Abstract
Since the last common ancestor shared by modern humans, chimpanzees and bonobos, the lineage leading to Homo sapiens has undergone a substantial change in brain size and organization. As a result, modern humans display striking differences from the living apes in the realm of cognition and linguistic expression. In this article, we review the evolutionary changes that occurred in the descent of Homo sapiens by reconstructing the neural and cognitive traits that would have characterized the last common ancestor and comparing these with the modern human condition. The last common ancestor can be reconstructed to have had a brain of approximately 300–400 g that displayed several unique phylogenetic specializations of development, anatomical organization, and biochemical function. These neuroanatomical substrates contributed to the enhancement of behavioral flexibility and social cognition. With this evolutionary history as precursor, the modern human mind may be conceived as a mosaic of traits inherited from a common ancestry with our close relatives, along with the addition of evolutionary specializations within particular domains. These modern human-specific cognitive and linguistic adaptations appear to be correlated with enlargement of the neocortex and related structures. Accompanying this general neocortical expansion, certain higher-order unimodal and multimodal cortical areas have grown disproportionately relative to primary cortical areas. Anatomical and molecular changes have also been identified that might relate to the greater metabolic demand and enhanced synaptic plasticity of modern human brain's. Finally, the unique brain growth trajectory of modern humans has made a significant contribution to our species’ cognitive and linguistic abilities.
Keywords: brain evolution, cognition, great ape, human evolution, language
‘What would have to be true – not only of the quaint folk across the river, but of chimpanzees, dolphins, gaseous extraterrestrials, or digital computers (things in many ways quite different from the rest of us) – for them to be correctly counted among us? ... When we ask, Who are we? or What sort of thing are we? the answers can vary without competing. Each one defines a different way of saying “we”; each kind of “we”-saying defines a different community, and we find ourselves in many communities. This thought suggests that we think of ourselves in broadest terms as the ones who say “we”.’ (Brandom, 1994)
Introduction
‘We’ are Homo sapiens and our species’ intellectual abilities distinguish us from all other animals. Our technological sophistication, capacity for introspection, and ability to create and manipulate symbols is unrivalled. We engage in behaviors that are profoundly unique, such as the production of personal ornamentation, language, art and music, and the performance of religious rituals. This behavioral discontinuity has prompted many to regard modern humans as standing apart from the rest of nature. Yet, despite our distinctiveness, ‘we’ are also one among several species of great ape, displaying more than 99% nonsynonymous DNA sequence similarity with chimpanzees (Wildman et al. 2003), having diverged from each other approximately 4–8 Ma (Bradley, 2008). Consequently, modern humans share many phenotypic traits with these close relatives through common descent. The tension between striking behavioral divergence in the face of phylogenetic continuity presents a puzzle. Although many authors have discussed the possible selective advantages and evolutionary processes underlying the emergence of modern human cognition (e.g. Holloway, 1967; Calvin, 1994; Dunbar, 1996; Tomasello, 1999; Tooby & Cosmides, 2005), it still remains a serious challenge to understand how the unique features of modern human behavior are mapped onto evolutionary changes in neural structure.
Considering the dramatic behavioral differences between modern humans and other animals, it is reasonable to expect similarly remarkable alterations in brain organization. As Darwin noted in The Descent of Man (1871), there appears to be a link between our intelligence and our expanded brain, which increased in size by roughly threefold since the last common ancestor (LCA) shared by hominins (the lineage including modern humans and our fossil close relatives and ancestors) and panins (the lineage including common chimpanzees, bonobos, and their fossil close relatives and ancestors). Because a large brain size so clearly distinguishes modern humans, many theories of human cognitive evolution consider only this single anatomical variable to account for the myriad specialized behaviors we exhibit (e.g. Jerison, 1973; Dunbar, 1996).
However, modern human-specific traits have been described at many different levels of neural organization, including gross brain size, the relative extent of neocortical areas, asymmetry, developmental patterning, the distribution of cell types, histology, and gene expression. Thus, while increased brain size, comprising mostly growth of the neocortex (Finlay & Darlington, 1995), undoubtedly has been central to the evolution of modern human cognition, other modifications to brain development, structure, and function are also certain to be significant. Furthermore, explaining modern human behavioral distinctiveness simply as a secondary byproduct of brain enlargement leaves unanswered fundamental questions regarding the computational substrates of our species-specific behavioral capacities (Holloway, 1968). Does it even make sense to ask how many ‘extra’ grams of neocortical tissue are necessary for the development of recursive syntax, pair-bondedness, or ‘theory of mind’? Indeed, abundant data from the neurosciences show that changes in structural modularity and connectivity interact with variation in molecular and neurochemical signaling to determine brain function. Subtle modifications in neural microstructure and gene expression can have a significant impact on behavior, even in the absence of large-scale changes in the size of brain parts (e.g. Hammock & Young, 2005). Evolutionary processes, therefore, can mold behavioral phenotypes using a host of strategies.
In this context, the aim of this article is to examine how changes in brain anatomy and physiology articulate with unique aspects of modern human cognition. We employ a multidisciplinary approach to trace evolutionary changes in mind and brain from the LCA to modern Homo sapiens, incorporating evidence from comparative psychology, neuroscience, genetics, paleoanthropology, and linguistics. By providing a detailed contrast between the mind of the LCA and Homo sapiens, it is our intent to bring into relief the distinctive characteristics of modern humans against the background of what is inherited from our most recent ancestry.
Although it would be desirable to trace the course of mental evolution through the succession of extinct species that fall along the lineage leading to modern humans, the fossil evidence is frustratingly scant. Unlike some other adaptations in human evolution that show reliable hard tissue correlates, such as the transition to habitual bipedalism (Lovejoy, 2005), behavior and soft tissue do not fossilize. Therefore, the paleonotological record for these traits in human evolution is limited to what can be gleaned from endocranial casts and archaeological evidence (Mithen, 1996; Holloway et al. 2004). As endocasts preserve only an impression of the external morphology of the brain, critical information regarding internal neuroanatomical organization cannot be determined. Behavioral abilities, furthermore, can only be glimpsed opaquely through material remains. However, the paleontological and archaeological records constitute the only direct evidence of temporal change in morphology and behavior, providing crucial insight regarding their association. Paleontological evidence, in fact, indicates that major innovations in cultural behavior were not always linked to upsurges in cranial capacity of fossil hominins. For example, early signs of animal butchery are found in association with Australopithecus garhi(Asfaw et al. 1999), suggesting that a small-brained (450 cm3 cranial capacity) East African hominin from 2.5 Ma might have had the capacity to fashion simple stone tools.
The comparative approach, although an indirect source of information regarding evolution, provides a greater opportunity to explore the relationship between biological diversification and its correlates. In addition, by analyzing the distribution of neuroanatomical, behavioral, and genetic character states that are present in contemporary species within an established phylogeny, the principle of parsimony may be used to make reasonable inferences concerning the condition of extinct ancestral taxa (Johnson et al. 1984; Northcutt & Kaas, 1995). Of course, all living species are the product of their own evolutionary trajectory and cannot be considered stand-ins for fossil ancestors. Nevertheless, when a character state is observed only in modern humans and not in any of the other extant hominids (the clade that includes the living great apes and modern humans), then it is reasonable to conclude that the modern human condition is derived compared to the symplesiomorphic state seen in the great apes. In this review, we rely heavily on comparative data and the types of inference used in cladistic analysis to reconstruct the natural history of the modern human mind (as such, unless stated otherwise, any subsequent reference to ‘humans’ pertains to modern humans).
To clearly distinguish what features are evolutionarily derived in recent human evolution, in the first part of this article we reconstruct the behavioral, cognitive, and neuroanatomical characteristics that we predict would have been present in the LCA. We draw particular attention to the features that generally distinguish hominids from other primates. In so doing, we highlight the cognitive and neural features that were derived character traits in our most recent evolutionary ancestry and which set the stage for the dramatic further modifications that were to take place in the Plio-Pleistocene within the lineage leading to Homo sapiens. This narrow focus on only recent human evolutionary history means that our account does not detail many important characteristics that arose at deeper nodes in our family tree. For example, the orientation of human cognition towards social problem solving is the product of a long primate heritage (Cheney & Seyfarth, 1990, 2007). Similarly, our capacity for fine-grained hand–eye coordination derives from selection long ago for visually guided reaching performance in stem primates (Cartmill, 1992).
Next, we review the cognitive and neural features that are uniquely present in Homo sapiens and which differ from the conditions that characterized the LCA. Because language is such a fundamental component of the human behavioral phenotype, we focus special attention on examining how this communication system differs from that used by other species. At the outset it should be acknowledged that establishing a clear causal link between evolutionary changes in brain structure and the emergence of species-specific behavior is complicated for a number of reasons – anatomical homology does not necessarily entail functional similarity; also, our present understanding of transcriptional, cyto- and chemoarchitectural scaling allometry is extremely rudimentary and some apparent human-specific differences may simply be related to maintaining functional equivalence in the context of biochemical, physiological, and geometric constraints at larger overall brain size. To compound the problem, because there are so few detailed studies of neuroanatomical organization comparing humans and great apes, many of the unique characteristics of the human brain are currently hidden from view. Therefore, we do not expect there to be a straightforward correspondence between every anatomical and every behavioral characteristic that we discuss.
We conclude by outlining a preliminary model to explain how changes in brain size and other aspects of neuroanatomical reorganization might yield domain-general cognitive specializations with emergent domain-specific skills.
Reconstruction of the hominin and panin LCA – the behavioral phenotype
Diet and social organization
It has proven easier to distinguish great apes from other primates based on dietary and ecological variables than on cognitive specializations. Because of the generalized dental anatomy of living hominids, these species rely heavily on mature, nonfibrous fruits with high sugar and calorie content. As a consequence of this diet, the great apes occupy a fairly narrow range of ecological habitats, being largely restricted to tropical and woodland forests (Foley & Lee, 1989; Potts, 2004). Paleoecological and dental evidence suggests that the middle Miocene hominids, presumably including the common ancestor of living great apes, consumed a varied frugivorous diet that incorporated opportunistic, perhaps seasonal, utilization of hard objects such as nuts and seeds (Singleton, 2004).
