Abstract
Many race- or isolate-specific disease resistance responses of plants toward pathogens (incompatible interactions) invoke hypersensitive response (HR)-like programmed cell death (PCD) and the coordinated activation of mitogen-activated protein kinases homologous with Arabidopsis (Arabidopsis thaliana) AtMPK6 and AtMPK3 (or tobacco [Nicotiana tabacum] SIPK and WIPK), respectively. Resistance of wheat (Triticum aestivum) leaves to the necrotrophic fungal pathogen Mycosphaerella graminicola can also operate at an isolate/cultivar-specific level. We confirm here that resistance is achieved without any sign of HR-like PCD during the incompatible interaction. Instead, PCD is strictly associated with the compatible interaction and is triggered during disease symptom expression. A strong transcriptional activation of TaMPK3, the wheat homolog of Arabidopsis AtMPK3, was observed immediately preceding PCD and symptom development in the compatible interaction. Generation and use of TaMPK3- and TaMPK6-specific antibodies on western blots and in coupled immunoprecipitation-protein kinase assays demonstrated that the TaMPK3 protein also accumulated, and was subsequently posttranslationally activated, during the compatible interaction in parallel to PCD. In contrast, no increase in expression, protein levels, or posttranslational activation of TaMPK6 was observed at any stage of either compatible or incompatible interactions. However, the protein levels of TaMPK6 became markedly reduced during the compatible interaction coincident with the onset of TaMPK3 protein accumulation. These data highlight the emerging similarity between the signaling pathways triggered in a host plant during successful infection by a necrotrophic fungal pathogen and the resistance responses normally effective against biotrophs.
Plant disease resistance to pathogens takes many forms. The most widespread form is referred to as nonhost resistance and operates at various levels to prevent infection of entire species of plants by entire species of pathogens (Nürnberger and Lipka, 2005). Below this level of control is race- or isolate-specific resistance, which operates between individual races or isolates of a pathogen species and individual cultivars or genotypes of a plant species. Resistance of this type is frequently regulated via a gene-for-gene interaction between complementary pathogen avirulence (Avr) genes and plant resistance (R) genes (Flor, 1971; Hammond-Kosack and Jones, 1997; Nimchuk et al., 2001; Martin et al., 2003). The host resistant reactions triggered as a consequence of Avr-R interactions are multifaceted and often include a rapid and strictly localized form of programmed cell death (PCD), referred to as the hypersensitive response (HR; Heath, 2000; Beers and McDowell, 2001; Nimchuk et al., 2003; Greenberg and Yao, 2004). This is particularly effective in inhibiting the growth of biotrophic pathogens, which require living host tissue in order to complete their infection cycle. In contrast, few reports have addressed plant race- or isolate-specific resistance toward necrotrophic pathogens, which complete their infection cycle in dead and/or dying host tissues and that have been suggested to benefit from the HR (Govrin and Levine, 2000; Lincoln et al., 2002; van Baarlen et al., 2004; van Kan, 2006).
In addition to the execution of HR-like cell death during resistance, many studies have also described the posttranslational activation of mitogen-activated protein kinases (MAPKs) homologous with Arabidopsis (Arabidopsis thaliana) AtMPK6/tobacco (Nicotiana tabacum) SIPK (herein referred to as MPK6 homologs belonging to the A2 subgroup; Ichimura et al., 2002) and Arabidopsis AtMPK3/tobacco WIPK (MPK3 homologs belonging to the A1 class subgroup). The simultaneous activation of MPK6 and MPK3 homologs has been reported during both Avr-R-mediated disease resistance reactions (Zhang and Klessig, 1998a; Romeis et al., 1999; Jin et al., 2003; Pedley and Martin, 2004; Stulemeijer et al., 2007) and also following the recognition of pathogen-associated molecular patterns, which can trigger non-host-mediated defenses (Ligterink et al., 1997; Zhang et al., 2000; Asai et al., 2002; Kroj et al., 2003). This activation has often been shown to be mediated by a single MAPK kinase (MKK), suggesting that in some situations the two MAPKs share the same regulatory control (Yang et al., 2001; Asai et al., 2002; Lee et al., 2004). It has also been shown that MPK6 and MPK3 homologs play direct or indirect roles in promoting disease resistance reactions of plants toward particular pathogens (Asai et al., 2002; Ekengren et al., 2003; Menke et al., 2004).
We are studying the interaction between wheat (Triticum aestivum) and its host-specific fungal leaf pathogen Septoria tritici (teleomorph Mycosphaerella graminicola), the causal agent of Septoria leaf blotch disease. Plant infection by M. graminicola exhibits characteristics shared by a number of related agriculturally important fungi (Goodwin, 2004). These pathogens all penetrate host leaves only via stomata and have long periods of symptomless association, ranging from weeks to months, before eventually triggering disease symptoms. For M. graminicola, the symptomless phase of wheat leaf infection can range from 1 week to several weeks. Key features of these fungi, which distinguish them from most current models, is the lack of any specialized feeding or penetration structures and the fact that they remain strictly extracellular with respect to host cells throughout the entire duration of infection. In all cases, successful plant infection by these pathogens involves a necrotrophic growth phase, and disease symptoms ultimately manifest themselves through the sudden appearance of leaf lesions bearing fungal sporulation structures.
Although quantitative trait loci are also known to play roles in some forms of plant resistance to M. graminicola, specific interactions between wheat cultivars and fungal isolates occur and have been shown to conform to the gene-for-gene hypothesis (Kema et al., 2000; Brading et al., 2002). However, neither the corresponding pathogen Avr nor host R genes (referred to as Stb genes) have yet been cloned. Of particular interest for this gene-for-gene system is that despite extensive studies performed at a number of laboratories using different approaches, HR-like cell death has never been shown to be associated with the resistant interaction (Cohen and Eyal, 1993; Kema et al., 1996; Ray et al., 2003; Shetty et al., 2003; Adhikari et al., 2004). Therefore, it remains to be determined how isolate-specific resistance operates against an initially slow-growing, strictly extracellular, ultimately necrotrophic fungal plant pathogen.
We recently described a M. graminicola-wheat seedling bioassay using attached leaves that allows the investigation of both host and pathogen physiology during a compatible disease interaction (Keon et al., 2000, 2005a, 2005b, 2007). A key feature of this bioassay was the use of high levels of fungal inoculum applied to a discrete region of the leaf blade, which introduces an element of synchronicity with respect to the subsequent development of disease symptoms. These studies demonstrated for the first time that successful fungal infection is associated with a host PCD response with features similar to the HR-like cell death more commonly seen during resistance to avirulent plant pathogens (Keon et al., 2007). Furthermore, fungal microarray transcription profiling during symptom development revealed that the induction of this host response relieved a nutritional starvation condition in the fungus, which correlated with an increase in growth rate and asexual sporulation (Keon et al., 2007).
This study describes and compares novel host signaling responses triggered during compatible and incompatible interactions between wheat and M. graminicola. The data we present demonstrate a strong correlation between the appearance of PCD markers and symptom expression only during the compatible interaction. Furthermore, we have identified three different levels of control on the wheat AtMPK6 and AtMPK3 homologs, referred to as TaMPK6 and TaMPK3, respectively, during these interactions. This includes transcriptional and posttranslational control and contrasting changes in steady-state protein levels. We discuss these findings in the context of the emerging similarity in host signaling responses triggered during successful attack by necrotrophic pathogens and those that occur during effective resistance toward biotrophs.
RESULTS
Selection of Experimental Cultivars and Fungal Isolates
The predominant fungal isolate used in this study is IPO323, which was selected for the M. graminicola genome sequencing project (http://genome.jgi-psf.org/Mycgr1/Mycgr1.home.html). IPO323 is avirulent on wheat cultivars possessing the Stb6 resistance gene, in accordance with the gene-for-gene hypothesis (Kema et al., 2000; Brading et al., 2002). The two experimental cultivars used in this study were Cadenza (Stb6 and resistant to IPO323) and Avalon, which is fully susceptible to IPO323 (Arraiano and Brown, 2006; Arraiano et al., 2007). The figures shown demonstrate the responses of these two cultivars toward isolate IPO323, which represent a fully characterized compatible and incompatible gene-for-gene-based resistant interaction. It is important to note that the specific resistance of Cadenza toward isolate IPO323, although strong, is weaker than in other Stb6-containing wheat cultivars and can result in the infrequent formation of lesions bearing fungal sporulation structures (Arraiano and Brown, 2006). Where appropriate, figures showing reciprocal interactions using isolate IPO88004, which exhibits the opposite specific resistance phenotype but has not yet been proven to operate in a gene-for-gene interaction, are presented as supplemental materials. All experiments were performed under controlled glasshouse conditions. However, it must also be noted that the precise time needed for the appearance of the first disease symptoms following fungal inoculation can vary between experiments by 1 to 3 d.
