Abstract
PURPOSE
High-radiation exposure occurs during computed tomographic (CT) fluoroscopy. Patient and operator doses during thoracic and abdominal interventional procedures were studied in the present experiment, and a novel shielding device to reduce exposure to the patient and operator was evaluated.
MATERIALS AND METHODS
With a 16-slice CT scanner in CT fluoroscopy mode (120 kVp, 30 mA), surface dosimetry was performed on adult and pediatric phantoms. The shielding was composed of tungsten antimony in the form of a lightweight polymer sheet. Doses to the patient were measured with and without shielding for thoracic and abdominal procedures. Doses to the operator were recorded with and without phantom, gantry, and table shielding in place. Double-layer lead-free gloves were used by the operator during the procedures.
RESULTS
Tungsten antimony shielding adjacent to the scan plane resulted in a maximum dose reduction of 92.3% to the patient. Maximum 85.6%, 93.3%, and 85.1% dose reductions were observed for the operator’s torso, gonads, and hands, respectively. The use of double-layer lead-free gloves resulted in a maximum radiation dose reduction of 97%.
CONCLUSIONS
Methods to reduce exposure during CT fluoroscopy are effective and should be searched for. Significant reduction in radiation doses to the patient and operator can be accomplished with tungsten antimony shielding.
Computed tomography (CT) has been used to guide interventional procedures of the chest and abdomen for nearly two decades (1,2). CT-guided procedures have continued to increase in number (3) and are less invasive than many surgical options. Despite advances in other areas of imaging, CT has remained the imaging study of choice for many procedures because of its inherently superior contrast and spatial resolution in comparison with conventional fluoroscopy and ultra-sonography (US). However, unlike other imaging modalities, conventional CT lacks real-time imaging capabilities. Therefore, CT-guided procedures generally take longer because the region of interest must be intermittently scanned to confirm safe adjustment and placement of the needle or catheter.
CT fluoroscopy is a technique that has been developed in the past decade (4). In this acquisition mode, CT images are reconstructed and displayed nearly in real time. This provides the interventionalist with immediate feedback during the procedure. CT fluoroscopy has been shown to reduce procedure time (5) and increase efficacy compared with standard CT guidance (6).
The inherent drawback of CT fluoroscopy is rather high radiation exposure to the patient and operator, which may explain why CT fluoroscopy has not been more broadly accepted (7). Radiation exposure for the patient is primarily along the scan plane. Because the exposure along the scan plane is cumulative, deterministic effects can be significant (8,9). Scattered radiation from the direct beam and collimator leakage also contribute to the patient dose (10).
For the operator, exposure is primarily a function of scattered radiation and collimator or gantry leakage (7,10). To make intraprocedural needle adjustments during CT fluoroscopy procedures, the operator’s hand must be in proximity to the scan plane. Kato et al (11) calculated that, with an annual dose limit of 500 mSv for the hands, a physician with hand exposure would be limited to performing only four CT fluoroscopy procedures a year. Regardless of scan time, exposure can be quite significant (12) because dose rates can exceed 1 mGy/sec with continuous exposure (13).
Because of high radiation doses to patients and personnel, in 1999, the United States Food and Drug Administration Radiation Safety Standards Committee expressed concern about CT fluoroscopy (14). Various methods including shielding (15), needle holders (11), and robotics (16) have been investigated to reduce CT fluoroscopy radiation exposure. We investigated the efficacy of a novel lead-free shielding device in the reduction of patient and operator exposure during simulated thoracic and abdominal procedures. Adult and pediatric anthropomorphic phantom models were tested. Additionally, we evaluated the effect of lead-free radiation protective gloves in reducing the dose to the operator’s hands.
MATERIALS AND METHODS
Phantom
Two anthropomorphic phantoms, known as Rando Phantoms (Phantom Laboratory, Salem, NY), were used in this study. This phantom is an assembly of an actual human skeleton cast inside a material that matches the elemental composition and density of the average human tissue. The phantom is composed of 35 axial slices that are 2.5 cm thick, which assemble into a figure that extends from the top of the head to the middle of the thigh. Adult and pediatric phantoms were used. The pediatric phantom is composed of 27 slices rather than 35 slices. Adult and pediatric models were tested in all phases of this study. The axial slices of the phantoms allowed the dosimeter detector to be placed between the slices, mimicking the actual anatomic location of the tissue of interest (eg, thyroid or ovary).