The social organization of modern panins reflects one solution to the foraging challenge posed by the hominid diet. Chimpanzee societies were first described as ‘fission-fusion’ by Jane Goodall (1986) to highlight the fluid nature of their associations. The unique fission-fusion social grouping of chimpanzees affords individuals the benefits of gregarious living, including predator defense, access to mates, and the opportunity to locate widely distributed foods, while simultaneously minimizing direct contest competition over food. In fission-fusion societies, individuals see each other infrequently, with some intervals of separation lasting as long as a week. Yet all individuals recognize each other and maintain their affiliations and alliances despite these relatively long separations (Goodall, 1986). Within the fission-fusion organization of chimpanzees certain age- and sex-specific subgroups appear to have particular social functions. For instance, groups of juveniles may serve as territory patrols for the community (Wrangham & Peterson, 1996; Mitani, 2006), adult males form hunting parties (Boesch, 2002; Mitani, 2006), and females and their offspring are largely associated with tool-use and other subsistence technologies such as nut-cracking (Boesch & Boesch, 1990). The segregation of individuals by sex and age for specific social activities is arguably unique to the great apes – specifically, chimpanzees – and virtually absent in monkeys, including those with societies that resemble a fission-fusion social organization, such as the hamadryas baboon.
Social learning and ‘traditions’
Another aspect of social behavior in great apes that appears to be unique among primates concerns regional traditions (Subiaul, 2007). All great apes, but no monkey species, possess a suite of behaviors that include gestures and styles of object manipulations that are distinctive for a given social group/community, persist from generation to generation, and are transmitted horizontally through social learning (Whiten, 2005; Horner et al. 2006). The traditions of chimpanzees are by far the best documented and also appear to be the most widespread and diverse of any nonhuman primate species. Whiten and colleagues (1999, 2001) reported 39 different traditions in various African chimpanzee communities that included tool use, grooming, and mating practices. Some of these traditions were customary or habitual in some chimpanzee groups but absent in others after controlling for ecological constraints (e.g. availability of certain raw materials). Using the same systematic approach to the documentation of traditions in nonhuman primates, van Schaik and colleagues (2003) reported at least 19 clearly defined traditions in orangutans. This stands in contrast to reports of traditions in monkeys, whales, birds, and fish, where at most a handful (usually only one or two) have been identified (e.g. song ‘dialect’ in birds and whales). In none of these cases has the number of behavioral variants reached double digits (Rendell & Whitehead, 2001; Fragaszy & Perry, 2003). Although there is always the possibility that such a result is due to over-representation of great apes in the sample, it is notable that despite many years of research on several monkey species (including macaques, baboons, and capuchin monkeys), only capuchin monkeys evidence behaviors that potentially meet the criteria for traditions (Panger et al. 2002; Perry et al. 2003, but see Subiaul, 2007). Previous claims of ‘proto-culture’ in Japanese macaques (Kawai, 1965), for example, are no longer considered to be ‘cultural’ as they do not conform to contemporary standards of non-human ‘traditions’ (e.g. Whiten et al. 1999; van Schaik et al. 2003).
One lingering question concerns how such complex and unique behaviors as hunting, patrolling, and cultural traditions map onto the various cognitive abilities known to distinguish the great apes from other primates. Below we discuss some cognitive skills that appear to be unique to great apes and that may shed some light on this question (for a more extensive review, please see Subiaul et al. 2006).
Self-awareness
Starting in the 1970s, a number of studies explored chimpanzees’ kinesthetic perception of the self via mirror self-recognition (Gallup, 1970). In these studies, it was demonstrated that chimpanzees use their reflections to explore body parts, such as the underarms, teeth, and anogenital region, which are difficult to see without the aid of a mirror. In contrast, after lengthy exposures to mirrors, monkeys continue to display social behaviors toward their mirror image, which suggests that they fail to see their reflections as representations of themselves. Additional research has reported mirror self-recognition in orangutans (Lethmate & Dücker, 1973; Suarez & Gallup, 1981), but most gorillas fail to recognize their mirror image (Suarez & Gallup, 1981; Ledbetter & Basen, 1982; Shilito et al. 1999), with one exception (Patterson & Cohn, 1994). Subsequent studies with monkeys confirmed Gallup's initial negative findings (e.g. Suarez & Gallup, 1981; Hauser et al. 2001; de Waal et al. 2005).
Povinelli & Cant (1995) have hypothesized that mirror self-recognition in great apes may be an emergent property of being a large-bodied primate that spends a significant amount of time navigating the complex three-dimensional environment of trees, constantly monitoring where to place limbs to support the body during travel. However, Povinelli & Cant's (1995) ‘clambering hypothesis’ has been challenged by evidence of mirror self-recognition in animals whose habitats do not require arboreal locomotion. Today there are reports that bottlenose dolphins (Reiss & Marino, 2001) may recognize themselves in the mirror – at the very least, they do not seem to treat their reflection as if it were another individual. Studies on elephants, however, are more equivocal. One study reported that elephants engaged in mirror-directed reaching but did not identify themselves in the mirror and behaved aggressively toward their image (Povinelli, 1989). However, Plotnik and colleagues (2006) reported that one of three elephants studied showed evidence of mirror self-recognition. The possibility that different mammalian lineages are ‘self-aware’ presents at least two possibilities: (1) mirror self-recognition is an emergent property present in species with large brain size and a complex social organization or (2) there are multiple adaptive functions to the cognitive ability that is measured by mirror-self recognition and, consequently, this skill emerged independently in numerous mammalian species.
Gaze-following
Great apes are acutely sensitive to the direction of others’ gaze. Determining the precise direction of another's attention is an important ability because it can provide salient information about the location of objects such as food and predators. In social settings, a great deal of information is communicated by means of following other individuals’ gaze to specific individuals or to call attention to specific events.
Many primate species engage in social activities, including tracking allies, that likely require following the gaze of conspecifics (e.g. Chance, 1967; Menzel & Halperin, 1975; Whiten & Byrne, 1988; Mitani, 2006). However, in field studies, it is often difficult to identify which object, individual, or event is the focus of two individuals’ attention and whether they arrived at the focal point by following one another's gaze. Laboratory studies have confirmed that great apes and, to a lesser extent, monkeys follow the gaze of others to objects (e.g. chimpanzees, mangabeys, and macaques; Emery et al. 1997; Itakura & Tanaka, 1998; Tomasello et al. 1993, 2001; Tomonaga, 1999). In one of the few explicitly comparative studies of this behavior, Itakura (1996) examined the ability of various primate species to follow a human experimenter's gaze. Notably, in this paradigm only chimpanzees and one orangutan responded above chance levels. Okamoto-Barth and colleagues (2007) have extended these results with a refined method that included barriers with and without windows. They conclude that chimpanzees, bonobos, and gorillas are more sensitive to another's line of sight than are orangutans.
One method commonly used in the laboratory to investigate nonhuman primates’ ability to use gaze cues is the ‘object-choice task’. In this task, an experimenter attends to one of two containers (controls usually include directing the face and eyes to one container or looking askance at one container, while facing forward) while subjects are given the opportunity to choose a container, only one of which is baited. The available research suggests that there is a significant difference between monkeys’ and great apes’ understanding of gaze cues in the object-choice task (see also Itakura & Anderson, 1996). Monkeys generally cannot be trained to use only the human experimenter's gaze cues to retrieve the concealed reward (Anderson et al. 1995, 1996), whereas great apes can (Itakura & Anderson, 1996). Povinelli & Eddy (1996a,b) have hypothesized that great apes outperform monkeys on this task because they possess an automatic response that forms part of a primitive orienting reflex triggered by a reward. This reflex, however, does not require the attribution of a mental state or an understanding of the psychological state underpinning ‘seeing’. Another possibility is that sensitivity to eyes, in particular, co-evolved with the ability to make inferences about certain psychological states such as seeing. In support of this latter hypothesis, Hare and colleagues (Hare et al. 2000, 2001, 2006; Hare & Tomasello, 2004) have argued that chimpanzees use the direction of gaze to reason about the intentions of conspecifics. Santos and her colleagues (Flombaum & Santos, 2005) have reached similar conclusions with rhesus macaques based on a comparable experimental paradigm. Although controversial, these tasks and results raise the possibility that catarrhine primates (including Old World monkeys and apes) either share a system that binds observable features (e.g. eyes) with unobservable concepts such as ‘seeing’ or that all primates share a primitive system that can only construct concepts based on observable features but not unobservable causes (Povinelli, 2000; Povinelli & Vonk, 2003).
Physical cognition
Some have argued that chimpanzees and other great apes have a more sophisticated understanding of physical causality than monkeys, as reflected by their tool-use in the wild (Visalberghi, 1990; Limongelli et al. 1995; Visalberghi et al. 1995; Westergaard, 1999). This conclusion is buttressed by the fact that traditions as they exist in chimpanzees and orangutans are mostly absent in monkeys. And where they exist, as appears to be the case in capuchin monkeys (Panger et al. 2002; Perry et al. 2003), they comprise only two or three behaviors, which lack the diversity and complexity that characterize chimpanzee and orangutan behavioral traditions (Subiaul, 2007; Whiten & van Schaik, 2007). But is there any evidence of differences in the physical cognition skills of apes and monkeys? Research with monkeys has shown that they disregard non-functional surface features, such as color and shape, when choosing a tool, but they fail to appreciate how changes in shape affect changes in function (Hauser, 1997a; Hauser et al. 1999, 2002b, 2002c; Santos & Hauser, 2002; Fujita et al. 2003; Santos et al. 2005; but see Santos et al. 2006). Povinelli (2000) reported similar results for chimpanzees. In a series of studies, Povinelli (2000) presented chimpanzees with tasks that involved actions commonly seen in the wild such as pulling, pushing, and poking. Following training, subjects were presented with a choice of method: one was consistent with a theory of intrinsic connection (transfer of force), whereas the other choice was consistent with a theory of superficial contact. With very few exceptions, superficial and/or perceptual contact guided the chimpanzees’ responses across the various tool tasks. Thus, great apes’ understanding of simple mechanics may not differ substantially from that of monkeys.
An important facet of physical cognition is the ability to quantify objects in one's environment. As such, many animals (birds, rodents and primates) have evidenced numerical knowledge (Brannon, 2006). Some of the most important work in this area has demonstrated that primates likely share a non-verbal system for ordering small and large numerosities (Cantlon & Brannon, 2006). Specifically, research suggests that monkeys, apes, and humans share a system for adding (chimpanzees: Rumbaugh et al. 1987; Boysen & Bernston, 1995; Boysen et al. 1993, 1996; Beran, 2001; Herrmann et al. 2007; rhesus macaques: Cantlon & Brannon, 2007) as well as subtracting quantities (chimpanzee: Beran, 2001; monkeys: Sulkowski & Hauser, 2001). Additionally, research with rhesus monkeys and chimpanzees has demonstrated that the ability to represent and quantify objects in one's environment is modality independent. In one study, rhesus monkeys in a laboratory setting matched the number of vocalizations that they heard with the number of faces that they saw (e.g. 2 vs. 3) (Jordan et al. 2005). This result corresponds with field research demonstrating that wild chimpanzees on patrol compare the number of vocalizations generated by ‘foreign’ chimpanzees with the number of individuals in their own group, retreating if the number of vocalizations exceeds the number of individuals in their own group (Wilson et al. 2002).