Differences in Visible Symptom Development during Compatible and Incompatible Interactions
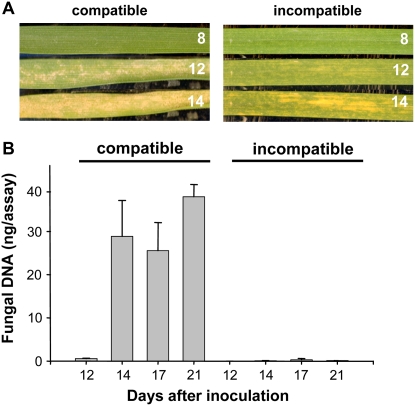
Figure 1A presents a time course of visual symptom development for a single leaf inoculated with an incompatible or compatible fungal isolate and illustrates that, for up to 8 d, both interactions occurred without visible disease symptoms. Symptoms subsequently appeared in both cases by day 12 and were restricted to the fungal inoculated areas of the leaf. In the compatible wheat leaves, these symptoms appeared as gray sunken necrotic regions across the inoculated area. In contrast, leaves of Cadenza when inoculated with isolate IPO323 developed a patchy chlorosis (yellowing) during this incompatible interaction. Only very discrete and infrequent necrosis-type lesions were seen at much later stages, and very few asexual sporulation structures (pycnidia) developed subsequently. Mock-inoculated leaves showed no visible symptoms (data not shown). Real-time quantitative PCR analysis of fungal genomic DNA throughout the infection time course, which reports the levels of fungal biomass, confirmed the strong resistance phenotype of the incompatible interaction (Fig. 1B; Supplemental Fig. S1). We were unable to detect any increase in the levels of fungal biomass during the presymptomatic phases (up to day 12) of either interaction, and subsequent increases after this time point were restricted to the compatible interaction (Fig. 1B; Supplemental Fig. S1). Both the visible symptoms and the lack of sporulation of isolate IPO323 in the resistant genotype (Stb6) segregated in a 1:1 ratio in 40 progeny lines generated from a double haploid population derived from an F1 cross between Avalon and Cadenza (K. Kanyuka, J. Keon, and J.J. Rudd, unpublished data). This confirmed the presence of a single segregating R gene locus.
Figure 1.
Visible phenotypes of the compatible and incompatible interactions between wheat and M. graminicola used in this study. A, Left panels show a single attached leaf of the susceptible cultivar Avalon inoculated with M. graminicola isolate IPO323 and photographed after 8, 12, and 14 d. Right panels show a single leaf of the resistant cultivar Cadenza (Stb6) inoculated with IPO323 and photographed after 8, 12, and 14 d. Note that both cultivars show no visible signs of infection until 12 d after inoculation. B, Determination of fungal biomass across the time course of symptom development using a quantitative PCR assay directed toward fungal genomic DNA. These data demonstrate the extreme susceptibility of Avalon and the resistance of Cadenza possessing the specific resistance gene Stb6.
Characteristic Features of PCD Are Associated with Symptom Expression during Compatible But Not Incompatible Interactions
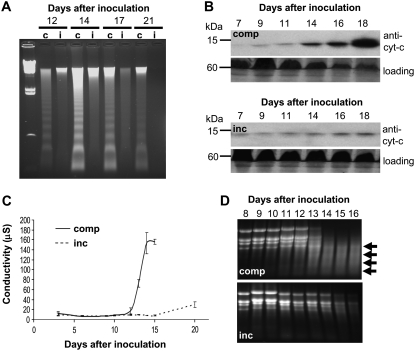
Previous work showed that physiological hallmarks of “apoptosis-like” PCD appeared during disease symptom development in a compatible interaction between Riband wheat and M. graminicola isolate IPO323. These included DNA laddering and translocation of cytochrome c from mitochondria to the cytosol (Keon et al., 2007). Figure 2A demonstrates that symptom development in the compatible interaction between Avalon and IPO323 was also associated with a DNA laddering response. This was not seen during an incompatible interaction, although isolation of genomic DNA proved difficult from Cadenza at the later stages, suggesting breakdown, although via a different mechanism (Fig. 2A). Similarly, western-blot analysis using anti-cytochrome c antiserum demonstrated that the development of disease symptoms in compatible leaves was clearly associated with an accumulation of cytochrome c in the cytosol. In contrast, the levels of cytochrome c in the cytosol of incompatible leaves remained consistently low despite the development of chlorotic symptoms (Fig. 2B).
Figure 2.
Apoptosis-like PCD markers are associated with symptom expression during compatible but not incompatible interactions. A, Analysis of the integrity of host genomic DNA during symptom development of compatible (c) and incompatible (i) interactions. A clear and prolonged DNA laddering response is only observed during symptom development in the compatible interaction (Avalon versus isolate IPO323). B, Western-blot analysis of cytochrome c in the cytoplasmic fraction of compatible (top panels; comp) and incompatible (bottom panels; inc) leaves after fungal inoculation. The relative protein loading is displayed in each case for the 60-kD region. C, A loss of host cell membrane integrity during symptom development is restricted to the compatible interaction. Electrolyte leakage assays were performed on leaves undergoing incompatible (inc) or compatible (comp) interactions with the fungus on various days after inoculation. Note that despite visible symptoms appearing at the same time point, a rapid and dramatic loss of host membrane integrity is restricted to the compatible interaction. D, Large-scale degradation of host total RNA is associated with symptom development during the compatible (comp) interaction. Total RNA was isolated on the indicated days after inoculation and analyzed on agarose gels. Arrows indicate the appearance of degraded RNA species during symptom expression in the compatible interaction.
Previous studies also demonstrated a rapid loss of host cell membrane integrity associated with symptom development during a compatible interaction. This was shown to result in large increases in both apoplastic electrolytes and metabolites (Keon et al., 2007). Therefore, we tested whether the symptoms observed during the incompatible interaction also involved a loss of host cell membrane integrity via the electrolyte leakage assay. In the compatible interaction, no increase in electrolyte leakage occurred until the first appearance of visible symptoms (9–11 d after fungal inoculation). The subsequent increase thereafter was dramatic and peaked by day 14 (Fig. 2C). In contrast, and despite the development of chlorotic symptoms, there was no measurable loss of host membrane integrity at any stage of the incompatible interaction, including the very early stages (<12 h after inoculation; data not shown). An analysis of the integrity of leaf RNA also highlighted that, in contrast to the compatible interaction, no obvious degradation of total RNA was observed during the appearance of chlorotic symptoms during the incompatible interaction (Fig. 2D). These data collectively demonstrate the strict association of the appearance of PCD markers with symptom expression only during the compatible disease interaction.
The Wheat MAPK TaMPK3 Is Strongly Transcriptionally Up-Regulated during the Immediate Presymptomatic Phase of the Compatible Interaction
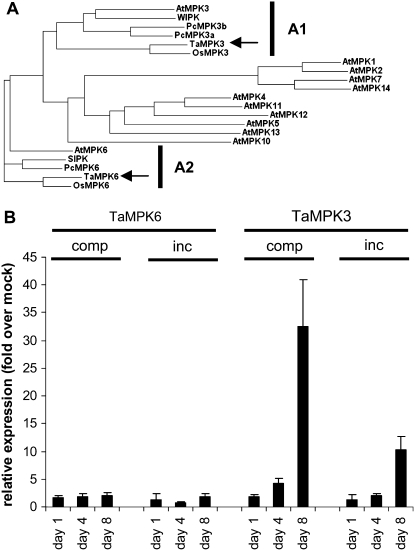
A preliminary wheat Affymetrix microarray experiment using leaf tissue collected prior to symptom development in both compatible and incompatible interactions identified a single MAPK as being transcriptionally up-regulated relative to mock-inoculated controls (J.J. Rudd, unpublished data). This gene encoded an extracellular signal-regulated kinase (ERK)-like MAPK previously referred to as WCK-1 (GenBank accession no. AY079318) and shown to be transcriptionally activated in a fungal elicitor-treated cell culture (Takezawa, 1999). Analysis of the amino acid sequence with MAPKs identified in various other plant species indicated the predicted protein to have most similarity to homologs of Arabidopsis AtMPK3 and tobacco WIPK (A1 class shown in Fig. 3A). In order to clarify this relationship, we will hereafter refer to WCK-1 as TaMPK3, according to the nomenclature suggested by the plant MAPK signaling group (Ichimura et al., 2002). We also identified a wheat homolog of Arabidopsis AtMPK6 and tobacco SIPK (A2 class), which did not change in expression in our microarray experiment (J.J. Rudd, unpublished data). This sequence was also present in GenBank under accession number AY173962. We now refer to this MAPK as TaMPK6 (Fig. 3A).
Figure 3.