Dosimeter
The dosimeter (EDD-30; Unfors Instruments, Billdal, Sweden) is an electronic device that uses a remote detector and meter, which provides an immediate readout of exposure that can be reset by turning it on and off. The detector has a spherical response system that can measure radiation dosages from all angles. The detector is connected to the dosimeter by a long wire. This arrangement allows the detector to be placed on the surface of the phantom or between individual slices to provide an accurate dose measurement to the organs of interest. The dose range of the dosimeter used was 10 nGy to 9,999 Gy with a start trigger level of 15 nGy/sec and an end trigger level of 10 nGy/sec.
Shielding
The shielding devices used were nonsterile RadPad drapes developed with a specific dimension for our study and custom sterile RadPad materials (Worldwide Innovations and Technologies, Overland Park, KS). They were composed of a tungsten antimony lead-free material in a proprietary polymer sheet. The nonsterile drapes measured 2 feet by 6 feet, were less than 1 mm thick, and weighed less than 3 lbs. The commercial pads were disposable and sterile and measured 12 inches by 17 inches, were less than 1 mm thick, and weighed less than 1 lb.
Radiation Protective Gloves
The lead-free radiation protective gloves were sterile bismuth oxide RadiaXon-model gloves that produce 55% attenuation of a 60-kVp beam (half value layer of 2.3 mm Al).
Computed Tomography
A Phillips Mx8000 IDT 16-slice CT scanner (Philips Medical Systems, Best, The Netherlands) was used. This scanner used a proprietary method of CT fluoroscopy termed continuous CT mode. When in continuous CT mode, this scanner uses a 240° scan arc centered below the patient. This is done to reduce scatter radiation to the operator. The scan parameters were 120 kVp and 30 mA.
Methods
Patient doses were measured at the lens, thyroid, breast, ovaries, and testicles. Operator doses were measured as the air-absorbed doses at various distances: from the scan plane (10–200 cm), at the height of the operator’s waist, and a single measurement 5 cm from the scan plan to estimate hand exposure. A limited pilot study on reproducibility of the doses (within a protocol) showed doses to be nearly identical to the two last digits displayed, which are likely greater than the variability of human doses and also greater than the error of the dosimeter used. Therefore, only a single dose measurement was performed.
First, patient dosages were recorded with and without shielding during simulated thoracic and abdominal CT fluoroscopy procedures of various lengths (1, 5, 15, and 30 frames at approximately 1.2 frames per second). To standardize the simulated procedures, the duration of the procedure was measured by the number of frames used rather than an actual length of time.
The thoracic procedure was chosen to be at the level of the sternomanubrial joint. The abdominal procedure was chosen to be at the level of the umbilicus. The phantom was then wrapped with the tungsten antimony shielding in various configurations and layers (180° single layer, 360° single layer, and 360° double layer; Fig 1). A 5-cm gap in shielding was left at the plane of imaging by placing the sterile drapes adjacent to the beam (2.5 cm cranial and 2.5 cm caudal to the procedure window).
Figure 1.
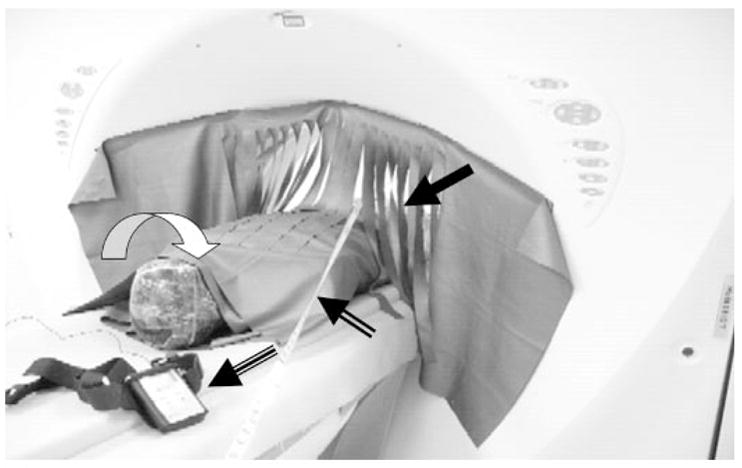
The fenestrated curtain drape (closed arrow) hangs from the CT gantry. The phantom is wrapped in a 360° double layer of shielding. The tape measure (two-line arrow) is used to determine the distances from the scan plane. The curved white arrow indicates the phantom head; the three-line arrowhead indicates the dosimeter.