Such experiments on physical and numerical cognition suggest that there are no significant qualitative differences between chimpanzees’ and monkeys’ understanding of the non-verbal aspects of number or of unobservable physical causes. Therefore, the differences between great apes’ and monkeys’ tool-use in the wild may reflect factors such as greater manual dexterity and fine motor coordination, as well as social-cognitive variables such as the ability to benefit from social conventions and copy novel motor rules.
Social tolerance
Affective and temperamental differences may also contribute to phylogenetic variation in behavioral performance (Hauser, 2003; Beran & Evans, 2006; Evans & Beran, 2007). In particular, great apes appear to be able to delay gratification longer than rhesus monkeys (Beran & Evans, 2006; Evans & Beran, 2007) and may be more tolerant of conspecifics than monkeys (Goodall, 1986; van Schaik et al. 1999; Brosnan et al. 2005). But there are also differences in tolerance among the great apes. These differences affect cognitive performance in certain domains. For instance, tolerance appears to play a major role in the frequency and diversity of cooperative behaviors in chimpanzees and bonobos. Specifically, in a cooperative feeding task, bonobos were found to be more tolerant of co-feeding than chimpanzees (Hare et al. 2007). However, when the task involved retrieving food that was difficult to monopolize, there were no differences between chimpanzees and bonobos. Aside from cooperation, tolerance and inhibitory control may similarly also affect performance in physical and spatial cognition tasks (Herrmann et al. 2007).
Such results point to subtle temperamental variables that in some instances have a significant effect on cognitive functioning. These results suggest that the great apes’ more sophisticated social and physical cognition skills rest in part on greater inhibitory control relative to monkeys – an executive function of the prefrontal cortex – which may allow them to better focus attention, in turn enhancing learning and memory (Call, 2007). Temperament and inhibitory control may be the target of directional selection insofar as they may result in greater behavioral flexibility and learning, offering individuals the opportunity to exploit new niches in their social or physical environments, resulting in increased fitness. It is possible that such subtle changes in temperament yield qualitatively distinct behavioral repertoires between species.
Given the differences between monkeys and great apes discussed above, what may be said about the LCA? We hypothesize that changes in the paleoenvironment in the late Miocene resulting in a wider distribution of woodlands produced a trend among the African great apes toward more stable relationships between the sexes and stronger associations between male kin (Foley & Lee, 1989). The LCA likely lived in an environment where the patchiness of food led to a wide distribution of females, selecting for male kin to form cooperative coalitions to defend an expansive home range (Foley & Lee, 1989). Given the evidence reviewed above, we conclude that the LCA displayed regional variation in certain behavioral traditions, ‘self-awareness’, and an enhanced ability to follow the gaze of other social agents. We further hypothesize that these behavioral characteristics are related to increased capacities of executive control to inhibit conventional responses in favor of social tolerance and seeking novel and flexible solutions to problems. These behavioral abilities would have been advantageous for a large-bodied, frugivorous, social primate in the late Miocene.
Reconstruction of the hominin and panin LCA – the neuroanatomical phenotype
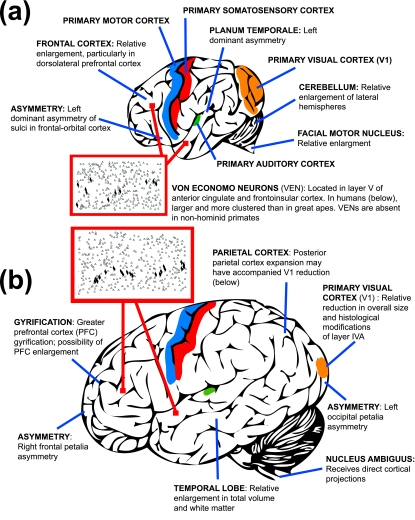
As a catarrhine primate, the LCA's brain would have been specialized for social cognition (Cheney & Seyfarth, 2007). In both macaque monkeys and humans, ventral premotor and inferior parietal cortex contain neurons that fire when an individual either performs or observes different goal-directed actions (Rizzolatti et al. 2002; Arbib, 2005). This ‘mirror neuron system’ potentially serves as a substrate for understanding others’ actions, imitating new skills, and simulating others’ intentions. Furthermore, catarrhine brains also contain separate populations of neurons in the temporal cortex that are selective for the direction of others’ gaze, facial expressions and identity (reviewed in Hauser, 1997), as well as species-specific vocal calls (Poremba et al. 2004; Gil-da-Costa et al. 2006). In addition to these, there are several features of extant great ape brains that distinguish them collectively from other primates (MacLeod, 2004; Sherwood & Hof, 2007). It is most parsimonious to conclude that these traits evolved in the great ape stem approximately 14 Ma and therefore would have also been present in the LCA. Here we focus on the neocortical phenotype of living hominids, with some reference to other brain systems, drawing particular attention to reorganization at the histological and molecular level (Fig. 1a).
Fig. 1.
A summary of several neuroanatomical features that are distinctive in the LCA (a) and in modern humans (b). Von Economo neurons are shown as black spindle-shaped cells among pyramidal neurons, represented as triangles. Modified in part from Nimchinsky et al. (1999).
Large brain size
All living hominids have large brain sizes in absolute terms and in some measures of relative size (Passingham, 1975a; Holloway, 1983; Martin, 1983). Similarly, the LCA is expected to have had a brain mass of approximately 300–400 g. This size is within the range of extant great apes and is close to the cranial capacity of the earliest hominins (Holloway et al. 2004). Furthermore, the cranial capacities of some late Miocene apes, such as Dryopithecus, are within the lower end of this range (300–330 cm3) (Begun, 2004). Comparative studies suggest that positive selection occurred in genes associated with primary microcephaly (i.e. ASPM and microcephalin) along the phylogenetic lineage leading to the LCA (Kouprina et al. 2004; Wang & Su, 2004); however, it is less clear whether these genetic variants are related to normal brain size variation and evolution (Woods et al. 2006). Accompanying enlarged absolute brain size, the LCA probably also had a high degree of cerebral cortical gyrification as compared with other primates (Connolly, 1950). Although not differing significantly from allometric scaling predictions (Zilles et al. 1989), the amount of gyral folding in living great apes suggests that there is relatively more associational connectivity between neighboring cortical regions, as gyri are thought to form due to tension-based mechanisms that bring strongly interconnected regions more closely together, achieving spatially compact wiring (van Essen, 1997).
Population-level gross anatomical asymmetries
The human brain displays strong population-level left hemisphere dominance for language functions, especially among right-handed individuals. Because language is clearly a unique behavioral innovation in humans, it has historically been thought that such lateralization is a requisite for the expression of this complex adaptation. Recent studies have shown that some of the cortical areas associated with language function in humans also display asymmetries in extant great apes, suggesting that they were present in the LCA. Specifically, population-level left hemisphere dominant asymmetry of the planum temporale, a surface feature of the cerebral cortex in the region of Wernicke's area, is shared by humans, chimpanzees, bonobos, gorillas, and orangutans (Gannon et al. 1998; Hopkins et al. 1998). A recent study, in fact, indicates that the volume of cytoarchitecturally defined area Tpt (the homologue of part of Wernicke's area) in macaque monkeys shows leftward dominance (Gannon et al. 2008). This finding suggests that the basis of planum temporale asymmetry can be traced back earlier to a common ancestor of catarrhine primates. Similarly, the sulci within the inferior frontal cortex, which contains Broca's area, display left hemisphere dominant asymmetry in their depth and length in humans, chimpanzees, bonobos, and gorillas (Cantalupo & Hopkins, 2001). Given the poor correspondence between external morphological landmarks and cytoarchitectural area borders in this region of great apes (Sherwood et al. 2003) and humans (Amunts et al. 1999), however, it remains to be determined how these gross asymmetries relate to the underlying organization of neural tissue.
Nonetheless, these neuroanatomical asymmetries suggest that some aspects of functional processing were already lateralized in the brain of the LCA prior to the evolution of language. This might be due to the computational demand to process sequential information, such as species-specific vocal calls and dexterous manual actions, with temporal fidelity by reducing conduction delay across hemispheres (Ringo et al. 1994). In fact, functional hemispheric dominance for processing communication signals was probably established much earlier in primate evolution. Species-specific vocalizations elicit responses in the homologues of Broca's area and Wernicke's area in macaque monkeys (Gil-da-Costa et al. 2006) and produce left-hemisphere dominant activity in the temporal cortex (Poremba et al. 2004). Taken together, these findings indicate that the neural machinery for processing complex acoustic signals contained in species-specific communication was present and lateralized in the LCA, providing a scaffold upon which language functions later evolved.
A relatively large percentage of neocortex comprises frontal cortex
The frontal cortex (which includes primary motor cortex, premotor cortex, supplementary motor area, and prefrontal cortex) is involved in numerous processes, ranging from simple motor execution to higher-order cognition. Regions of the prefrontal cortex are implicated in functions such as decision-making, planning, working memory, and emotional regulation. As a fraction of total neocortex volume, the frontal cortex of hominids is large (36%) in comparison with other primates (29% in gibbons and 31% in capuchin and macaque monkeys) (Semendeferi et al. 2002). Analyses of scaling relative to the rest of the neocortex indicate that the frontal cortex shows a positive allometric relationship in primates, such that it comprises an increasing percentage of the neocortex as overall brain size enlarges (Bush & Allman, 2004). Thus, proportionally larger frontal cortex is to be expected in primates with big brains such as hominids.
In addition, the dorsal frontal cortex of hominids has a more complex pattern of gyral folding than in other primates, with distinct precentral and superior frontal sulci evident. Comparisons of macaques, chimpanzees, and humans based on sulcal landmark registration suggest that the enlargement of frontal cortex in hominids has involved mostly the dorsolateral prefrontal cortex (van Essen, 2007). Recent studies have shown that the signaling protein Fgf17, which regulates the expression of transcription factors in the developing neuroepithelium, is particularly important for specifying the size of dorsolateral frontal cortex in mammals (Cholfin & Rubenstein, 2007), pointing to one possible developmental mechanism responsible for the relatively increased size of this cortical region in great apes and humans.