Identification of wheat TaMPK6 and TaMPK3 and analysis of their expression during the symptomless phase of incompatible and compatible interactions. A, Candidate wheat homologs of A1 and A2 class plant MAPKs were identified by BLASTP analysis of GenBank and a proprietary wheat EST collection. ClustalW analysis was then performed with the sequences against all 12 Arabidopsis MAPKs containing the TEY activation loop domain (genes At1g10210, At1g59580, At3g45640, At4g01370, At4g11330, At2g43790, At2g18170, At3g59790, At1g01560, At2g46070, At1g07880, and At4g36450) in addition to A1 and A2 class MAPKs from rice (GenBank accession nos. NP_001056846 and ABH01189), tobacco (AAB58396 and BAA09600), and parsley (Petroselinum crispum; AAN65179 and AAN65181/CAA73323). Arrows indicate the positions of the TaMPK6 and TaMPK3 homologs. B, Real-time SYBR Green RT-PCR analysis of TaMPK3 and TaMPK6 expression at 1, 4, and 8 d after inoculation of Cadenza wheat with either isolate IPO323 (incompatible [inc]) or isolate IPO88004 (compatible [comp]).
The relative mRNA levels of TaMPK3 and TaMPK6 were studied across an infection time course taken at 1, 4, and 8 d after fungal inoculation preceding visible symptom development. Both experimental wheat cultivars were inoculated separately with both fungal isolates in order to generate reciprocal specific interactions for each. No significant change in the relative expression of TaMPK6 was detected during this analysis. In contrast, increases in levels of the TaMPK3 transcript were detected during the course of both compatible and incompatible interactions. However, for each cultivar-fungal isolate combination tested, this increase was strongest during the compatible interaction at 8 d after inoculation (Fig. 3B; Supplemental Fig. S2). Transcriptional activation of TaMPK3 was also seen at the same time point during incompatible interactions but was notably weaker (Fig. 3B; Supplemental Fig. S2).
ERK-Like MAPKs Are Posttranslationally Activated during Disease Symptom Development in the Compatible Interaction
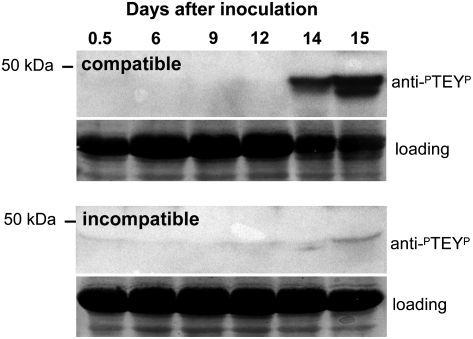
The strong transcriptional activation of TaMPK3 preceding disease symptom expression led us to test whether ERK-type MAPKs became posttranslationally activated at any point during either compatible or incompatible interactions with M. graminicola. Protein extracts from fungus-inoculated leaves were subjected to western-blot analysis using an anti-active MAPK antibody (anti-ERK-PTEYP) that specifically detects the dual phosphorylation on Thr and Tyr residues that is an essential feature of their posttranslational activation. Figure 4 shows that at least two (as indicated by two discrete bands) ERK-type MAPKs were strongly activated in this experiment at 14 d after fungal inoculation during the compatible interaction. This was detected coincident with the appearance of disease symptoms and PCD markers (Fig. 2). Only a very slight increase in MAPK activity was detected at the corresponding time point of the incompatible interaction between Cadenza and isolate IPO323 (Fig. 4). No MAPK activation was detected at any tested earlier stage of either compatible or incompatible interactions within the first few hours after fungal inoculation (Supplemental Fig. S2).
Figure 4.
ERK-type MAPKs are strongly posttranslationally activated during symptom expression in the compatible interaction. Western-blot analysis of the activation of ERK-type MAPKs. Protein extracts from leaves inoculated with compatible or incompatible fungal isolates were probed with an anti-active ERK antiserum that recognizes the dual phosphorylated motif present in the active form (PTEYP). These data demonstrate that at least two ERK-type MAPKs are activated during a compatible interaction between Avalon and isolate IPO323 (top panel). The relative protein loading (Ponceau S staining of the nitrocellulose membrane) is displayed in each case for the 60-kD region.
Differences in TaMPK6 and TaMPK3 Protein Levels Contrast Markedly during the Course of the Compatible Interaction
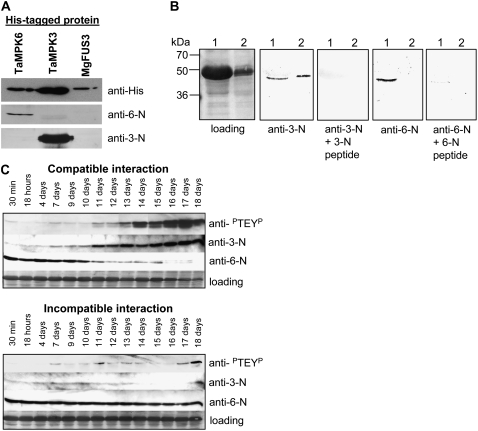
In order to determine whether any of the MAPK activities stimulated during the compatible disease interaction corresponded to either TaMPK3 or TaMPK6, we generated kinase-specific peptide antibodies recognizing the N terminus of either protein. The resulting antisera had the correct specificity on western blots against the recombinant His-tagged TaMPK3 and TaMPK6 proteins (Fig. 5A). Neither antiserum cross-reacted with an ERK-like MAPK from M. graminicola, MgFus3 (Fig. 5A; Cousin et al., 2006).
Figure 5.
Western-blot analysis with TaMPK3- and TaMPK6-specific antibodies reveals dynamic and contrasting changes in the respective protein levels during fungal infection. A, The specificity of peptide antibodies directed against the N terminus of TaMPK3 (anti-3-N) and TaMPK6 (anti-6-N) against the recombinant TaMPK3, TaMPK6, and MgFus3 His-tagged proteins. B, Specific detection of the TaMPK proteins in leaf extracts generated from tissue taken 14 d after mock inoculation (lane 1) or inoculation with a compatible fungal isolate (lane 2). Note that the 6-N antibody fails to detect the specific TAMPK6 protein in the extract generated from leaves undergoing a compatible interaction. C, Time course western-blot analysis for MAPK activity levels (anti-PTEYP) and TaMPK3 (anti-3-N) and TaMPK6 (anti-6-N) protein levels across a compatible (top series) or incompatible (bottom series) fungal inoculation time course. Protein loading levels are shown in each case for the 60-kD region. Note the opposite responses of TaMPK3 and TaMPK6 protein levels specifically across the time course of the compatible interaction.
The specificity of both antisera was then tested against extracts from mock-inoculated leaves (Fig. 5B, lane 1) and from leaves collected 14 d after challenge with a compatible fungal isolate (Fig. 5B, lane 2). Both antisera recognized a single specific band (shown by peptide competition) of the correct size in the mock-inoculated leaf extract (Fig. 5B, lane 1). The TaMPK3-N antiserum also strongly recognized its antigen in the leaf extract generated 14 d after inoculation with a compatible fungal isolate (Fig. 5B, lane 2). However, the TaMPK6-N antiserum no longer detected its specific target in the same extract. This suggested that the TaMPK6 protein levels had fallen beyond detectable levels in this extract. To test this further, we analyzed a more detailed time course following fungal inoculation to determine the overall MAPK activity levels (using the anti-PTEYP antibody) and the TaMPK3 and TaMPK6 protein levels (using their specific antisera) during both compatible and incompatible interactions. Figure 5C shows the results of this analysis for a time course ranging from 30 min to 18 d after fungal inoculation. Protein levels of TaMPK3 were found to be relatively low during the early stages of the compatible interaction but began to increase at approximately 7 d after inoculation. A subtle increase in overall MAPK activity was detected at the same time point. A more significant increase in MAPK activation became obvious by day 11 and then increased strongly for up to 18 d after inoculation. This pattern was mirrored by the corresponding levels of the TaMPK3 protein in the same extracts (Fig. 5C, top). In contrast, the TaMPK6 protein was relatively abundant in extracts that contained no appreciable MAPK activity and low TaMPK3 protein levels (up to 11 d after inoculation). However, from that point on the protein levels of TaMPK6 began to diminish to undetectable levels by 18 d after inoculation.
No significant change in overall MAPK activity or increase in TaMPK3 protein levels was observed across the time course of the incompatible interaction (Fig. 5C, bottom). In addition, there was no dramatic decrease in levels of the TaMPK6 protein, which was clearly detectable at all time points. These data demonstrate that the strong activation of ERK-like MAPKs in wheat leaves specifically during the compatible disease interaction with M. graminicola is accompanied by inverse changes in the levels of the TaMPK3 and TaMPK6 proteins.
