents. Jama. 1989;262:649–652. [PubMed] [Google Scholar]
- Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2007 doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, Sheriff MH, Szinicz L, Dawson AH, Buckley NA. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Phillips MR. Self poisoning with pesticides. Bmj. 2004;328:42–44. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer F, Meischner V, Kiderlen D, Thiermann H, Worek F, Haberkorn M, Felgenhauer N, Zilker T, Eyer P. Human parathion poisoning. A toxicokinetic analysis. Toxicol Rev. 2003;22:143–163. doi: 10.2165/00139709-200322030-00003. [DOI] [PubMed] [Google Scholar]
- Fears R, Ferres H, Glasgow E, Standring R, Hogg KJ, Gemmill JD, Burns JM, Rae AP, Dunn FG, Hillis WS. Monitoring of streptokinase resistance titre in acute myocardial infarction patients up to 30 months after giving streptokinase or anistreplase and related studies to measure specific antistreptokinase IgG. Br Heart J. 1992;68:167–170. doi: 10.1136/hrt.68.8.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne I, Sutherland TD, Harcourt RL, Russell RJ, Oakeshott JG. Identification of an opd (organophosphate degradation) gene in an Agrobacterium isolate. Appl Environ Microbiol. 2002;68:3371–3376. doi: 10.1128/AEM.68.7.3371-3376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CJ, Carr PD, Kim HK, Liu JW, Herrald P, Mitic N, Schenk G, Smith CA, Ollis DL. Anomalous scattering analysis of Agrobacterium radiobacter phosphotriesterase: the prominent role of iron in the heterobinuclear active site. The Biochemical journal. 2006;397:501–508. doi: 10.1042/BJ20060276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat Q. 1990;43:139–144. [PubMed] [Google Scholar]
- Lewis VE, Donarski WJ, Wild JR, Raushel FM. Mechanism and stereochemical course at phosphorus of the reaction catalyzed by a bacterial phosphotriesterase. Biochemistry. 1988;27:1591–1597. doi: 10.1021/bi00405a030. [DOI] [PubMed] [Google Scholar]
- Mazur A. An enzyme in animal tissue capable of hydrolysing the phosphorusfluorine bond of alcyl fluorophosphates. J Biol Chem. 1946;164:271–289. [PubMed] [Google Scholar]
- Ortigoza-Ferado J, Richter RJ, Hornung SK, Motulsky AG, Furlong CE. Paraoxon hydrolysis in human serum mediated by a genetically variable arylesterase and albumin. Am J Hum Genet. 1984;36:295–305. [PMC free article] [PubMed] [Google Scholar]
- Peng A, Meng FQ, Sun LF, Ji ZS, Li YH. Therapeutic efficacy of charcoal hemoperfusion in patients with acute severe dichlorvos poisoning. Acta Pharmacol Sin. 2004;25:15–21. [PubMed] [Google Scholar]
- Phillips MR, Li X, Zhang Y. Suicide rates in China, 1995–99. Lancet. 2002;359:835–840. doi: 10.1016/S0140-6736(02)07954-0. [DOI] [PubMed] [Google Scholar]
- Sogorb MA, Vilanova E, Carrera V. Future applications of phosphotriesterases in the prophylaxis and treatment of organophosporus insecticide and nerve agent poisonings. Toxicology letters. 2004;151:219–233. doi: 10.1016/j.toxlet.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Van der Hoek W, Konradsen F, Athukorala K, Wanigadewa T. Pesticide poisoning: a major health problem in Sri Lanka. Soc Sci Med. 1998;46:495–504. doi: 10.1016/s0277-9536(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Yang H, Carr PD, McLoughlin SY, Liu JW, Horne I, Qiu X, Jeffries CM, Russell RJ, Oakeshott JG, Ollis DL. Evolution of an organophosphate-degrading enzyme: a comparison of natural and directed evolution. Protein Eng. 2003;16:135–145. doi: 10.1093/proeng/gzg013. [DOI] [PubMed] [Google Scholar]