Even though it is predicted by allometric scaling, the relatively increased frontal cortex size of hominids could have significant functional implications. It has been hypothesized that, through axon competition and sorting in development, brain regions that are relatively enlarged might influence activity in more widespread targets (Deacon, 1997; Striedter, 2005). Given the role of dorsolateral prefrontal cortex in executive functions, such as selection among alternative cognitive strategies when faced with novel problems, it is an intriguing possibility that the enhanced capacity of great apes to inhibit their behavioral responses, delay gratification, and demonstrate a higher degree of behavioral flexibility might be related to the increased size of this part of frontal cortex relative to the rest of the neocortex.
Specializations of projection cell types
Several different neuron types have evolved biochemical and morphological specializations in the hominid lineage (Sherwood & Hof, 2007). For example, in layer V of anterior cingulate and frontoinsular cortex, neurons with a spindle-shaped cell body are only found in great apes and humans among primates (Nimchinsky et al. 1995, 1999; Allman et al. 2005). This class of cell, called the von Economo neuron, has a very large soma that displays a distinctive tapering towards the apical dendrite and basal axon. Golgi impregnation studies in human brains show that von Economo neurons have a narrow dendritic field and a thick axon that descends into the white matter (Watson et al. 2006). Based on the location, neurochemistry, and morphological characteristics of von Economo neurons, it has been hypothesized that they transmit rapid outputs to subcortical regions (Allman et al. 2005). It is interesting that these specialized projection neuron types have been identified in cortical areas that are positioned at the interface between emotional and cognitive processing. Given their characteristics, it has been speculated that von Economo neurons are designed for quick signaling of an appropriate response in the context of social ambiguity (Allman et al. 2005). Enhancements of this ability would be particularly important in the context of fission-fusion communities, such as those of panins and possibly the LCA, with complex networks of social interactions and potential uncertainties at reunions. Quite interestingly, von Economo neurons have now also been identified in large-brained cetaceans (Hof & van der Gucht, 2007), indicating that they have independently evolved in multiple lineages. Given the distribution of mammalian species in which they are found, it seems that they may differentiate from a common precursor pool to perform important social cognitive functions in species that have both large brain size and complex social organization.
Modifications of axons in the neocortex arising from extrinsic neurotransmitter systems
Diffusely projecting neurotransmitter systems in the neocortex have an enormous influence on cognitive processing by modulating attention, working memory, states of arousal, and motivation. These systems have the capacity to adjust the excitability and synchronicity of large ensembles of postsynaptic neurons. Due to their widespread effects, deficits in neocortical innervation by these neuromodulators result in the decline of cognitive functions, notably learning and memory, and are associated with neurodegenerative conditions in humans such as Alzheimer's and Parkinson's disease. A recent comparative examination of axons that contain serotonin, dopamine, and acetylcholine in the frontal cortex of humans, chimpanzees, and macaque monkeys has revealed significant phylogenetic variation (Raghanti et al. 2008a; Raghanti et al. 2008b; Raghanti et al. in review). In general, these neuromodulators provide more extensive innervation to layers V and VI in prefrontal cortical areas associated with cognition (Brodmann's areas 9 and 32) of chimpanzees and humans relative to macaques, but there are no species differences in primary motor cortex (Brodmann's area 4). Given the role of these neuromodulatory afferents in attention and learning, it is tempting to speculate that the increased prefrontal innervation of hominids is involved in the unique behavioral characteristics of this clade, particularly enhanced gaze-following and the existence of socially transmitted traditions.
In addition, a remarkable morphological feature present in chimpanzee and human neocortex, but not in macaques, is the accumulation of ‘coils’ of varicose axons for serotonin, dopamine, and acetylcholine. Based on previous observations of such cholinergic axon ‘coils’ in human neocortex, these morphological features might represent episodes of plasticity and synaptic reorganization (Mesulam et al. 1992). These findings suggest that enhanced facilitation of intracortical processing in prefrontal cortex by neuromodulatory systems would have characterized in the LCA.
Molecular adaptations for high neuronal activity level
Brain tissue is metabolically expensive because of the high energetic costs associated with ion pumps and the synthesis and packaging of neurotransmitters (Aiello & Wheeler, 1995). Over and above these general costs, available evidence suggests that the mass-specific energetic demands of neocortex might be even higher in apes (including hylobatids) than in other primates. For example, a duplication of the glutamate dehydrogenase gene took place in the stem hominoid (Burki & Kaessmann, 2004). The hominoid-specific isoform of this enzyme, GLUD2, which is expressed by astrocytes, is activated during high glutamate flux. These modifications, which provide a greater capacity for glutamate metabolism, presumably occurred to enable relatively high levels of excitatory neurotransmission in ape brains. Additionally, several electron transport chain genes (COX4-1, COX5A, COX8A, ISP, NDUFA1, NDUFC2, and NDUFA4) underwent positive selection in the hominoid stem (Grossman et al. 2004; Uddin et al. 2008). The amino acid substitutions characterizing the hominoid variants of these proteins have been demonstrated in some cases to involve changes in electrical charge, suggesting that they confer functional enhancements of the mitochondrial aerobic metabolism pathway to provide energy substrates to cells that are highly active, such as neurons. Taken together, these studies suggest that relatively high rates of overall neuronal activity level in the LCA would have been supported by evolutionary changes in biochemical pathways for glutamate uptake and energy production.
Other features of interest
Outside of the neocortex, several other neuroanatomical traits distinguish apes (including hylobatids) compared with other primates. Specifically, the lateral cerebellar hemisphere of hominoids is larger than would be predicted by allometry in monkeys (Rilling & Insel, 1998; MacLeod et al. 2003). This portion of the cerebellum participates in a variety of functions including planning of complex motor patterns, sensory discrimination, attention shifting, and procedural learning (Leiner et al. 1993). The cerebellar specializations of apes are most likely associated with their suspensory mode of locomotion to allow for feeding on fruits at the tips of branches given a large body size. Such adaptations require excellent physical coordination in addition to visuo-spatial mapping skills to track seasonal resource availability, both functions involving lateral cerebellar connections. Hence, the relatively greater size of the lateral cerebellum in the LCA might have provided a neuroanatomical basis for refining motor behavior and consequentially also supporting other higher-order cognitive processes.
Even the brainstem of apes exhibits distinctive features in comparison with other primates. Perhaps in conjunction with the enlarged neocortex subsuming a greater diversity of functions, the dorsal cochlear nucleus of hominoids has lost the granular layer and stratified cellular organization that is evident in other primates (Moore, 1980). The functional significance of this simplified dorsal cochlear nucleus in hominoids is unclear. Conversely, the facial nucleus of the great ape and human clade displays a larger volume and a greater number of neurons than would be expected for a non-hominid primate of the same medulla size (Sherwood, 2005; Sherwood et al. 2005a). These changes in facial nucleus anatomy might subserve the increased facial mobility of hominids compared to other primates (Dobson, 2006). Additionally, a neurochemically distinct nucleus has been described in the brainstem of humans, the nucleus paramedianus dorsalis, which is absent in all other mammals investigated, including macaque monkeys – great apes have not yet been studied (Baizer et al. 2007). It is hypothesized that this nucleus receives vestibular inputs related to eye movements.
Finally, many life history characteristics relevant to brain growth show modifications in hominoids relative to other primates. Skeletal, dental, and sexual maturation are delayed and the lifespan is elongated. In fact, Miocene fossil hominoids (Sivapithecus and Dryopithecus) appear to have matured at rates and durations similar to those of the living apes (Kelley, 2004). Prolonged life history phases have been shown to relate to the evolution of large brain size (Sacher, 1982; Smith, 1989; Allman et al. 1993; Deaner et al. 2002). The extended period of offspring dependency, combined with more slowly maturing neuronal pathways in the LCA, would have provided an opportunity for learning in a social environment to strongly shape plastic changes in the developing brain.
Specializations in Homo sapiens– the behavioral phenotype
Comparative research has shed light on the cognitive specializations of modern humans (Subiaul et al. 2006). Here we will focus on those findings that have received the most empirical support, including those pertaining to joint or shared attention, the understanding of minds, and imitation learning.
Gaze-following and joint attention
Various studies (for reviews see Povinelli, 2000; Subiaul et al. 2006) have revealed that chimpanzees and humans share many aspects of gaze-following behavior including: (1) the ability to extract specific information about the direction of gaze from others; (2) displaying the gaze-following response whether it is initiated by movements of the hand and eyes in concert or by the eyes alone; (3) using another's gaze to visually search into spaces outside their immediate visual field in response to eye plus head/upper torso movement, eye plus head movement or eye movement alone; (4) not requiring direct visual perception of the shifts in another's gaze direction to follow it into a space outside their immediate visual field; and (5) possessing at a minimum a tacit understanding of how another's gaze is interrupted by solid, opaque surfaces.
But important differences exist alongside these similarities in the gaze-following behavior of humans and great apes. For example, Okamoto and colleagues (2002, 2004) reported a case study in which an infant chimpanzee failed to look back at the experimenter after following her gaze to an object located behind him. This type of triadic interaction, which exists between mother, child, and an object of interest, has been widely reported in the human developmental literature but it is largely absent in the animal literature. Researchers have offered various explanations for these differences. Among humans, a number of changes in social communication occur around 9 months of age (Carpenter et al. 1998). For instance, by 6 months, human infants interact dyadically with objects or with a person in a turn-taking reciprocally exchanging sequence. However, they do not interact with a person who is manipulating objects (Tomasello, 1999). From 9 months on, infants start to engage in triadic exchanges with others. Their interactions involve both objects and persons, resulting in the formation of a referential triangle of infant, adult, and object to which they share attention (Tomasello, 1999; Rochat, 2001).
Theory of mind
Mirror self-recognition (Gallup, 1970) and the many parallels between human and chimpanzee gaze-following (Povinelli & Eddy, 1996c) have been widely interpreted to indicate that chimpanzees and other great apes have a ‘theory of mind’ (Premack & Woodruff, 1978). One way researchers have attempted to assess whether an agent possesses an understanding of unobservable mental states is to measure whether individuals associate certain observable features such as eyes with unobservable psychological states such as ‘seeing.’ In a series of classic studies, Povinelli & Eddy (1996c) used the chimpanzee's natural begging gesture – an out-stretched hand, palm facing up –to make requests to a human experimenter. When chimpanzees were confronted with two experimenters, one whose eyes were visible and therefore could respond to their gestures, and another whose eyes were covered or closed and therefore could not respond to their gestures, results revealed that chimpanzees showed no preference for gesturing toward the experimenter who could see them. There was one exception. Subjects responded correctly in the condition where one experimenter faced forward and another faced backwards. In a follow-up experiment to exclude the possibility that chimpanzees were using a global cue (e.g. body orientation) to guide their responses, Povinelli & Eddy (1996c) had two human experimenters turn their backs to a chimpanzee subject, but one looked over their shoulder. In this condition, performance dropped to chance. Many aspects of these results have now been independently replicated by other comparative psychologists working with captive chimpanzees (e.g. Kaminsky et al. 2004).