Operator dose was recorded with and without phantom shielding as follows: from the epicenter of the radiation field (ie, CT gantry) at 5, 10, 20, 30, 40, 50, 75, 100, 150, and 200 cm at the levels of the waist (114 cm) and gonads (84 cm) with the sensor of the dosimeter placed and secure on a tape measure extending from the epicenter to each of these lengths. Several types of gantry drapes were studied (Fig 1). The effect of the gantry drape in addition to patient shielding was evaluated. A 90° “corner shield” (Fig 2) consisted of single- and double-layer tungsten antimony sheets placed at the junction of the gantry and the table, from the level of the operator’s waist to the operator’s ankles, to evaluate dose reduction to the operator.
Figure 2.
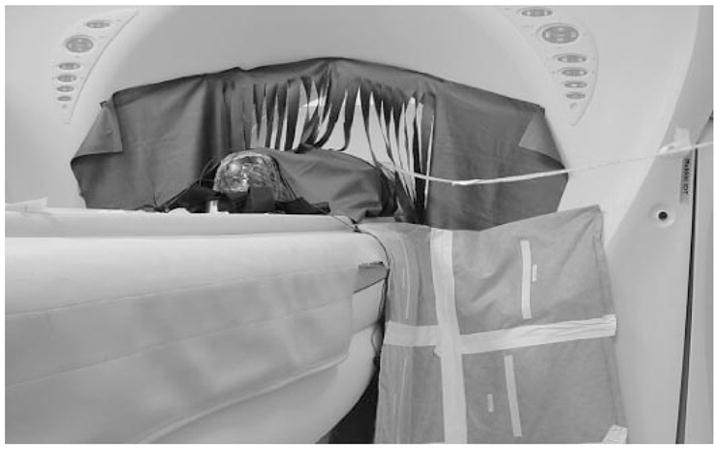
The 90° corner drape at the junction of the gantry and table.
The effect of the radiation protective gloves was measured at various distances from the scan plane (ie, 5–10 cm). This effect was examined with and without phantom shielding and with and without a fenestrated gantry shield.
RESULTS
The benefit in dose reduction to the patient was minimal with 180° single-layer shielding and maximized with a 360° double layer of shielding (Table 1). During an adult chest procedure (30 frames) with a double layer of 360° shielding, patient dose reductions of 86.6% and 89% were noted in the lens and testicle, respectively (Fig 3). For an adult abdominal procedure (30 frames) with a double layer of 360° shielding, patient dose reductions of 92.3% and 75.5% were observed in the lens and breast, respectively (Fig 4). Similar results were seen in the pediatric models (Figs 5, 6).
Table 1.