Changes in MAPK Activities and TaMPK3 and TaMPK6 Protein Levels Occur Almost Exclusively in the Fungus-Inoculated Leaf Areas
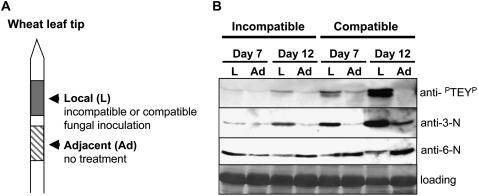
M. graminicola infection of wheat leaves is typified by the appearance of localized leaf lesions that do not spread appreciably from the initial inoculation site. The ability to inoculate discrete regions of a single leaf with fungal spores permits the study of both the immediate local host responses and also the responses that might occur beyond these regions, for example in immediately adjacent leaf areas (Fig. 6A). Therefore, we tested whether changes in MAPK activity levels, and TaMPK3 and TaMPK6 protein levels, occurred outside the fungus-inoculated leaf region at both 7 d (before symptoms) and 12 d after inoculation with compatible or incompatible fungal isolates (Fig. 6B). MAPK activation (anti-PTEYP) again appeared strongest during the compatible interaction at day 12 and was restricted to the fungus-inoculated (local or L) region exhibiting disease symptoms. No increase in MAPK activity was detectable in the adjacent (Ad) noninoculated leaf areas. Protein levels of TaMPK3 were also highest in the immediate local region 12 d after inoculation with a compatible fungal isolate; however, some increase in protein level was also detected in the adjacent noninoculated region specifically at this time point (Fig. 6B).
Figure 6.
Changes in MAPK activity levels and TaMPK3 and TaMPK6 protein levels during the compatible interaction are largely restricted to the fungus-inoculated regions. A, Diagrammatic representation of the material taken to investigate whether changes in MAPK activity levels and/or protein levels were restricted to the fungus-inoculated leaf areas (local [L]) or were also detectable outside the fungus-inoculated leaf regions (adjacent [Ad]). B, Western-blot analysis of the tissue areas shown in A at 7 and 12 d after inoculation with compatible or incompatible fungal isolates. Extracts were probed with anti-PTEYP, anti-TaMPK3-N (anti-3-N), and anti-TaMPK6-N (anti-6-N), and protein loading is shown for the 60-kD region for each extract. These data highlight that strong MAPK activation is restricted to the compatible fungus-inoculated regions and that changes in the relative levels of the TaMPK3 and TaMPK6 proteins are also greatest in these regions.
A slight activation of MAPKs was also detected at the 12-d incompatible time point and was restricted to the local region while also appearing to correlate positively with the amount of TaMPK3 protein detected in this region (Fig. 6B). This slight increase in MAPK activity and possibly TaMPK3 protein levels may be explained by the subtle chlorotic symptom development in these areas at this time point in Cadenza (Fig. 1A). In all cases in which MAPK activity and TaMPK3 protein levels were high, a corresponding and noticeable reduction in TaMPK6 protein levels was apparent. This was evident in the compatible fungus-inoculated local regions and was not seen in the adjacent regions. These data demonstrate that the changes in TaMPK3 and TaMPK6 protein levels and MAPK activity states are strongly affected in the fungus-inoculated areas (local areas) that subsequently undergo PCD and develop into restricted lesions as part of the compatible disease interaction.
TaMPK3 Is Posttranslationally Activated during Disease Symptom Development in the Compatible Interaction
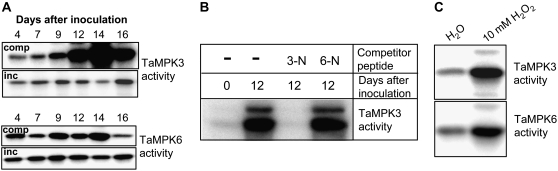
The TaMPK3- and TaMPK6-specific antibodies were then used in coupled immunoprecipitation-protein kinase assays using the myelin basic protein as an artificial substrate to determine whether any change in the corresponding protein activity occurred at any stage of compatible or incompatible interactions. Figure 7A demonstrates that immunoprecipitation-protein kinase assays using the TaMPK3 antibody identified a strong activation of the protein during the compatible interaction, peaking at 14 d after fungal inoculation. This occurred in parallel to disease symptom development, the appearance of PCD markers, and its own protein accumulation (Figs. 5C and 6B). In contrast, TaMPK3 was not posttranslationally activated during the incompatible interaction (Fig. 7A). Immunoprecipitation-protein kinase assays with the TaMPK6 antibody failed to detect any change in its activity during either interaction, with the exception of a notable decrease in the activity level detected at the 16-d compatible time point (Fig. 7A). This probably occurs as a consequence of the diminishing levels of TaMPK6 protein during the compatible interaction (Fig. 5C). No change in either TaMPK6 or TaMPK3 activity was seen during any of the early stages (within hours) of compatible or incompatible interactions.
Figure 7.
TaMPK3, but not TaMPK6, is posttranslationally activated during disease symptom expression during a compatible interaction. A, Coupled immunoprecipitation-protein kinase assays show that TaMPK3 is posttranslationally activated coincident with PCD responses only during the compatible interaction (comp). TaMPK6 is not activated during this response. Neither TaMPK3 nor TaMPK6 is activated during an incompatible interaction (inc). B, Immunoprecipitation-protein kinase assays on extracts from leaves undergoing a compatible interaction (12 d after inoculation) in the presence of the TaMPK3-N peptide competitor (3-N at 50 μg mL−1) establish the specificity of the assay. The equivalent amount of the TaMPK6-N peptide (50 μg mL−1) failed to block immunoprecipitation of the activated kinase. C, Coupled immunoprecipitation-protein kinase assays demonstrate that both TaMPK6 and TaMPK3 can be immunoprecipitated in an activated state from wheat leaf extracts generated 10 min after leaf infiltration with 10 mm H2O2.
The specificity of the immunoprecipitation-TaMPK3 activation assay was further demonstrated by peptide competition, in which addition of an excess of the TaMPK3 peptide, but not the TaMPK6 peptide, to plant extracts from compatible leaves undergoing PCD blocked the immunoprecipitation of protein kinase activity (Fig. 7B). Finally, in order to ensure that the TaMPK6 antibody was able to immunoprecipitate its activated protein target, we tested whether both kinases would respond to leaf infiltration with hydrogen peroxide (H2O2), a known activator of MPK6 and MPK3 homologs in various plant species (Kovtun et al., 2000; Kroj et al., 2003). A strong activation of both kinases was detected at 10 min after leaf infiltration with 10 mm H2O2 (Fig. 7C), confirming the ability of the TaMPK6 antiserum to immunoprecipitate activated TaMPK6. These data identify TaMPK3 as an ERK-type MAPK posttranslationally activated in parallel to PCD specifically during the course of the compatible disease interaction with M. graminicola.
DISCUSSION
Isolate-Specific Cultivar Resistance in the Absence of HR-Like Cell Death
Race- or isolate-specific plant resistance toward pathogens is widely regarded to operate at the level of a gene-for-gene interaction between pathogen Avr genes and cognate host R genes. In many well-established systems, particularly with respect to defense against biotrophic pathogens, HR-like cell death has frequently appeared as a feature of incompatible interactions (Stakman, 1915; Ryerson and Heath, 1996; Tada et al., 2004). This HR-like cell death often exhibits characteristic features of PCD, including, for example, the DNA laddering response (Ryerson and Heath, 1996; Tada et al., 2004). The importance of HR-like PCD in many incompatible disease resistance reactions, however, has been questioned and may depend on the specific host-pathogen interaction and the nuances of the experimental setup (Greenberg and Yao, 2004).
There is convincing genetic evidence that isolate-specific resistance of wheat toward M. graminicola operates at the gene-for-gene level (Kema et al., 2000; Brading et al., 2002), although the corresponding Avr and R genes remain to be identified. The visible symptoms of isolate-specific resistance of wheat to M. graminicola can vary from none whatsoever to some form of reaction, including leaf chlorosis, as shown here for the resistant interaction between isolate IPO323 and Cadenza. Our own studies, along with excellent published electron microscopy- and light microscopy-based analyses of the resistant reaction, have failed to detect HR-like cell death at any stage of an incompatible interaction, including very early time points (minutes to hours) after fungal inoculation (Cohen and Eyal, 1993; Kema et al., 1996). Given the high levels of fungal inoculum (1 × 107 spores mL−1) directly applied onto the leaf surface in our assays, if HR-like cell death were to be triggered during resistance it would probably be visible to the naked eye and/or detected in our electrolyte leakage assays. Therefore, how resistance is achieved against this strictly apoplastic, initially slow-growing, fungal plant pathogen is still largely unclear. Previous studies have identified changes in levels of reactive oxygen species (Shetty et al., 2003, 2007) and defense gene expression (Ray et al., 2003; Adhikari et al., 2007) in wheat leaves during the symptomless phase of both incompatible and compatible interactions. However, it remains unclear whether these responses kill or merely contain the fungus, and recent evidence suggests that in some M. graminicola-wheat interactions, host defense responses can be triggered very late (several weeks) after inoculation with an incompatible isolate (Adhikari et al., 2007). Therefore, in addition to these events, a likely significant contributing factor toward achieving resistance may also be a failure of the fungus to trigger host cell death signaling events that are features of compatibility.