This pattern of performance sharply contrasted with the performance of human children. In an experiment similar to the one described above, where children were trained to gesture to an experimenter to request brightly colored stickers (Povinelli & Eddy, 1996c). They were tested on several of the same conditions used with the chimpanzees, and the youngest children (2-year-olds) were correct in most or all of the conditions from their very first trial.
Hare, Call, Tomasello and their associates have challenged Povinelli & Eddy's (1996c) conclusion that theory of mind is unique to modern humans (Hare et al. 2000, 2001, 2006; Hare, 2001). Hare and colleagues used a ‘competitive paradigm’, where individuals must compete with conspecifics or human experimenters for food, because they argue that this paradigm is more ecologically valid than the ‘cooperative paradigm’, where subjects gesture to an experimenter, used by Povinelli and Eddy (Hare, 2001; Hare & Tomasello, 2004). In the competitive paradigm, a dominant and a subordinate chimpanzee were placed in opposite sides of a large enclosure. In certain trials both the subordinate and the dominant animal were in view of one another and food was placed in a position that was visible to both. In other trials, food was strategically placed in a position that only was visible to the subordinate. In this experiment, the subordinate animals avoided the food that was visible to the dominant animal but not the food that had been positioned so that only the subordinate animal could see. The authors interpret these results as evidence that chimpanzees have the capacity to infer some aspects of mental states such as ‘seeing’ (Hare et al. 2000, 2001, 2006; Hare, 2001). These results have been further supported by Hostetter and colleagues (2007) who presented over 100 chimpanzees with treatment conditions similar to those presented by Povinelli and Eddy (1996c). They report that chimpanzees produced more overt behaviors (e.g. vocalizations) when the experimenter's eyes were visible than when the experimenter's eyes were covered or not visible.
However, the see/not-see paradigm (whether competitive or cooperative) poses several distinct problems as a way of addressing whether nonhuman animals are capable of mental state attribution (for a review see Vonk & Povinelli, 2006; Subiaul, 2007). The main problem involves whether or not Povinelli and Eddy's (1996c) and Hostetter et al.'s (2007) ‘cooperative paradigm’ or Hare et al.'s (2000, 2001, 2006) ‘competitive paradigm’ can adequately isolate nonhuman primates’ understanding of unobservable psychological states such as ‘seeing’ from their use of non-psychological, observable cues such as eyes. Certainly, reasoning about mental states or any other ‘unobservable’ is premised on a correlation between a behavioral or physical feature (e.g. eyes) and an underlying cognitive state (e.g. ‘seeing’). However, it is possible that these are decoupled in the minds of nonhuman primates. This presents the distinct possibility that an individual could reason adeptly about the visibility of eyes (or other observable features) without simultaneously reasoning about what eyes do (‘see’). In such an instance, the individual that reasons about eyes only, would behave remarkably like someone who associates eyes with the psychological state of ‘seeing.’ For these reasons, Povinelli and colleagues (Povinelli, 2000; Vonk & Povinelli, 2006) have argued that the see/not-see paradigms (e.g. Hare et al. 2000, 2001; Hostetter et al. 2007; Povinelli & Eddy, 1997) are fundamentally flawed and cannot be used as evidence that animals have an understanding of a mental state such as seeing. The development of an experimental paradigm that overcomes this interpretational challenge continues to elude researchers.
Imitation
Given the varied and dynamic ability of modern humans to learn new behaviors, is it possible that our species deploys a dramatically different cognitive mechanism when learning new skills? To what extent is ‘learning by imitation’ (i.e. ‘novel imitation’) unique to humans? To date, 10 studies have directly compared novel imitation – or imitation learning, where individuals must copy responses or rules that do not already exist in their behavioral repertoire – in human and nonhuman adult primates using analogous procedures (Nagell et al. 1993; Tomasello et al. 1993; Call & Tomasello, 1995; Whiten et al. 1996; Horowitz, 2003; Horner & Whiten, 2005; Herrmann et al. 2007; Subiaul et al. 2007). Only one study has compared novel imitation in monkeys and children (Subiaul et al. 2007). Six studies have reported that on an operational task, where a tool or object had to be manipulated in a certain manner to retrieve a reward, humans reproduced the demonstrator's actions with greater fidelity than did great apes who either reproduced only the outcome of the modeled actions or did not imitate at all (Nagel, et al. 1993; Tomasello et al. 1993; Call & Tomasello, 1995; Call et al. 2005; Hermann et al. 2007; Horner & Whiten, 2007). The other four studies reported both similarities and differences between humans and chimpanzees when executing specific actions on an object following a demonstration (Whiten et al. 1996; Horner & Whiten, 2004). Two studies, one that involved an operational-tool task (Horowitz, 2003) and another that used a cognitive imitation paradigm (Subiaul et al. 2007), found no differences between the performance of humans and nonhuman primates. Thus, ‘imitation’ is not a singular cognitive mechanism. Different aspects of the imitation faculty in humans are shared with chimpanzees and other primates, whereas other characteristics appear to be unique in our species (Subiaul, 2007).
The newborn's ability to copy the orofacial expressions of a model, for instance, appears to be a behavioral trait that is shared among humans, chimpanzees, and rhesus macaques. Neonatal chimpanzees and rhesus macaques, like human infants (e.g. Meltzoff & Moore, 1977), reproduce tongue protrusions and mouth openings in response to a model displaying the same expression (Myowa-Yamakoshi et al. 2004;Ferrari et al. 2006). There are also striking parallels in the developmental trajectory of orofacial imitation in these species. Similar to humans (Abravanel & Sigafoos, 1984), the incidence of orofacial imitation in chimpanzees slowly disappears after 9 weeks of age (Myowa-Yamakoshi et al. 2004).
Recent experiments, however, appear to show that both cooperation and imitation come more ‘naturally’ to human children than to young chimpanzees (Horner & Whiten, 2005, 2007; Herrmann & Tomasello, 2006). For example, it has been shown that human children, but not nonhuman animals, learn in a ‘ghost condition’, which is an experimental social learning control condition where the actions of a model are removed and the target object and the consequent results occur automatically (i.e. as if executed by a ghost) (Subiaul et al. 2004; Thompson & Russell, 2004; Huang & Charman, 2005). Other studies have also demonstrated that human children learn from others’ mistakes (Want & Harris, 2001; Subiaul et al. in review) but nonhuman primates do not (Horner & Whiten, 2007). These results may be indicative of fundamental differences between species where only humans are capable of differentiating between a correct ‘intentional’ response and an incorrect ‘unintentional’ response, and of engaging in counterfactual reasoning (Want & Harris, 2002; Subiaul et al. in review). As such, the human imitation faculty likely has added more functions or possibly become functionally linked with other psychological faculties, such as theory of mind, granting it greater flexibility and power to copy a broad range of rules and responses, including the ability to reason about physical capability and counterfactual information (learning from others’ mistakes).
Specializations in Homo sapiens– language
Language and the thought that it expresses constitute arguably the most distinctive feature of human behavior. Yet, consensus about the course of language evolution is elusive. Controversies pervade not only speculation about the phylogeny of distinctively human language, but also the characterization of what has evolved, the linguistic component of the human behavioral phenotype. There is at least this much agreement about human language – it is a form of communication that is unique in the natural world (Hockett, 1960; Bickerton, 1990; Hauser, 1997b; Christiansen & Kirby, 2003a,b). Unlike systems of communication employed by other species, human language is said to have: (1) modality/stimulus independence, (2) duality of patterning, (3) shared, arbitrary symbols, capable of displaced reference, (4) generalized systematicity/domain independence, and (5) hierarchical/recursive structure or syntax.
Modality/stimulus independence
Unlike nonhuman animal communication systems, human language is both modality and stimulus independent (Hockett, 1960; Hauser, 1997b; Hauser et al. 2002a). It is modality independent because it can take oral, visual, gestural, and even tactile forms. In other words, human language is abstract enough to be communicated in radically different media. In contrast, the communication signals of nonhuman animals do not display similar flexibility of modality. For example, mating or threat displays always employ the same sequences of bodily movements, and cannot be conveyed in different modalities.
The stimulus independence of human language refers to its freedom from specific environmental triggers. Human language users can speak about anything in virtually any circumstances. For example, we can talk of food even without food present or without being hungry. This contrasts with animal communication systems, which for the most part are under the control of very specific environmental or endogenous triggers. Both the modality and stimulus independence of human language imply that it is under far more voluntary control than typical, nonhuman animal communication systems (Deacon, 1997).
Although no form of nonhuman animal communication approaches the extreme stimulus- and modality independence of human language, there is evidence that nonhuman primate species, and even some non-primates, are capable of a certain degree of voluntary control over communicative acts. The best-studied examples are the use of gestures to indicate intentions in various great ape species, and the use of referential vocalizations in various monkey species (Tomasello & Call, 1997). Chimpanzees, bonobos, and gorillas have been shown to use ‘ontogenetically ritualized’ gestures to express intentions to conspecifics. These gestures begin as direct behavioral manipulations that are gradually truncated as interactions recur. For example, the initiation of play by a chimpanzee might start off as a play-hit, and then become ritualized, such that merely raising an arm comes to indicate the intention to play (Tomasello & Call, 1997). Such gestures are often used for very different communicative purposes, indicating some intentional, voluntary control, and stimulus independence.
Perhaps the best-known example of intentional vocal communication in nonhuman primates is the vervet monkey's system of warning calls (Cheney & Seyfarth, 1990; Tomasello & Call, 1997). Vervets produce different vocalizations depending on whether they see predatory cats, birds, or snakes. Other members of a vervet troop respond to such signals with avoidance behavior appropriate to the indicated predator. The proper use of these signals must be learned during subadulthood. Also, there is anecdotal evidence that vervets are capable of tailoring these calls to specific audiences, indicating stimulus independence, and some degree of intentional, voluntary control (Cheney & Seyfarth, 1990; Tomasello & Call, 1997). It is worth noting that several other nonhuman primate species, as well as non-primates, including domestic chickens, are capable of comparable control over predator-warning vocalizations (Hauser, 1997b; Tomasello & Call, 1997; Zuberbühler, 2006).