Dose Reduction to the Patient with Single- and Double-layer Shielding
| Shielding | None | Single 180º | Single 360º | Double 360º | Reduction (%): None vs Double 360º |
|---|---|---|---|---|---|
| Adult chest procedure | |||||
| Eye (μGy) | 61.24 | 49.82 | 15.55 | 8.21 | 86.6 |
| Thyroid (μGy) | 183.2 | 183.9 | 154 | 148.7 | 18.8 |
| Breast (mGy) | 4.507 | 4.500 | 4.500 | 4.473 | 0.8 |
| Umbilicus (μGy) | 39.89 | 43.16 | 17.79 | 17.59 | 56 |
| Ovary (μGy) | 8.606 | 4.543 | 2.502 | 1.748 | 79.7 |
| Testicle (μGy) | 3.9 | 2.997 | 0.810 | 0.429 | 89 |
| Adult abdomen procedure | |||||
| Eye (μGy) | 5.353 | 4.683 | 0.916 | 0.41 | 92.3 |
| Thyroid | 7.139 μGy | 4.674 μGy | 2.805 mGy | 2.112 μGy | 70.4 |
| Breast (μGy) | 64.66 | 47.59 | 20.4 | 15.86 | 75.5 |
| Umbilicus (mGy) | 4.561 | 4.744 | 4.237 | 4.331 | 5 |
| Ovary (μGy) | 244.7 | 198.4 | 120.5 | 161.9 | 33.8 |
| Testicle (μGy) | 31.78 | 27.81 | 22.99 | 21.93 | 31 |
| Child chest procedure | |||||
| Eye (μGy) | 63.64 | 96.06 | 30.01 | 14.12 | 77.8 |
| Thyroid (μGy) | 585.5 | 389.4 | 454.1 | 259.9 | 55.6 |
| Breast (mGy) | 5.005 | 6.204 | 4.748 | 3.265 | 34.8 |
| Umbilicus (μGy) | 239 | 226.4 | 146.7 | 161.8 | 32.3 |
| Ovary (μGy) | 82.9 | 36.13 | 29.23 | 36.7 | 55.7 |
| Testicle (μGy) | 17.04 | 9.437 | 7.117 | 4.923 | 71.1 |
| Child abdomen procedure | |||||
| Eye (μGy) | 13.85 | 13.1 | 4.252 | 2.109 | 84.8 |
| Thyroid (μGy) | 53.17 | 38.01 | 29.57 | 18.38 | 65.4 |
| Breast (μGy) | 226.8 | 156.6 | 137.8 | 134.2 | 40.8 |
| Umbilicus (mGy) | 6.634 | 6.712 | 6.517 | 5.879 | 11.4 |
| Ovary (μGy) | 873.5 | 564.6 | 794.9 | 574.9 | 34.2 |
| Testicle (μGy) | 123.04 | 97.31 | 110.9 | 87.61 | 28.8 |
Figure 3.
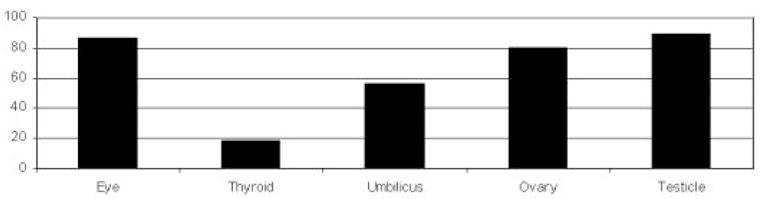
Percentage radiation reduction to various organs with a double layer 360° of shielding to the patient during a simulated adult chest procedure.
Figure 4.
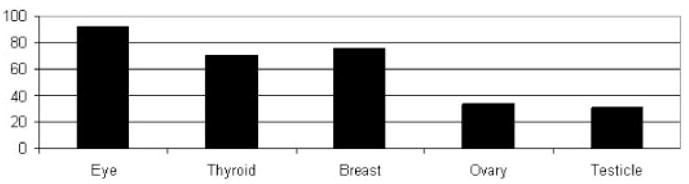
Percentage radiation reduction to various organs with a double layer 360° of shielding to the patient during a simulated adult abdominal procedure.
Figure 5.
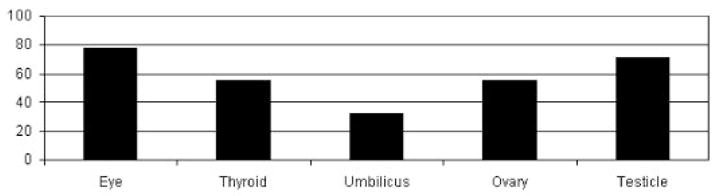
Radiation reduction to various organs with a double layer 360° of shielding to the patient during a simulated pediatric chest procedure.
Figure 6.
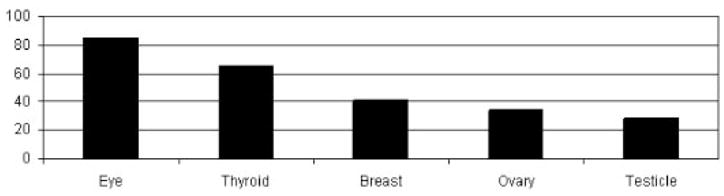
Radiation reduction to various organs with a double layer 360° of shielding to the patient during a simulated pediatric abdominal procedure.