This work and our previous studies have shown the compatible disease interaction to be associated with a PCD response of the host leaf exhibiting various apoptosis-like features and similarities to HR-like cell death. The latter point is particularly emphasized by the strict localization of the response to the area of initial fungal inoculation (Keon et al., 2007). Microarray analysis has shown that the execution of this cell death response allows the fungus to transition away from a nutrient-limiting state, enabling an increase in growth rate and the generation of its asexual sporulation structures (Keon et al., 2007). The apparent importance of host cell death signaling for this process suggests that the Stb resistance genes probably play an important role in either directly of indirectly preventing these events, thereby holding the fungus in a nutrient-limited stasis in the leaf apoplast and preventing its asexual sporulation.
The execution of host PCD during a compatible interaction is the opposite of what one immediately associates with a gene-for-gene resistance mechanism, as these features are more characteristic of incompatible interactions. With respect to plant HR-like responses occurring during a successful pathogen infection, M. graminicola infection of wheat leaves shares features that have previously only been described for a small number of broad host range, cell-penetrating, necrotrophic fungi (Govrin and Levine, 2000; Lincoln et al., 2002; van Baarlen et al., 2004).
Various Levels of Differential Regulation Are Imposed upon TaMPK3 and TaMPK6 during Compatible and Incompatible Interactions
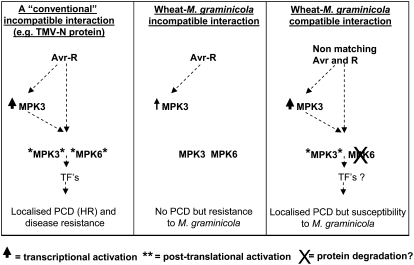
Intriguingly, during our gene expression studies, TaMPK3 mRNA levels strongly accumulated immediately preceding symptom development in the compatible interaction, while the protein was subsequently posttranslationally activated in parallel to the development of symptoms and the appearance of PCD markers. The strong association between posttranslational activation of TaMPK3 and a compatible interaction is clearly distinct from the MAPK signaling responses observed in several model systems for studying race-specific disease resistance (summarized in Fig. 8). For example, the disease resistance reactions triggered toward Cladosporium fulvum and Psuedomonas syringae pv tomato by tomato (Solanum lycopersicum) and toward Tobacco mosaic virus by tobacco were each shown to involve simultaneous activation of MPK6 and MPK3 homologs only during the incompatible interaction. These responses were triggered via pathogen recognition through the Cf-9 (Romeis et al., 1999), Cf-4 (Stulemeijer et al., 2007), Pto (Pedley and Martin, 2004), and N (Zhang and Klessig, 1998a; Jin et al., 2003) disease resistance genes. It is perhaps noteworthy, though, that the activation of HR-like cell death is also often a feature of the disease resistance reactions in these systems.
Figure 8.
Summary of the data presented in this study in comparison with an established model for an incompatible disease resistance reaction. The scheme at left illustrates the responses detected during an incompatible interaction between Tobacco mosaic virus (TMV) and tobacco leaves mediated through pathogen recognition via the N disease resistance protein (Zhang and Klessig, 1998a). The two protein kinases referred to for clarity as MPK3 and MPK6 represent WIPK and SIPK, respectively, in this system. TFs refer to transcription factors that function downstream of the MAPK cascades. The middle scheme depicts the events that occur during an incompatible interaction between wheat and M. graminicola as described in this study. The scheme at right describes the events that occur during a compatible disease reaction between wheat and M. graminicola. Note the overall similarity between the N protein-mediated resistance responses to Tobacco mosaic virus and the responses that occur during the compatible disease interaction between wheat and M. graminicola.
The fact that TaMPK3 is strongly transcriptionally up-regulated during the immediate presymptomatic phase of compatible interactions with M. graminicola suggests that it may have responded to a “general elicitation” caused by the presence of the fungus growing slowly within the leaf, despite the fact that no visible symptoms of infection are seen. The transcriptional activation of MPK3 homologs has been reported in various systems following exposure to a range of both biotic and abiotic stimuli (Zhang and Klessig, 1998b; Liu et al., 2003; Ahlfors et al., 2004; Mayrose et al., 2004; Reyna and Yang, 2006), and a number of studies have specifically demonstrated the transcriptional activation of MPK3 homologs in association with HR-mediated incompatible disease resistance reactions (Zhang and Klessig, 1998a; Mayrose et al., 2004). This contrasts remarkably with the responses of wheat leaves during interactions with M. graminicola, in which transcriptional activation of TaMPK3 is strongest preceding the PCD responses that feature in the compatible disease reaction.
It is noteworthy that many of the pathogen signals shown to activate plant MAPKs transcriptionally and posttranslationally are often described as “general elicitors” or pathogen-associated molecular patterns (Ligterink et al., 1997; Zhang et al., 2000; Lee et al., 2001; Asai et al., 2002; Kroj et al., 2003; Liu et al., 2003). It is possible, therefore, that the subsequent posttranslational activation of TaMPK3 occurs as a consequence of the recognition of specific fungal elicitor(s), effector(s), or toxin(s) that may be produced to support compatibility by inducing host PCD. The tight temporal association of the posttranslational activation of TaMPK3 with the appearance of PCD markers during a compatible interaction also suggests a functional link between the two events, as has been suggested in a number of other studies (Zhang et al., 2000; Ren et al., 2002, 2006; Jin et al., 2003; Liu et al., 2007). Intriguingly, pharmacological studies have implicated protein kinase signaling in the cell death responses of plants to various fungal toxins (Yao et al., 1991; Rasmussen et al., 2004). However, in none of these examples was the precise protein kinase activity identified. We must also emphasize that, based upon the data shown in Figure 4, additional TEY domain-containing activated MAPKs remain to be identified in the wheat-M. graminicola pathosystem.
A limited number of studies have addressed MAPK function in monocot plants during interactions with fungal pathogens. For example, OsMAPK5a, the rice (Oryza sativa) homolog of TaMPK3, was shown to play an important role in disease lesion formation induced by the hemibiotrophic rice blast fungus and was also shown to be transcriptionally activated during this interaction (Xiong and Yang, 2003; Reyna and Yang, 2006). Our initial attempts to silence TaMPK3 transiently in wheat leaves in combination with fungal inoculation have so far failed due to technical difficulties with this type of combined pathoassay involving a fungus that has a long symptomless phase of host colonization. Whether or not the stage-specific prolonged posttranslational activation of TaMPK3 during compatible interactions with M. graminicola is functionally linked with the activation of PCD remains an important question for ongoing and future research.
Clear differences were detected in the comparative levels and activities of the TaMPK3 and TaMPK6 proteins during the compatible interaction. The TaMPK3 protein is initially present at relatively low levels in leaves but accumulates prior to the appearance of first disease symptoms and its own subsequent posttranslational activation. This series of events is remarkably similar to what has been reported for WIPK in tobacco leaves following N gene-mediated recognition of Tobacco mosaic virus and the activation of HR. The difference is that these responses were features of an incompatible reaction and therefore the opposite of what we have described here (summarized in Fig. 8).
Intriguingly, in the wheat-M. graminicola pathosystem, the levels of the TaMPK6 protein fell dramatically in extracts that began to accumulate active TaMPK3. The two most obvious explanations for this are (1) that TaMPK6 is specifically degraded in plant cells in the period leading up to the execution of PCD, or (2) that the degradation of TaMPK6 is a general feature of this response, but instead TaMPK3 is somehow stabilized or continually replenished via its strong transcriptional activation. Precedent exists for the selective degradation of plant signaling proteins during pathogen attack. For example, studies on the interaction of barley (Hordeum vulgare) with the biotrophic stem rust fungus identified a specific proteolysis of the RPG1 receptor-like kinase that preceded the execution of HR-mediated disease resistance responses (Nirmala et al., 2007). During our time course studies, there was no obvious change in the overall profile of stained total proteins on the blots at the time points when the TaMPK6 protein levels began to fall (see Ponceau S stain for loading in Fig. 5C). Thus, it remains possible that TaMPK6 is selectively degraded around the time that the TaMPK3 protein begins to accumulate.
The various changes imposed upon the activities and levels of the TaMPK6 and TaMPK3 proteins were largely restricted to the immediate fungus-inoculated leaf areas and much more pronounced in the areas undergoing PCD during the compatible interaction. This again suggests some form of functional relationship between the two events. The one exception to this was an increase in the level of inactive TaMPK3 protein, which was detected in the leaf area adjacent to the developing lesion of the compatible interaction (Fig. 6B). This may suggest that the strictly localized lesions that form during the compatible interaction signal to uninoculated surrounding leaf areas in a similar, or identical, manner to how the development of HR-like lesions has been shown to trigger local and systemic acquired resistance reactions in many other pathosystems (Durrant and Dong, 2004).