Although it is clear that various nonhuman species are capable of voluntary, intentional, stimulus- and modality independent communication, these capacities are extremely limited when compared with the human capacity for language. The ontogenetically ritualized gestures observed in various great ape species are limited to intention expression, attention getting, or other conspecific-manipulative functions, so the range of stimuli that can elicit them is relatively limited. The modalities available for expressing such communicative acts are also limited to ritualized versions of the kinds of manipulative behaviors from which they originate. There is very little evidence that, for example, chimpanzees can accomplish with vocalization the same sorts of communicative acts as they can accomplish with gesture (Pollick & de Waal, 2007). Monkey vocalizations appear even more limited in these respects. Vervet referential vocalizations are limited to the function of warning conspecifics about predators, so the range of stimuli that elicit them is also relatively narrow. Furthermore, vervets do not use gestures, for example, to communicate the same warning signals, indicating a lack of modality independence.
Duality of patterning
Alone among natural communication systems, human language is compositional at two levels (Hockett, 1960; Pinker & Jackendoff, 2005). At the first level, neural representations of a finite set of meaningless gestures, known as ‘phonemes’, can be systematically combined into a much larger set of meaningful units, like representations of words, called ‘morphemes.’ At the second level, this set of morphemes can be systematically combined into an infinite set of larger meaningful units, e.g. phrases, clauses, and sentences. The meanings of these larger units are systematic functions of the meanings of the morphemes that compose them, computed via recursive, syntactic rules.
Some argue that certain nonhuman animal communication systems, like birdsong and whalesong, have an analog of the first compositional level (Hauser, 1997b; Okanoya, 2002; Fitch, 2004, 2005; Pinker & Jackendoff, 2005) – a finite set of meaningless sounds can be systematically combined into a much larger set of sequences of such sounds. However, these sequences of sounds are not meaningful in the way that morphemes of human language are, i.e. they are not words with referential meaning, and they are not themselves combined into higher-order meaningful units like phrases, clauses, and sentences (Fitch, 2004, 2005; Pinker & Jackendoff, 2005).
Shared, arbitrary symbols capable of displaced reference
Human language consists of shared, arbitrary symbols capable of displaced reference, i.e. the capacity to refer to events and objects that are not perceptually present, like spatially or temporally distant objects and events (Hauser, 1997b; Hauser et al. 2002a; Pinker & Jackendoff, 2005). For example, a word like ‘star’ is an arbitrary symbol that has no connection, e.g. resemblance or correlation, with what it stands for. It is shared in that both the producer and the consumer of the symbol ‘star’ understand it to stand for the same object. This is made possible by the consumer's capacity to infer the communicative intention of the producer, premised on a well developed theory of mind. Finally, the reference of the symbol need not be perceptually salient or restricted to the here-and-now. In fact, language can be used to talk about things with which it is not possible to have perceptual contact, either because they are spatio-temporally too removed, e.g. the beginning of the universe, or because they are not located in space and time, e.g. abstractions like the number two.
There is some controversy about the degree to which nonhuman animals are capable of shared, symbolic, displaced reference (Hauser et al. 2002a; Pinker & Jackendoff, 2005). Honeybees appear capable of encoding spatially removed locations using a ‘dance-language’, components of which correspond systematically to distance and direction (Von Frisch, 1967; Hauser, 1997b). However, the honeybee dance is not symbolic, i.e. it stands in a non-arbitrary relation to the locations it encodes. Because components of the dance, e.g. ‘waggles’, correspond systematically to distance and direction (Hauser, 1997b), the dance is more of an iconic representation, like a map, than a symbolic representation.
Another example of nonhuman shared, symbolic, displaced reference comes from studies of language-trained great apes. The most famous of these, the bonobo Kanzi, has learned to use visual symbols, with arbitrary referents, to communicate with his handlers (Savage-Rumbaugh et al. 1998). Kanzi appears capable of inferring simple communicative intentions, and can use symbols to refer to objects that are spatially displaced. However, this feat was heavily reliant on scaffolding by Kanzi's handlers (Tomasello & Call, 1997). There is very little evidence of such capacity in wild populations. So even if nonhuman animals are capable of some rudimentary, symbolic communication, it does not come easily or naturally.
Generalized systematicity/domain independence
Human language is characterized by two distinctive, related and, as far as we know, unparalleled semantic properties. Firstly, human language is generally systematic: it can represent any object for which it has a term as possessing any property for which it has a term (Evans, 1982; Fodor & Pylyshyn, 1988). This property gives language a boundless, creative capacity for representing unobserved and unobservable situations, for example, cats that walk upright in boots and talk, etc. Such talk and the thought it expresses also enable the discovery of the hidden mechanisms of nature – light can be thought of as a wave, for example (see Mithen, 1996; Carruthers, 2003; Camp, 2004). Second, human language is task-domain neutral; it represents information about the world as independent of any task to which it might be put. For example, the sentence ‘There are fruit trees beyond the hill’ has no immediate implications for action; it puts no constraints on what the speaker or hearer can do with this information. These two properties are closely related. A sentence that systematically combines words for concepts from two different cognitive domains cannot itself be domain specific. It must transcend the proprietary task domains of its components, and achieve a kind of task-domain neutrality.
There are good reasons to doubt that nonhuman animals are capable of generally systematic and task-domain neutral communication or thought. Nonhuman primates show little evidence of it (Cheney & Seyfarth, 1990; Tomasello & Call, 1997; Hurley, 2003) and it is difficult to imagine ecological circumstances that could have selected for this kind of thought in nonhuman species. Indeed, there is little archaeological evidence that even our relatively recent hominin ancestor, Homo erectus, was capable of such thought (Mithen, 1996). For example, prior to the origin of modern Homo sapiens, hominin tools lacked totem-like, symbolic decoration. By contrast, the archaeological record that dates roughly from the speciation of Homo sapiens shows clear evidence of domain neutral cognition – tools incorporate animal products, and display increasingly complex, symbolic decoration, often depicting fauna. Mithen (1996) argues that this demonstrates a capacity to integrate information from disparate cognitive domains, including knowledge of the natural world, tool-making capacity, and social cognition. It is unclear how and why such domain-neutral cognition evolved in humans. One suggestion is that once the capacity to encode complex information in a public medium, i.e. complex language, evolved, a ‘common code’ in which information from previously isolated cognitive modules could be integrated, became available. On this view, domain-neutral, generally systematic thought is a kind of ‘thinking for speaking’ (Slobin, 1991) made possible by the prior evolution of a capacity for complex, public language (Mithen, 1996; Zawidzki, 2006).
Hierarchical/recursive structure or syntax
Since Chomsky's early work (Chomsky, 1957), the claim that natural language exhibits hierarchical, recursive structure has had the status of orthodoxy. Sentences of natural language are composed of nested hierarchies of sub-sentential units. For example, consider the sentence, ‘The herd, beyond the woods, north of the plain, west of the hills is on the move.’ This sentence consists of more than just words; it consists of sub-sentential compounds of these words known as phrases. Because phrases can be nested within other phrases, we can construct sentences of arbitrary length, conveying information of arbitrarily precise specificity. This indefinitely extendable hierarchical nesting of phrases constitutes the recursive, syntactic structure of human language (Pinker & Jackendoff, 2005).
Many theorists consider recursive syntax the most distinctive and important property of human language, on which many of its other unique properties depend (Bickerton, 1990, 1998, 2000, 2003; Hauser et al. 2002a). This is currently a topic of great controversy (Hauser et al. 2002a; Fitch et al. 2005; Jackendoff & Pinker, 2005; Pinker & Jackendoff, 2005). Bickerton (1990) argues that recursive syntax explains the creativity, or the ‘generalized systematicity/domain independence’ of language. Deacon (1997, 2003) argues that communication is truly symbolic only if communicative acts constitute a system governed by internal structural relations, much like recursive syntax. Similarities between the syntactic structure of human language and the hierarchically-organized action sequences of Acheulean toolmaking in later hominins have been noted (Holloway, 1969), with recent functional neuroimaging evidence suggesting a shared neural substrate related to complex, goal directed action (Stout et al. in press). However, others downplay the importance of syntax –Wray (2000), Hurford (2003), Tomasello (2003), Christiansen & Kirby (2003b) all suggest that syntax is an artifact of cultural evolution since the emergence of Homo sapiens and that it is not as biologically central as some of the other properties of language discussed here.
There is evidence that nonhuman animals are incapable of both comprehending and producing hierarchically recursive structures (Fitch & Hauser, 2004). Indeed, Kanzi, the most successful of the language-trained apes, is conspicuously incapable of acquiring syntax (Corballis, 2002).
Specializations in Homo sapiens– the neuroanatomical phenotype
Large brain size
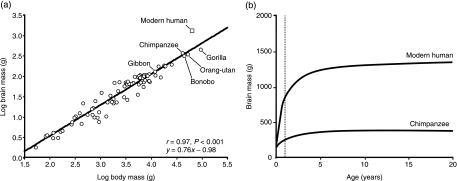
The single most obvious neuroanatomical specialization of Homo sapiens is large absolute and relative size of the brain. Figure 1b summarizes this and other unique features of the human brain. Averaging about 1400 g, human brains are approximately three times larger than those of great apes. This indicates that a significant amount of brain mass increase occurred along the hominin lineage since its origination from the LCA. Humans also outrank all other animals in measures of encephalization, the degree to which brain mass exceeds expectations based on allometric scaling for body mass (Fig. 2a) (Holloway & Post, 1982; Martin, 1990). Fossil evidence indicates that in the hominin lineage since the LCA there have been periods of gradual increases in cranial capacity that were occasionally accompanied by increases in body mass. However, starting at about 1.8 Ma, beginning with Homo erectus, brain expansion in hominins occurred at a much more rapid pace (Holloway et al. 2004).
Fig. 2.
Allometric scaling plot of brain mass versus body mass in 86 species of primates based on Holloway (1996), with bonobo data from Rilling & Insel (1999), showing the least squares regression line fit to the non-human data (a). Modern humans have brains that are approximately three times larger than would be predicted for a primate of the same body mass. Brain growth trajectories in modern humans and chimpanzees in the first 20 years of life, modified from data in Leigh (2004) (b). The dashed line indicates the end of the first postnatal year.