Exposure to the operator at various distances from the scan plane exponentially decreased with distance (Figs 7, 8). With the phantom wrapped in two layers of 360° shielding, dose reductions of 81.9% and 85.1% to the operator were seen at 10 cm and 20 cm from the scan plane, respectively, during a chest procedure. Similar results were seen during an abdominal procedure.
Figure 7.
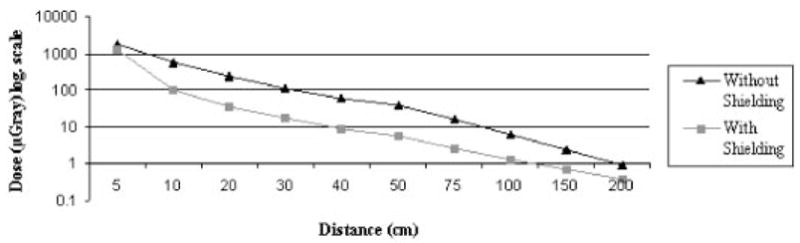
Radiation dose to the operator at various distances from the scan plane without shielding and with a double layer of 360° shielding to the patient during a simulated adult chest procedure. A distance of 10 cm from the beam with shielding in place results in a considerable dose reduction to the operator.
Figure 8.
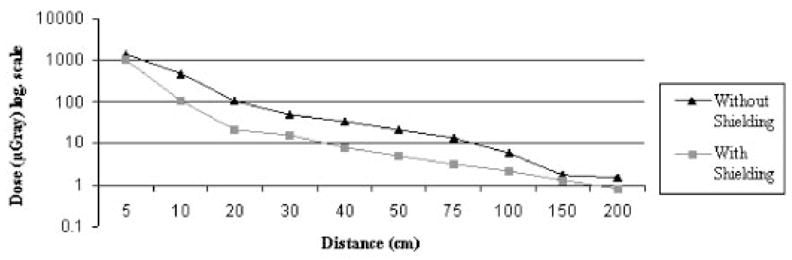
Radiation dose to the operator at various distances from the scan plane without shielding and with a 360° double layer of shielding to patient during a simulated adult abdominal procedure. A distance of 10 cm from the beam with shielding in place results in a significant dose reduction to the operator.
The dose to the operator’s unshielded testicle after the acquisition of 30 frames of CT fluoroscopy was 25.41 μGy. Single- and double-layer 90° corner drapes provided significant dose reductions of 93.3% and 98.7%, respectively, in this region (Fig 9).
Figure 9.
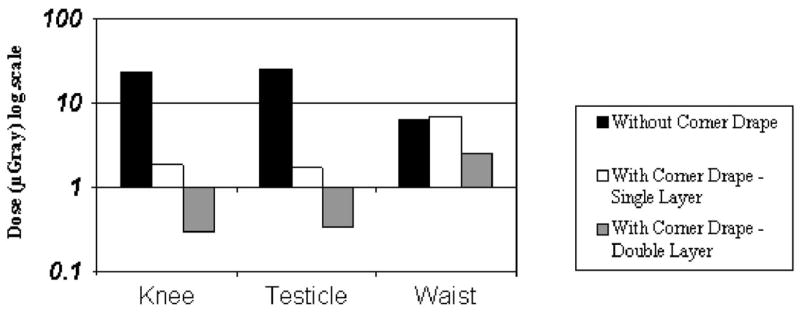
Effects of single and double 90° corner shields on dose reduction to the operator’s knees, testicles, and waist.
Several designs of gantry shields were tested. A vertical fenestrated drape hung from the gantry yielded dose reductions of only 21.3% and 34.7%, respectively, to the operator at 10 cm and 20 cm from the scan plane.
Double-layer radiation protective gloves in addition to double-layer 360° phantom shielding and a fenestrated gantry drape garnered dose reductions to the operator’s hand of approximately 97.1% at 5 cm (from 1,792 μGy to 51.77 μGy) and 93.1% at 10 cm (from 564.6 μGy to 38.81 μGy) from the scan plane after a 30-frame acquisition (Table 2).