The differences in protein levels between TaMPK6 and TaMPK3 throughout the course of the compatible interaction in the M. graminicola-wheat pathosystem also has implications for the upstream activators of the cascade(s). In many dicot systems, MPK6 and MPK3 homologs are activated simultaneously, often via the same MKK, in response to pathogen signals (Yang et al., 2001; Asai et al., 2002; Lee et al., 2004). This is not strictly the case, however, as during Arabidopsis responses to abiotic stresses, particular MKKs have also been shown to have a discriminatory specificity toward these two MAPKs (Teige et al., 2004). Whether or not a single MKK is/can be responsible for the activation of MPK6 and MPK3 homologs in monocots remains to be determined. From this perspective, and given the opposite regulation of the levels of TaMPK3 and TaMPK6 proteins, it will be interesting to identify the MKK responsible for activating TaMPK3 during the compatible disease interaction. It will also be interesting to test whether the same MKK can act on TaMPK6 when this kinase is activated in wheat leaves following direct infiltration with H2O2 (Fig. 7C).
Emerging Similarities between Susceptibility Signaling toward Necrotrophs and Resistance Signaling toward Biotrophs
PCD events in plants have been shown to be triggered in response to the application of fungal toxins, and many of these responses have been demonstrated to be host and/or cultivar specific (Wolpert et al., 2002). This has led to the suggestion that avirulence factors and toxins trigger essentially the same host cell signaling pathways; therefore, whether a molecule is referred to as an avirulence determinant or a toxin depends largely on the nutritional lifestyle of the invading pathogen. This concept implies that necrotrophic fungi essentially hijack a host PCD signaling response pathway that is often deployed effectively against biotrophs (Wolpert et al., 2002). This idea is significantly strengthened by the recent identification of the Arabidopsis LOV1 gene encoding a NBS-LRR “resistance” protein homolog, but which conferred susceptibility toward the necrotrophic fungal pathogen Cochliobolus victoriae. Host susceptibility to the fungus is mediated via the interaction of LOV1 with the fungal host-specific toxin victorin. This triggered host responses, including HR-like cell death, that are more commonly associated with disease resistance reactions (Lorang et al., 2007).
Small proteinaceous toxins were also recently shown to play key roles in determining the outcome of interactions between wheat leaves and the necrotrophic fungi Pyrenophora tritici-repentis and Stagonospora nodorum. The ability of these fungi to cause full disease has been shown to be positively influenced by the host sensitivity toward the proteinaceous fungal toxin ToxA, which has been horizontally transferred between the two species (Friesen et al., 2006). Host sensitivity to ToxA is conferred by the presence of an as yet unidentified single dominant gene, Tsn1 (Ciuffetti et al., 1997; Gamba et al., 1998). Wheat cultivars harboring this gene are sensitive to the toxin and undergo a cell death reaction that involves a marked loss of membrane integrity and activation of cell signaling events involving unidentified protein kinases (Kwon et al., 1998; Rasmussen et al., 2004). No homologs of ToxA are present in the sequenced genome of M. graminicola isolate IPO323 (http://genome.jgi-psf.org/Mycgr1/Mycgr1.home.html). Irrespective of this, the possibility remains that fungal host-specific toxin(s)/elicitor(s) play key roles in determining the outcome of interactions between M. graminicola and wheat (Kema et al., 1996). It is conceivable that they may function by initiating host PCD and MAPK signaling cascades that are more commonly associated with disease resistance responses. In conclusion, our data support the idea that the mechanisms used by necrotrophic plant pathogens to infect their hosts will prove to be more sophisticated than has hitherto been appreciated.
MATERIALS AND METHODS
Plant and Fungal Material and Handling
Mycosphaerella graminicola isolates IPO323 and IPO88004 were used in all experiments. The isolates were stored at −80°C in 50% (v/v) glycerol. Fungal spores for plant inoculation were harvested from 7-d-old cultures growing (budding) on yeast peptone dextrose plates (Oxoid) at 15°C.
For plant infection, the second leaf of 17-d-old wheat (Triticum aestivum) seedlings (cultivar Avalon or cultivar Cadenza) were attached, adaxial side up, to Perspex sheets using double-sided tape. The inoculation procedure was as described previously (Keon et al., 2007). The leaves were inoculated evenly with fungal spores at a density of 1 × 107 cells mL−1 in water containing 0.1% (v/v) Tween 20. Following 72 h of incubation at 100% relative humidity, inoculated plants were incubated at 16°C with a 16-h light period at 88% relative humidity for up to 21 d. Leaf tissues were excised at various time points after inoculation and stored at −80°C for DNA and RNA isolation, or immediately used to generate protein extracts, or used in electrolyte leakage assays.
Generation of Recombinant His-Tagged TaMPK3, TaMPK6, and MgFus3 Proteins
Full-length MAPK-encoding genes were obtained by reverse transcription (RT)-PCR on wheat leaf cDNA using the following primers. For TaMPK6, a first round of PCR was done with the primers MPK6FwdEST (5′-AAATCCACCGCACGGGCTTTC-3′; binds in the 5′ untranslated region of the TaMPK6 transcript) and MPK6XhoRev (5′-CTCGAGCTGGTAATCAGGGTTGAACGTGATG-3′). The PCR product was cloned into vector pGEM-T Easy (Promega) and sequenced. A second round of PCR was then done to enable in-frame cloning via BamHI and XhoI into expression vector pET-28b(+) (Novagen) using MPK6BamsiteFwd (5′-ACGCGCGGGATCCGGCGGAGATGGAC-3′) and MPK6XhoRev (as above). TaMPK3 was amplified in a single round of RT-PCR using primers MPK3BamFwd (5′-GGATCCGATGGACGGCGCTCCGGTGGCCGA-3′) and MPK3XhoRev (5′-CTCGAGGTATCGGAAGTTGGGGTTCAACTC-3′) for BamHI and XhoI cloning into the expression vector. MgFus3 was also cloned into the expression vector (via BamHI and HindIII) following a single round of PCR with primers MgFus3BamFwd (5′-GGATCCGATGTCGAGAACCGCACAGCAACAG-3′) and MgFus3HindRev (5′-AAGCTTCCGCATGATCTCTTCGTAAATCAG-3′). Recombinant protein expression was driven in BLR(DE3) pLysS cells (Novagen) by 0.1 m isopropylthio-β-galactoside, and proteins were purified from cell extracts using His-Select Nickel Affinity Gel (Sigma) according to the supplier's guidelines.
Generation of MAPK-Specific Antisera
Peptides corresponding to the N-terminal regions of TaMPK3 and TaMPK6 were used to immunize rabbits. For TaMPK3-N, the following amino acid sequence was used corresponding to residues 2 to 15 of the mature protein (GenBank accession no. AY079318): 5′-DGAPVAEFRPTMTHG-3′. For TaMPK6-N, amino acids 1 to 12 (accession no. AY173962) were used: 5′-MDAGGAQPPDSE-3′. Immunizations were performed by Eurogentec.
Protein Extraction
For cytochrome c assays, crude cytosolic and microsomal fractions were generated as described previously (Krause and Durner, 2004; Keon et al., 2007). Eight inoculated leaves (5 cm each) were homogenized and filtered through two layers of Miracloth. The extract was then centrifuged at 13,200 rpm for 15 min at 4°C to pellet the crude microsomal fraction. The supernatant was taken as the cytosolic fraction. For the MAPK activity assays, three to four leaves were collected on various days after inoculation and homogenized in kinase extraction buffer as described previously (Kroj et al., 2003; Ahlfors et al., 2004). Following centrifugation (13,200 rpm for 15 min at 4°C), the supernatant was collected and immediately analyzed by western blotting with anti-active ERK antibody (see below) or used in immunoprecipitation-protein kinase assays.
Western Blotting
For cytochrome c release assays, approximately 100 μg of protein was separated on 15% SDS-PAGE gels and blotted onto Hybond ECL nitrocellulose (Amersham Pharmacia). Blots were probed with a 1:2,000 dilution of monoclonal anti-cytochrome c antiserum (BD Biosciences clone 7H8.2C12) and subsequently a 1:5,000 dilution of anti-mouse IgG horseradish peroxidase conjugate (Sigma). Blots were developed using chemiluminescence (Amersham ECL-plus). For the investigation of ERK-type MAPK activity during plant infection, western blots were probed with Phospho-p44/42 MAP Kinase (Thr-202/Tyr-204) antibody according to the supplier's guidelines (Cell Signaling Technology). For western blots using the MAPK-specific antibodies, 1:1,000 dilutions of TaMPK3-N (affinity purified) and TaMPK6-N were used against His-tagged recombinant proteins or leaf extracts. To detect recombinant protein expression, an anti-His(C-term) antibody (Invitrogen) was used according to the supplier's guidelines to detect the C-terminal His tag generated following expression in pET-28c(+).