Recent progress has been made in identifying possible genetic mechanisms underlying the expansion of the neocortex in human evolution (Bradley, 2008). Several genes that are known to participate in regulating the dynamics of proliferation and programmed death of cerebral precursor cells show evidence of positive selection in the hominin clade since the LCA (Evans et al. 2004; Gilbert et al. 2005; Vallender & Lahn, 2006). These putative changes in the division, differentiation, and migration of cerebral progenitor cells might explain why the modern human brain growth trajectory deviates from the pattern typical of most primates. While the neonatal brain in Homo sapiens makes up only about 27% of its adult size, other primates have relatively more mature brains at birth – newborn macaque brains are approximately 70% of adult size and newborn chimpanzee brains are 36% of adult size (Martin, 1983; Robson & Wood, 2008). At the time of birth, human brains are already about two times larger than great ape brains (Martin, 1983; Robson & Wood, 2008). Subsequently, postnatal brain growth in humans continues at its fetal rate through the first year, whereas in other primates, brain growth rates decrease shortly after birth (Fig. 2b) (Leigh, 2004). This unique human brain growth schedule is critical to achieving a high level of encephalization in the face of the obstetric constraints associated with pelvic adaptations for bipedality and provides a richer set of social and environmental stimuli to the developing infant while the brain's connections are still highly malleable. In this context, it is notable that the onset of joint attention in human infants occurs within this critical first year of life, providing the opportunity for intensive social facilitation of learning to influence synapse establishment during this period.
Across mammals, as overall brain size enlarges, various parts do not increase at the same rate. Because of the particularly steep allometric scaling slope of the neocortex relative to other brain parts, larger brains become comprised of progressively more neocortex (Finlay & Darlington, 1995). Going even beyond this general trend, the human neocortex (including both gray and white matter) exceeds predictions for a hominoid of the same total brain size (Rilling, 2006). Thus, brain size enlargement in human evolution might have led to a greater degree of functional ‘neocorticalization’, with this structure taking on more direct influence of other brain regions, allowing for greater voluntary control over actions (Deacon, 1997; Striedter, 2005). There is some evidence to support this hypothesis. Firstly, as overall brain size increases in primates, a larger proportion of the brainstem becomes occupied by structures related to or receiving descending neocortical projections, such as the pyramidal tract, red nucleus, and pontine nuclei (Tilney, 1928). Second, tracing studies have shown that neocortical axons form synapses directly onto motoneurons of the vocal folds in the nucleus ambiguus only in humans (Kuypers, 1958a;Iwatsubo et al. 1990), but not other primates (Kuypers, 1958b;Simonyan & Jürgens, 2003). Accordingly, functional MRI studies in humans have demonstrated the existence of an expanded representation of the intrinsic muscles of the larynx located in the dorsal precental gyrus adjacent to the representation of the lips (Brown et al. in press). In squirrel monkeys and rhesus macaques, the cortical laryngeal area occupies a position anterior to the precental gyrus, in the opercular part of the premotor cortex, and it does not contribute to vocalization (Simonyan & Jürgens, 2003). This greater extent of direct cortical involvement in the activation of the vocal folds may be important in the voluntary motor control needed to learn and execute the articulatory sequences of speech. More generally, such enhanced involvement of the neocortex in voluntary control over actions might contribute to other human-specific behavioral abilities, such as the modality and stimulus independence of language.
Increased size of higher-order unimodal and multimodal areas of the neocortex and their connections to other brain structures
A large number of neocortical areas in humans have been shown to have functional and structural homologues in macaque monkeys, including many higher-order multimodal and language related areas (Preuss & Goldman-Rakic, 1991; Petrides & Pandya, 1994, 1999; Grefkes & Fink, 2005; Petrides et al. 2005). Currently, the most compelling evidence for ‘new’ neocortical areas in humans that are not homologous with macaques include regions within posterior parietal cortex which provide additional central visual field representations and greater sensitivity to extract three-dimensional form related to motion (Orban et al. 2006). Quite interestingly, these very same regions in the dorsal interparietal sulcus are activated in positron emission tomography imaging of humans learning how to fashion Oldowan-style stone tools (Stout & Chaminade, 2007). In the absence of comparable data from apes, however, it is not clear whether these posterior parietal areas are specific to Homo sapiens or whether they are shared with our close relatives.
Although the layout of the human cortical map shares many similarities with other catarrhine primates, it is possible that some territories have changed in relative size. One long-held idea is that human neocortical expansion has involved enlargement of higher-order multimodal areas to a greater degree than primary sensory and motor areas. Anatomically, this can be seen as an increase in the relative amount of ‘generalized’ or eulaminate cortex. However, humans have as much total eulaminate cortex as expected for a primate of our brain size, with slightly fewer eulaminate neurons (Shariff, 1953; Passingham, 1975b; Armstrong, 1990). Despite this finding, it remains possible that particular higher-order unimodal and multimodal cortical fields have enlarged disproportionately in humans.
Because parts of frontal cortex are implicated in executive control functions, it has long been assumed that this region was a focal point for volumetric expansion in human evolution. Recent data, however, have shown that total frontal cortex size in humans is no greater than expected based on apelike scaling trends for brain size and that it occupies a similar fraction of the cerebral cortex as in great apes (Semendeferi et al. 2002; Bush & Allman 2004). But is the prefrontal portion of the frontal cortex relatively large in humans? Circumstantial evidence suggests that this is the case. The human prefrontal cortex exhibits more gyrification than expected for an anthropoid primate of the same brain size (Rilling & Insel, 1999). Furthermore, primary motor and premotor cortex in humans occupy a smaller proportion of the frontal lobe compared with other primates, suggesting that the remainder is comprised of a relatively large prefrontal cortex (Preuss, 2004). Yet, comparative studies that have directly examined whether prefrontal cortex or any of its subdivisions are enlarged in humans have yielded contradictory results (Brodmann, 1912; Blinkov & Glezer, 1968; Holloway, 1968, 2002; Passingham, 1973; Uylings & van Eden, 1990; Deacon, 1997; Semendeferi et al. 2001; Schoenemann et al. 2005; Sherwood et al. 2005b). Unfortunately, these studies are typically based on small sample sizes of only one or two individuals per species. No doubt, the current lack of consensus on this critical topic will be alleviated once a rigorous comparative study of prefrontal cortex volume is performed with larger samples.
Besides the prefrontal cortex, other cortical regions appear to have undergone human-specific reorganization in size. For example, human primary visual cortex is only about one and half times larger in humans than in great apes, while the rest of neocortex is about three times larger (Stephan et al. 1981). Human primary visual cortex is also substantially smaller than predicted by allometry for total human brain size (Holloway, 1996). The relatively small size of primary visual cortex in humans suggests that adjacent areas of the posterior parietal cortex have disproportionately increased in volume (Holloway, 1996; Holloway et al. 2004). Several endocasts of Australopithecus afarensis and Australopithecus africanus from approximately 4–2.5 Ma show evidence that the lunate sulcus, which marks the border between primary visual cortex and parietal cortex, had already shifted to a more humanlike configuration (Holloway et al. 2004). This suggests that early hominins had evolved a relatively enhanced representation of visuospatial and sensorimotor integration in the posterior parietal cortex prior to dramatic brain size expansion. Because posterior parietal cortex is active in object manipulation tasks and motor planning (Shibata & Ioannides, 2001; Stout & Chaminade, 2007), it is possible that this cortical reorganization opened the door to stone tool production in later hominins. Further geometric expansion of the parietal lobes appears to distinguish modern Homo sapiens endocasts from other hominins (Bruner, 2004).
Another region that shows extraordinary enlargement in humans is the temporal lobe. Based on measurements of MRI scans, Semendeferi & Damasio (2000) and Rilling & Seligman (2002) demonstrated that the human temporal lobe, especially the underlying white matter, exceeds allometric predictions based on hominoids. The relative increase in white matter interconnectivity in humans appears to be concentrated in the region immediately beneath gyri rather than within the central core (Schenker et al. 2005), suggesting that the human temporal lobe is specifically characterized by increased local connectivity between neighboring cortical fields. The relative increase in the volume of the human temporal lobe, furthermore, is related to coordinated reorganization of nuclei in the amygdala (Barger et al. in press). Enlargement of the temporal lobe and its axonal connectivity in humans is intriguing in light of the key role of this region in functions such as language comprehension, naming, verbal memory, and face recognition. Compared with great apes, an anteriorly expanded and laterally pointing temporal lobe characterizes endocasts of Australopithecus africanus, suggesting reorganization of the cortical areas involved in some aspects of the these multimodal functions might have preceded brain size enlargement in human evolution (Falk et al. 2000).
In parallel with these changes in the size of higher-order cortical areas in humans, there has also been differential enlargement of certain thalamic nuclei with which they share reciprocal connections. Humans have more neurons than other hominoids in several dorsal thalamic nuclei, including the anterior principal (anteroventral) nucleus, mediodorsal nucleus, and pulvinar, while neuron numbers in sensory relay nuclei are generally similar across these species (Armstrong, 1982). Recent data indicate that pulvinar size scales against brain volume in anthropoid primates with positive allometry, explaining the proportionally larger nucleus complex in humans (Chalfin et al. 2007). During human brain development, the pulvinar and other dorsal thalamic nuclei attract migrating neurons from the telencephalic ganglionic eminence, which mature to become GABAergic interneurons (Letinic & Rakic, 2001). This migration stream has not been observed in any other species, including macaque monkeys, where cells of exclusively diencephalic origin take up positions in the dorsal thalamus. The possibility that greater numbers of interneurons uniquely characterize the human thalamus is supported by the observation that only humans show a bimodal distribution of neuron sizes in the pulvinar, whereas apes show a unimodal distribution (Armstrong, 1981). Thus, fundamental developmental processes might have been modified in the evolution of the human brain to accommodate expanded representation of higher-order systems in the thalamus.
Also concomitant with the elaboration of certain neocortical areas in humans, the cerebellum is large relative to body size (Rilling, 2006). Cerebellar enlargement in humans is not surprising given that it is linked by extensive connections with the neocortex and these two structures have evolved as a coordinated system across primates (Whiting & Barton, 2003). Interestingly, the ventral portion of the cerebellum's dentate nucleus is relatively larger in humans than in the great apes (Matano, 2001). This part of the dentate nucleus projects to non-motor regions of the frontal lobe by way of the ventrolateral thalamus. Therefore, the human cortico-cerebellar circuit may be distinguished from other primates in having a greater development of the connections with frontal association areas that play a role in cognition and language (Leiner et al. 1993).
More elaborate asymmetries
As described above, many neuroanatomical asymmetries would have been present in the LCA. However, humans have elaborated on these asymmetries and evolved a much greater degree of hemispheric lateralization. Across Homo sapiens the incidence of right-handedness is approximately 90%, particularly for fine motor tasks involving precision grip and manipulation of tools. In contrast, most other primates do not display such pronounced bias for hand use at the population level (McGrew & Marchant, 1997). When tested with an experimental coordinated bimanual task, for example, captive common chimpanzees (n = 467) display population-level right-handedness at an incidence of approximately 67%, captive gorillas (n = 31) show a non-significant trend towards right-handedness, and 79% of captive orangutans (n = 19) are significantly left-handed (Hopkins et al. 2003, 2004). Thus, to date there is no evidence that any great ape species displays the same high degree of population-level handedness that is present in humans.