Table 2.
Radiation Protection with Double-layer Protective Gloves, Double-layer 360° Phantom Shielding, and Fenestrated Gantry Drape
| Shielding | No Protection | Patient Double 360º | Patient Double 360º plus Single-layer Glove | Patient Double 360º plus Double-layer Glove | Reduction (%): None vs Patient Double 360º plus Double-layer Glove |
|---|---|---|---|---|---|
| Protective glove without fenestrated gantry drape | |||||
| 5 cm (μGy) | 1,792 | 1262 | 355.8 | 138.8 | 92.3 |
| 10 cm (μGy) | 564.6 | 102.3 | 66.79 | 56.78 | 90 |
| Protective glove with fenestrated gantry drape | |||||
| 5 cm (μGy) | 1,792 | 1262 | 139 | 51.77 | 97.1 |
| 10 cm (μGy) | 564.6 | 102.3 | 50.81 | 38.81 | 93.1 |
DISCUSSION
During the past 20 years, interventional radiology has established an important role in patient care, traditionally using ionizing radiation as a common means of image guidance. Any benefits must be weighed against potential risk. Diagnostic CT examinations have become more frequently used and involve increased radiation dosage to patients (17). Analysis of radiation shielding during diagnostic CT is under way. The hazard of direct and scattered radiation also exists for the interventional radiology staff (18). For the patient, scatter radiation to radiation-sensitive organs is of great concern. These organs include male and female gonads, thyroid, breast, and lens of the eye, as well as other organs that were not studied directly in our study, like the lung and gastrointestinal tract.
Deterministic and stochastic effects could be developed if the radiation exposure exceeds 500 mSv per year and 50 mSv per year, respectively (19). Also, a “linear no-threshold” risk model showed that continuous low doses of radiation (0–100 mSv) have the potential to cause a slight increase in cancer risk. These low doses are responsible for approximately 1% of the development of cancers in humans, whereas the other 42% result from other causes (20).
Compared with adults, pediatric patients are at a higher risk with high radiation dosages because they are more susceptible to the carcinogenic effects of radiation (21).
For radiologists who perform these imaging procedures frequently, the cumulative risk may be greater and is being redefined with new data (22). Our data show that tungsten antimony seems to be an excellent shielding material for CT fluoroscopy and potentially for diagnostic CT. This material is lightweight and durable. Wrapping the phantom with two layers of tungsten was effective in reducing the dose not only to the phantom but also to the operator. The addition of the 90° corner shield resulted in drastic dose reduction to the operator’s gonads.
The vertically fenestrated gantry shield provided less dramatic results, with a dose reduction of less than 30%. This potentially could be related to the fact that our CT scanner scanned a 240° arc below the phantom, thereby limiting the use of a gantry shield that is above the phantom. Another possibility is that collimator leakage may arise above the level of the gantry drape. It should be noted that the effect of the gantry drape may be more effective on CT fluoroscopy units of different vendors, particularly in those that use 360° scanning. Although the fenestrated drape may add complexity to the procedure and may be impractical at this phase, as collimation widens with increasing detector rows on multislice CT scanners it could become important in the future. Further studies to optimize dose reduction for different manufacturers’ CT fluoroscopy equipment need to be performed.
It is recognized that the operator’s hand is not within the beam during the procedure, but it will be in its proximity during the procedure. Although not standard practice, routine glove use may afford more safety. Such techniques could result in CT fluoroscopy being more widely used, for example, during tumor ablation, when precise placement and repositioning affect outcome. The use of double-layer radiation-protective gloves combined with a double-layer 360° phantom shield (without the fenestrated gantry drape) still significantly reduced the operator’s hand dose by 92.3% at 5 cm and by 90% at 10 cm from the beam (Table 2). Therefore, the use of this combined shielding technique is strongly suggested for operator safety. A significant dose decrease does occur beyond 10 cm from the beam, which would also come with a loss of dexterity. Manual assist devices become cumbersome and hard to control with greater length, which suggests that 10–20 cm is an ideal length for such devices. Robotic assist devices that allow remote needle insertion are being developed. New methods of radiation reduction to the patient and physician should be developed so patients can safely benefit from this powerful technology for accurate image guidance.