Immunoprecipitation-Protein Kinase Assays
Leaf extracts containing 100 μg of soluble protein were immunoprecipitated with Protein A-Sepharose CL-4B (Amersham Biosciences) bound antiserum for 1 h at 4°C. All subsequent washing and radioactive steps were as described previously (Ligterink et al., 1997; Kroj et al., 2003; Ahlfors et al., 2004). Reactions were stopped by the addition of 2× SDS loading buffer, and the phosphorylation of an artificial substrate, the myelin basic protein, was detected following SDS-PAGE and phosphorimaging (Typhoon 8600; Molecular Dynamics).
Electrolyte Leakage Assays
All assays were performed in triplicate. Two inoculated leaf segments (approximately 5 cm) were added to 10 mL of deionized water (bathing solution). Leaves were then vacuum infiltrated (4 × 30 s) at 25 mbar pressure with complete release of vacuum in between. The infiltrated leaves were allowed to stand in the bathing solution for 1 h at room temperature. The leaves were then removed, the bathing solution was vortexed, and the ionic strength was monitored for conductivity as an indication of host membrane integrity.
Real-Time Quantitative PCR on Fungal DNA
Genomic DNA was isolated from 100 mg of infected leaf tissue (2- × 6-cm leaf segments) harvested on various days after inoculation, using a DNeasy Plant Mini Kit (Qiagen), following the supplier's instructions. Real-time quantitative PCR was performed in order to monitor levels of fungal biomass in infected leaf tissues using a Cy5-labeled probe to quantify the presence of the cytochrome b gene of M. graminicola (Fraaije et al., 2005). A 50-ng aliquot of DNA was used in a 20-μL PCR. Results were obtained from three replicate time course experiments.
RNA Isolation and Real-Time RT-PCR with SYBR Green Detection
Total RNA was isolated from leaf tissues infected by M. graminicola using the Trizol procedure (Invitrogen), following the supplier's protocol and incorporating the suggested additional procedures for polysaccharide-containing tissues. Further purification of the total RNA was achieved by precipitation from a solution of 4 m lithium chloride. Total RNA was used for all real-time RT-PCR analyses. Where appropriate, RNA species were analyzed by agarose gel electrophoresis using MOPS buffer and formaldehyde. For RT-PCR analysis, first-strand cDNA was synthesized from total RNA using the SuperScript III First_Strand Synthesis System for RT-PCR (Invitrogen). Five micrograms of total RNA primed with oligo(dT)20 was used in a 20-μL reaction, following the supplier's instructions. The resulting cDNA was analyzed using the QuantiTect SYBR Green PCR Kit (Qiagen), following the supplier's instructions. Then, 0.5 μL of cDNA was used in a 20-μL PCR, with an annealing temperature of 56°C. Primers were added at a final concentration of 0.25 μM. PCR was run and analyzed using an ABI 7500 Real Time PCR System, with β-tubulin acting as the endogenous control.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Quantification of fungal biomass in cultivars Cadenza and Avalon following inoculation with M. graminicola isolate IPO88004.
Supplemental Figure S2. Gene expression of TaMPK3 and TaMPK6 at 1, 4, and 8 d after inoculation of Avalon with isolate IPO323 (compatible) or isolate IPO88004 (incompatible).
Supplemental Figure S3. Western-blot analysis of MAPK activity levels during the early time points following mock, fungal compatible, and fungal incompatible inoculations.
Supplemental Figure S4. Posttranslational activation of TaMPK3 is also observed during symptom development in a compatible interaction between Riband wheat and isolate IPO323.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Gert Kema (Plant Research International) for the provision of M. graminicola isolates IPO323 and IPO88004. We gratefully acknowledge the help of Bart Fraaije and Hans Cools for real-time PCR analyses and John Lucas for critical reading of the manuscript.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom to Rothamsted Research and by non-BBSRC funds provided for preliminary Affymetrix microarray analyses and antibody production.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jason J. Rudd (jason.rudd@bbsrc.ac.uk).
The online version of this article contains Web-only data.
References
- Adhikari TB, Balaji B, Breeden J, Goodwin SB (2007) Resistance of wheat to Mycosphaerella graminicola involves early and late peaks of gene expression. Physiol Mol Plant Pathol 71 55–68 [Google Scholar]
- Adhikari TB, Cavaletto JR, Dubcovsky J, Gieco JO, Schlatter AR, Goodwin SB (2004) Molecular mapping of the Stb4 gene for resistance to Septoria tritici blotch in wheat. Phytopathology 94 1198–1206 [DOI] [PubMed] [Google Scholar]
- Ahlfors R, Macioszek V, Rudd J, Brosche M, Schlichting R, Scheel D, Kangasjarvi J (2004) Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis during ozone exposure. Plant J 40 512–522 [DOI] [PubMed] [Google Scholar]
- Arraiano LS, Brown JKM (2006) Identification of isolate-specific and partial resistance to Septoria tritici blotch in 238 European wheat cultivars and breeding lines. Plant Pathol 55 726–738 [Google Scholar]
- Arraiano LS, Chartrain L, Bossolini E, Slatter HN, Keller B, Brown JKM (2007) A gene in European wheat cultivars for resistance to an African isolate of Mycosphaerella graminicola. Plant Pathol 56 73–78 [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 977–983 [DOI] [PubMed] [Google Scholar]
- Beers EP, McDowell JM (2001) Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr Opin Plant Biol 4 561–567 [DOI] [PubMed] [Google Scholar]
- Brading PA, Verstappen ECP, Kema GHJ, Brown JKM (2002) A gene-for-gene relationship between wheat and Mycosphaerella graminicola, the Septoria tritici blotch pathogen. Phytopathology 92 439–445 [DOI] [PubMed] [Google Scholar]
- Ciuffetti LM, Touri RP, Gaventa JM (1997) A single gene encodes a selective toxin causal to the development of tan spot on wheat. Plant Cell 9 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Eyal Z (1993) The histology of processes associated with the infection of resistant and susceptible wheat cultivars with S. tritici. Plant Pathol 42 737–743 [Google Scholar]
- Cousin A, Mehrabi R, Guilleroux M, Dufresne M, Van der Lee T, Waalwijk C, Langin T, Kema GHJ (2006) The MAP kinase-encoding gene MgFus3 of the non-appressorium phytopathogen Mycosphaerella graminicola is required for penetration and in vitro pycnidia formation. Mol Plant Pathol 7 269–278 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42 185–209 [DOI] [PubMed] [Google Scholar]
- Ekengren SK, Liu YL, Schiff M, Dinesh-Kumar SP, Martin GB (2003) Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J 36 905–917 [DOI] [PubMed] [Google Scholar]
- Flor HH (1971) Current status of gene-for-gene concept. Annu Rev Phytopathol 9 275–276 [Google Scholar]
- Fraaije BA, Cools HJ, Fountaine J, Lovell DJ, Motteram J, West JS, Lucas JA (2005) Role of ascospores in further spread of QoI-resistant cytochrome b alleles (G143A) in field populations of Mycosphaerella graminicola. Phytopathology 95 933–941 [DOI] [PubMed] [Google Scholar]
- Friesen TL, Stukenbrock EH, Liu ZH, Meinhardt S, Ling H, Faris JD, Rasmussen JB, Solomon PS, McDonald BA, Oliver RP (2006) Emergence of a new disease as a result of interspecific virulence gene transfer. Nat Genet 38 953–956 [DOI] [PubMed] [Google Scholar]
- Gamba FM, Lamari L, Brule-Babel AL (1998) Inheritance of race-specific necrotic and chlorotic reactions induced by Pyrenophora tritici-repentis in hexaploid wheats. Can J Plant Pathol 20 401–407 [Google Scholar]
- Goodwin SB (2004) Minimum phylogenetic coverage: an additional criterion to guide the selection of microbial pathogens for initial genomic sequencing efforts. Phytopathology 94 800–804 [DOI] [PubMed] [Google Scholar]
- Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10 751–757 [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Yao N (2004) The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol 6 201–211 [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG (1997) Plant disease resistance genes. Annu Rev Plant Physiol Plant Mol Biol 48 575–607 [DOI] [PubMed] [Google Scholar]
- Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44 321–334 [DOI] [PubMed] [Google Scholar]
- Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang SQ, Hirt H, Wilson C, et al (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7 301–308 [DOI] [PubMed] [Google Scholar]
- Jin HL, Liu YD, Yang KY, Kim CY, Baker B, Zhang SQ (2003) Function of a mitogen-activated protein kinase pathway in N gene-mediated resistance in tobacco. Plant J 33 719–731 [DOI] [PubMed] [Google Scholar]
- Kema GHJ, Verstappen ECP, Waalwijk C (2000) Avirulence in the wheat Septoria tritici leaf blotch fungus Mycosphaerella graminicola is controlled by a single locus. Mol Plant Microbe Interact 13 1375–1379 [DOI] [PubMed] [Google Scholar]