One possible limitation of our study was the assumption that the anthropomorphic phantom was a real patient. Also, a limited sample of procedure locations were used in our study as an estimation only. Other procedure locations could yield different results. Also, there is a wide variety of commercially available and developing CT fluoroscopy techniques with different arcs or degrees of use (240° in our study), number of detector rows (16 in our study), frames, rates, and display modes. This variability complicates the extrapolation of the findings of this study to other systems or techniques. However, some generalization can be made, as more detector rows or degrees of use may signify more radiation leakage (10). Attention and definition from the industry regarding these issues is needed.
Various methods have been sought to reduce radiation doses during interventional procedures (23–27). The aggressive use of US alone for the guidance of biopsies may be the most important way to reduce radiation. The ALARA principle (“as low as reasonably achievable”) is used to ensure that radiation exposures will be well below the accepted limit. Methods of radiation reduction may be inherent to the equipment or achieved with post-marketing adaptations, and vendors should consider this issue during future scanner development.
The use of tungsten antimony shielding and radiation-protective gloves significantly reduces exposure to the patient and operator during CT fluoroscopy procedures in a user-friendly and likely cost-effective manner. The sterile pad is currently commercially available for $39 per pad (oral communication, Worldwide Innovations and Technologies). The quantity needed depends on the procedure and the layers of shielding to use. A prototype of nonsterile shielding produced for our study is shown in Figure 2. Such a configuration not only would be easy to use (ie, the sterile field could be adjusted) but would not interfere with the use of monitoring equipment (eg, electrocardiography).
In summary, the lead-free, lightweight, and disposable tungsten antimony shielding used in the present investigation protect the patient and operator from scatter radiation in a simple, reasonable, and efficient manner.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health Clinical Center and was also supported in part by Worldwide Innovations and Technologies, Overland Park, KS.
References
- 1.Mueller PR, Van Sonnenberg E. Interventional radiology in the chest and abdomen. N Engl J Med. 1990;322:1362–1372. doi: 10.1056/NEJM199005103221906. [DOI] [PubMed] [Google Scholar]
- 2.Haaga JR, Alfidi RJ. Precise biopsy localization by computed tomography. Radiology. 1976;118:603–607. doi: 10.1148/118.3.603. [DOI] [PubMed] [Google Scholar]
- 3.Carlson SK, Bender CE, et al. Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology. 2001;219:515–520. doi: 10.1148/radiology.219.2.r01ma41515. [DOI] [PubMed] [Google Scholar]
- 4.Katada K, Anno H, Koga S, et al. Initial trial with CT fluoroscopy [abstract] Radiology. 1994;190(suppl):662. [Google Scholar]
- 5.Sheafor DH, Paulson EK, Kliewer MA, et al. Comparison of sonographic and CT guidance techniques: does CT fluoroscopy decrease procedure time? AJR Am J Roentgenol. 2000;174:939–942. doi: 10.2214/ajr.174.4.1740939. [DOI] [PubMed] [Google Scholar]
- 6.Gianfelice D, Lepanto L, Perreault P, et al. Value of CT fluoroscopy for percutaneous biopsy procedures. J Vasc Interv Radiol. 2000;11:879–884. doi: 10.1016/s1051-0443(07)61805-3. [DOI] [PubMed] [Google Scholar]
- 7.Stoeckelhuber BM, Leibecke T, Schulz E, et al. Radiation dose to the radiologist’s hand during continuous CT fluoroscopy-guided interventions. Cardiovasc Intervent Radiol. 2005;28:589–594. doi: 10.1007/s00270-005-0104-2. [DOI] [PubMed] [Google Scholar]
- 8.Shope TB. Regulations and recommendations relevant to interventional radiology. In: Balter S, Shope T, editors. Syllabus: a categorical course in physics—physical and technical aspects of angiography and interventional radiology. Oak Brook, Ill: Radiological Society of North America; 1995. pp. 195–205. [Google Scholar]
- 9.Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high x-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71–84. doi: 10.1016/s1051-0443(94)71456-1. [DOI] [PubMed] [Google Scholar]
- 10.Bushberg J, Seibert AJ, Leidholdt EA, et al. The essential physics of medical imaging. 2. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 11.Kato R, Katada K, Anno H, et al. Radiation dosimetry at CT fluoroscopy: physician’s hand dose and development of needle holders. Radiology. 1996;201:576–578. doi: 10.1148/radiology.201.2.8888264. [DOI] [PubMed] [Google Scholar]
- 12.ImPACT Group; UK National Health Service Purchasing and Supplies Agency. London: ImPACT; October 5, 2001, [Accessed April 2006]. Technology Update No. 2: Real Time CT and CT Fluoroscopy. Version 1.11. Available at: www.impactscan.org/download/ctfluoro.pdf. [Google Scholar]
- 13.Katada K, Kato R, Anno H, et al. Guidance with real-time CT fluoroscopy: early clinical experience. Radiology. 1996;200:851–856. doi: 10.1148/radiology.200.3.8756943. [DOI] [PubMed] [Google Scholar]
- 14.Gagne RM. Status report on computed tomography fluoroscopy (CTF): radiation protection and control for new applications of existing technology. Food and Drug Administration–Technical Electronic Product Radiation Safety Standards Advisory Committee Meeting Summary. Silver Spring, MD: Office of Science and Technology of the Food and Drug Administration; September 15–16, 1999. [Google Scholar]
- 15.Nawfel RD, Judy PF, Silverman SG, et al. Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology. 2000;216:180–184. doi: 10.1148/radiology.216.1.r00jl39180. [DOI] [PubMed] [Google Scholar]
- 16.Solomon S, Patricu A, Bohlman M, et al. Robotically driven interventions: a method of using CT fluoroscopy without radiation exposure to the physician. Radiology. 2002;225:277–282. doi: 10.1148/radiol.2251011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rehani Berry. Radiation doses in computed tomography. Br Med J. 2000;320:593–594. doi: 10.1136/bmj.320.7235.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nawfel R, Judy P, Silverman S, et al. Patient and personnel exposure during CT fluoroscopy–guided interventional procedures. Radiology. 2000;216:180–184. doi: 10.1148/radiology.216.1.r00jl39180. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Nuclear Regulatory Commission. Maximum permissible radiation levels. Report No 97–083; May 1997. [Google Scholar]
- 20.BEIR VII. Health risks from exposure to low levels of ionizing radiation. The National Academies Report in Brief. [Accessed April 2006];June 2005; Available at: http://www. nap.edu/reportbrief/11340/11340rb.pdf. [PubMed]
- 21.Fricke B, Donnely L, Frush D, et al. In-plane bismuth breast shields for pediatric CT: effects on radiation dose and image quality using experimental clinical data. AJR Am J Roentgenol. 2003;180:407–411. doi: 10.2214/ajr.180.2.1800407. [DOI] [PubMed] [Google Scholar]
- 22.Haskal ZJ. Interventional radiology carries occupational risk for cataracts. [Accessed August 1, 2006];RSNA News. June 2004; Available at: http://www.rsna.org/Publications/rsnanews/upload/jun2004.pdf.
- 23.Miotto D, Feltrin G, Calamosca M. A radiation protection device for use during percutaneous transhepatic examinations. Radiology. 1984;151:799. doi: 10.1148/radiology.151.3.6718743. [DOI] [PubMed] [Google Scholar]
- 24.Young AT, Morin RL, Hunter DW, et al. Surface shield: device to reduce personnel radiation exposure. Radiology. 1986;159:801–803. doi: 10.1148/radiology.159.3.3704160. [DOI] [PubMed] [Google Scholar]
- 25.Miller DL, Vucich JJ, Cope C. A flexible shield to protect personnel during interventional procedures. Radiology. 1985;155:825. doi: 10.1148/radiology.155.3.4001386. [DOI] [PubMed] [Google Scholar]
- 26.Yafae MY, Mawdsley GE, Lilley M, et al. Composite materials for X-ray protection. Health Phys. 1991;60:661–664. doi: 10.1097/00004032-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Webster EW. Addendum to “Composite materials for x-ray protection” [letter] Health Phys. 1991;61:917–918. [PubMed] [Google Scholar]