- Kema GHJ, Yu DZ, Rijkenberg FHJ, Shaw MW, Baayen RP (1996) Histology of the pathogenesis of Mycosphaerella graminicola in wheat. Phytopathology 86 777–786 [Google Scholar]
- Keon J, Antoniw J, Carzaniga R, Deller S, Ward JL, Baker JM, Beale MH, Hammond-Kosack KE, Rudd JJ (2007) Transcriptional adaptation of Mycosphaerella graminicola to programmed cell death of its susceptible wheat host. Mol Plant Microbe Interact 20 178–193 [DOI] [PubMed] [Google Scholar]
- Keon J, Antoniw J, Rudd JJ, Skinner W, Hargreaves J, Hammond-Kosack KE (2005. a) Analysis of expressed sequence tags from the wheat leaf blotch pathogen Mycosphaerella graminicola (anamorph Septoria tritici). Fungal Genet Biol 42 376–389 [DOI] [PubMed] [Google Scholar]
- Keon J, Bailey A, Hargreaves J (2000) A group of expressed cDNA sequences from the wheat fungal leaf blotch pathogen, Mycosphaerella graminicola (Septoria tritici). Fungal Genet Biol 29 118–133 [DOI] [PubMed] [Google Scholar]
- Keon J, Rudd JJ, Antoniw J, Skinner W, Hargreaves J, Hammond-Kosack KE (2005. b) Metabolic and stress adaptation by Mycosphaerella graminicola during sporulation in its host revealed through microarray transcription profiling. Mol Plant Pathol 6 527–540 [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Durner J (2004) Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol Plant Microbe Interact 17 131–139 [DOI] [PubMed] [Google Scholar]
- Kroj T, Rudd JJ, Nürnberger T, Gabler Y, Lee J, Scheel D (2003) Mitogen-activated protein kinases play an essential role in oxidative burst-independent expression of pathogenesis-related genes in parsley. J Biol Chem 278 2256–2264 [DOI] [PubMed] [Google Scholar]
- Kwon CY, Rasmussen JB, Meinhardt SW (1998) Activity of Ptr ToxA from Pyrenophora tritici-repentis requires host metabolism. Physiol Mol Plant Pathol 52 201–212 [Google Scholar]
- Lee J, Klessig DF, Nürnberger T (2001) A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell 13 1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Rudd JJ, Macioszek VK, Scheel D (2004) Dynamic changes in the localization of MAPK cascade components controlling pathogenesis-related (PR) gene expression during innate immunity in parsley. J Biol Chem 279 22440–22448 [DOI] [PubMed] [Google Scholar]
- Ligterink W, Kroj T, zur Nieden U, Hirt H, Scheel D (1997) Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276 2054–2057 [DOI] [PubMed] [Google Scholar]
- Lincoln JE, Richael C, Overduin B, Smith K, Bostock R, Gilchrist DG (2002) Expression of the antiapoptotic baculovirus p35 gene in tomato blocks programmed cell death and provides broad-spectrum resistance to disease. Proc Natl Acad Sci USA 99 15217–15221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YD, Jin HL, Yang KY, Kim CY, Baker B, Zhang SQ (2003) Interaction between two mitogen-activated protein kinases during tobacco defense signaling. Plant J 34 149–160 [DOI] [PubMed] [Google Scholar]
- Liu YD, Ren DT, Pike S, Pallardy S, Gassmann W, Zhang SQ (2007) Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J 51 941–954 [DOI] [PubMed] [Google Scholar]
- Lorang JM, Sweat TA, Wolpert TJ (2007) Plant disease susceptibility conferred by a “resistance” gene. Proc Natl Acad Sci USA 104 14861–14866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GB, Bogdanove AJ, Sessa G (2003) Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol 54 23–61 [DOI] [PubMed] [Google Scholar]
- Mayrose M, Bonshtien A, Sessa G (2004) LeMPK3 is a mitogen-activated protein kinase with dual specificity induced during tomato defense and wounding responses. J Biol Chem 279 14819–14827 [DOI] [PubMed] [Google Scholar]
- Menke FLH, van Pelt JA, Pieterse CMJ, Klessig DF (2004) Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell 16 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchuk Z, Eulgem T, Holt BE, Dangl JL (2003) Recognition and response in the plant immune system. Annu Rev Genet 37 579–609 [DOI] [PubMed] [Google Scholar]
- Nimchuk Z, Rohmer L, Chang JH, Dangl JL (2001) Knowing the dancer from the dance: R-gene products and their interactions with other proteins from host and pathogen. Curr Opin Plant Biol 4 288–294 [DOI] [PubMed] [Google Scholar]
- Nirmala J, Dahl S, Steffenson BJ, Kannangara CG, von Wettstein D, Chen X, Kleinhofs A (2007) Proteolysis of the barley receptor-like protein kinase RPG1 by a proteasome pathway is correlated with Rpg1-mediated stem rust resistance. Proc Natl Acad Sci USA 104 10276–10281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger T, Lipka V (2005) Non-host resistance in plants: new insights into an old phenomenon. Mol Plant Pathol 6 335–345 [DOI] [PubMed] [Google Scholar]
- Pedley KF, Martin GB (2004) Identification of MAPKs and their possible MAPK kinase activators involved in the Pto-mediated defense response of tomato. J Biol Chem 47 49229–49235 [DOI] [PubMed] [Google Scholar]
- Rasmussen JB, Kwon CY, Meinhardt SW (2004) Requirement of host signaling mechanisms for the action of Ptr ToxA in wheat. Eur J Plant Pathol 110 333–335 [Google Scholar]
- Ray S, Anderson JM, Urmeev FI, Goodwin SB (2003) Rapid induction of a protein disulfide isomerase and defense-related genes in wheat in response to the hemibiotrophic fungal pathogen Mycosphaerella graminicola. Plant Mol Biol 53 741–754 [DOI] [PubMed] [Google Scholar]
- Ren DT, Yang HP, Zhang SQ (2002) Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem 277 559–565 [DOI] [PubMed] [Google Scholar]
- Ren DT, Yang KY, Li GJ, Liu YD, Zhang SQ (2006) Activation of Ntf4, a tobacco mitogen-activated protein kinase, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol 141 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna NS, Yang Y (2006) Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Mol Plant Microbe Interact 19 530–540 [DOI] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Zhang SQ, Klessig DF, Hirt H, Jones JDG (1999) Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11 273–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson DE, Heath MC (1996) Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty NP, Kristensen BK, Newman MA, Møller K, Gregersen PL, Jørgensen HJL (2003) Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol Mol Plant Pathol 62 333–346 [Google Scholar]
- Shetty NP, Mehrabi R, Lutken H, Haldrup A, Kema GHJ, Collinge DB, Jørgensen HJL (2007) Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol 174 637–647 [DOI] [PubMed] [Google Scholar]
- Stakman EC (1915) Relations between Puccinia graminis and plants highly resistant to its attack. J Agric Res 4 193–199 [Google Scholar]
- Stulemeijer IJE, Stratmann JW, Joosten M (2007) Tomato mitogen-activated protein kinases LeMPK1, LeMPK2, and LeMPK3 are activated during the Cf-4/Avr4-induced hypersensitive response and have distinct phosphorylation specificities. Plant Physiol 144 1481–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Mori T, Shinogi T, Yao N, Takahashi S, Betsuyaka S, Sakamoto M, Park P, Nakayashiki H, Tosa Y, et al (2004) Nitric oxide and reactive oxygen species do not elicit hypersensitive cell death but induce apoptosis in the adjacent cells during the defense response of oat. Mol Plant Microbe Interact 17 245–253 [DOI] [PubMed] [Google Scholar]
- Takezawa D (1999) Elicitor- and A23187-induced expression of WCK-1, a gene encoding mitogen-activated protein kinase in wheat. Plant Mol Biol 40 921–933 [DOI] [PubMed] [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Doczi F, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15 141–152 [DOI] [PubMed] [Google Scholar]
- van Baarlen P, Staats M, van Kan JAL (2004) Induction of programmed cell death in lily by the fungal pathogen Botrytis elliptica. Mol Plant Pathol 5 559–574 [DOI] [PubMed] [Google Scholar]
- van Kan JAL (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11 247–253 [DOI] [PubMed] [Google Scholar]
- Wolpert TJ, Dunkle LD, Ciuffetti LM (2002) Host-selective toxins and avirulence determinants: what's in a name? Annu Rev Phytopathol 40 251–285 [DOI] [PubMed] [Google Scholar]
- Xiong LZ, Yang YN (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 15 745–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K-Y, Liu Y, Zhang S (2001) Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA 98 741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Tada Y, Park P, Nakayashiki H, Tosa Y, Mayama S (1991) Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. Plant J 28 13–26 [DOI] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (1998. a) Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc Natl Acad Sci USA 95 7433–7438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (1998. b) The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc Natl Acad Sci USA 95 7225–7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Klessig DF (2000) Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J 23 1–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.