An additional feature of asymmetry that has increased in human evolution is a pattern of combined left-occipital and right-frontal petalias. These asymmetric lateral protrusions at the frontal and occipital poles of the cerebral hemispheres are a common feature of human brains. Although recent voxel-based MRI studies do not support previous findings of an association between this petalial torque pattern in humans and right-handedness (Good et al. 2001; Herve et al. 2006), it is nonetheless significant that this pattern of cerebral asymmetry is not consistently present in nonhuman primates or in hominin fossil brain endocasts until Homo erectus (Holloway & De La Coste-Lareymondie, 1982).
Beyond these asymmetries of the human brain's gross morphology, there are additional aspects of neuroanatomical asymmetry in the cellular organization of the neocortex that have not yet been found in other species. Human brains tend to have a greater proportion of neuropil in the left hemisphere of Broca's area (Brodmann areas 44 and 45) (Amunts et al. 1999, 2003), Wernicke's area (area Tpt) (Buxhoeveden et al. 2001), primary motor cortex hand area (Amunts et al. 1996, 1997), as well as primary visual cortex and extrastriate areas (Amunts et al. 2007). Neuropil is the space between cell bodies occupied by dendrites, axons, and synapses. In contrast, investigations of chimpanzee brains have not revealed such interhemispheric differences in the amount of neuropil in area Tpt (Buxhoeveden et al. 2001) or the primary motor cortex representation of the hand (Sherwood et al. 2007). Thus, this aspect of histological asymmetry appears to be unique to humans. Although the adaptive significance of such brain asymmetry is poorly understood, it is possible that humans have evolved a greater extent of cerebral lateralization in the context of specialization for computationally demanding functions, such as language, to avoid bilateral duplication of circuitry and interhemispheric conflict (Corballis, 1991).
Changes in histology, neuronal metabolism, and synaptic plasticity
Increased neocortex size in humans is not the result of a simple multiplication of uniform processing units. Shariff (1953) reported that human cerebral cortex volume is 2.75 times larger than in chimpanzees, but has only 1.25 times more neurons. This suggests that much of the increased mass of the neocortex derives from alterations within the space between cell bodies. Recent studies that make detailed comparisons of the fine structure of the neocortex among humans and their close relatives indicate that microanatomical changes have occurred in the course of human brain evolution. For example, the patterned arrangement of dendrites and local-circuit interneurons in layer IVA of primary visual cortex of humans is distinctive relative to other hominids (Preuss et al. 1999; Preuss & Coleman, 2002), potentially relating to changes in the motion-processing pathway. Additionally, in humans the spindle-shaped von Economo neurons located in layer V of anterior cingulate and frontoinsular cortex are especially large in size, more numerous, and show a greater tendency to be aggregated in clusters than in great apes (Nimchinsky et al. 1995, 1999). If, as hypothesized, von Economo neurons furnish a projection pathway that integrates interoceptive feedback and cognitive monitoring of conflict to mediate rapid non-rational behavioral selection in ambiguous social interactions, then these anatomical differences might be important in allowing humans to navigate ever more complex social networks (Allman et al. 2005).
Studies of gene expression using microarray techniques have shown that the human cerebral cortex is also distinguished from chimpanzees and other primates in displaying up-regulation of genes related to neuronal signaling, plasticity, and metabolic activity (Cáceres et al. 2003; Preuss et al. 2004; Uddin et al. 2004). These observations are further supported by findings that various subunits of the mitochondrial electron transport chain show evidence of natural selection in the human terminal lineage (Grossman et al. 2004; Uddin et al. 2008a). Some of the increased mass-specific metabolic demand of human neocortex is expected given the energetic costs of maintaining membrane potentials in neurons that have expanded dendritic arbors and longer axonal projections in a large brain (Elston et al. 2006). Congruent with this idea, there are increasing numbers of glial cells relative to neurons in the primate neocortex as a function of brain size, and humans have the highest glia-neuron ratio (Sherwood et al. 2006). Other findings indicate that two thrombospondins, THBS2 and THBS4, have elevated expression in the neuropil of the adult human neocortex and striatum (Cáceres et al. 2007). These proteins are astrocyte-secreted factors that have the capacity to induce synapse formation. Therefore, their increased expression suggests that the human brain might be distinguished by enhanced synaptic plasticity in adulthood, comprising a possible molecular substrate of greater flexibility of behavior and capacity for learning.
Insights from genes
In recent years, a tremendous amount of progress has been made in understanding the underpinning of human brain evolution through the examination of changes in gene sequences (Enard et al. 2002a; Dorus et al. 2004; Fisher & Marcus, 2006; Bradley, 2008). These comparative studies have revealed many intriguing human-specific genetic differences relative to chimpanzees and other primates. While some have clear implications for the human brain phenotype, the significance of others remains mysterious.
One of the first brain-important genes reported to show sequence changes in human evolution was the transcription factor FOXP2 (Enard et al. 2002b). Based on the association of certain point mutations of this gene with grammatical impairment and orofacial dyspraxia, accompanied by functional under-activation and structural abnormalities of language-related brain regions, FOXP2 has been suggested to play a role in the development of language and speech in humans (Fisher & Marcus, 2006). Although the FOXP2 gene is highly conserved across mammals (with identical amino acid sequences in rhesus macaques, gorillas, and chimpanzees), humans have fixed mutations that yield two amino acid substitutions in comparison with other primates, suggesting positive selection for its function. The human variant of FOXP2 has been reported to also be present in Neandertals (Krause et al. 2007). As of yet, however, it is not clear how these genetic changes might relate to modifications in human neuroanatomy relevant to language or speech.
AHI1 is another gene that shows evidence of positive selection in human evolution (Ferland et al. 2004). This gene is required for normal axonal pathfinding in development that leads to decussation of the corticospinal tract and superior cerebellar peduncles. Deleterious mutation of this gene in human patients leads to Joubert syndrome, a condition that presents with abnormalities of motor coordination and gait, mental retardation, and antisocial behavior. The evidence for AHI1 gene evolution indicates that some particular aspects of neuronal interconnectivity were selectively modified in human evolution, possibly in support of our species’ derived mode of gait and posture.
Using novel search strategies, other studies are identifying new genomic regions that might play a role in the evolution of human brain structure and function. For example, a previously unknown gene was recently identified that is markedly amplified in the human brain (Popesco et al. 2006). This gene, which encodes the DUF1220 protein domain, shows much higher copy number in humans than in other primate species. Although it has been demonstrated that the DUF1220 protein is expressed in neuronal somata and dendrites, its function is not yet understood. In another study, Pollard and colleagues (2006) identified several genomic regions that show rapid evolution in the human lineage since the LCA but are otherwise highly conserved across mammals. The most highly accelerated of these, dubbed HAR1, is a 118-bp region in the last band of chromosome 20q that encodes a stable secondary RNA structure expressed in Cajal-Retzius cells during weeks 7–9 of gestation in humans. These cells types, which also express reelin, are critical in the early specification and migration of cerebral cortical neurons into their correct layers. Further studies of the products of these genes might shed light on the mechanisms leading to the development of distinctive microanatomical features of the human brain.
Finally, recent genome-wide surveys indicate that non-coding cis-regulatory sequences in close proximity to genes involved in neuronal cell adhesion (Prabhakar et al. 2006) and neurogenesis (Haygood et al. 2007) have undergone accelerated evolution in both human and chimpanzee lineages. Of special significance, despite the overrepresentation of these gene categories in both human and chimpanzee terminal lineages, these studies have found little overlap among the specific genes underlying these shared enriched annotations. These findings suggest that independent accelerated evolution of sequences in these categories since the divergence of the human and chimpanzee lineages has contributed to distinct neural and cognitive phenotypes through differential regulation of genes involved in coordinating developmental process.
A model
Our current view of human mental evolution is like a jigsaw puzzle where many of the pieces have been taken out of the box, but they have not yet been put together to form a coherent picture. In the preceding sections, we have enumerated many changes that have taken place in the descent of humans from the LCA. However, while many differences can be described that distinguish humans at genetic, behavioral, and neuroanatomical levels, we are still woefully ignorant about how these apparent specializations are bound together. Modern humans differ from the reconstructed LCA, and from all other living animals, dramatically in cognition and language. In many other lineages that show such profound divergence in behavior or sensory capacities there is a comparably obvious specialization at the neuroanatomical level. Well-described examples include the increased allocation of cortical somatosensory representation for the bill in the platypus or the magnification of auditory cortex in echolocating bats (Krubitzer, 2007). Can such a direct parallel between structure and function be drawn in the case of modern humans? In the concluding section of this article, we offer a preliminary model to account for some links between brain and behavior in human evolution.
We hypothesize that subtle shifts in the genetically programmed processes underlying brain development, cellular physiology, and neurochemistry, in conjunction with biases in temperament, perception, and sensation that epigenetically affect learning processes, are sufficient to yield significant changes in the architecture and function of the modern human brain (Fig. 3). According to our model, domain-specific behavioral capacities emerge from deep shifts in the weight of basic behavioral processes like attention, executive control, working memory, and inhibition, as they guide the individual's interactions with the environment. In humans, this process unfolds through a unique postnatal ontogeny that includes especially rapid brain growth and synaptogenesis in the first year of life. The neural substrates of these changes in bias are, to some extent, emergent from allometric changes in microstructure related to brain enlargement and, to some extent, determined by non-allometric mosaic changes in neural structure and physiology.
Fig. 3.
A model for the evolution of species-typical, domain-specific skills through interactions among genetically programmed biases in neural pathways. This figure shows one possible set of relationships among perception, sensation, and executive control for illustration; however, other domain-general capacities, such as attention, motivation or arousal, might also be modified in a similar manner. Selection acts on the performance of the organism, manifest as a repertoire of domain-specific skills, via modification of heritable traits encoded in the genome. Such traits might include, for example, adjustment to the graded expression of growth factors, cell adhesion, or regulation of cell cycle dynamics in neural precursors. These genetically determined traits establish biases within networks of connections that guide perception and sensation throughout ontogeny and, therefore, powerfully shape the organism's behavioral development by directing exploration and learning. We envision that multiple tiers of domain-specific skill sets might emerge from shifting such biases early in development.
General correlates of enlarged brain size
Given the high energetic cost of neural tissue (Aiello & Wheeler, 1995), brain enlargement could only have arisen in human evolution if offset by significant fitness benefits (Barrickman et al. in press). The fact that the human neocortex tripled in size since the LCA is almost certainly related to increased general intelligence in our species. Absolute brain size has been shown to predict interspecific variation on measures of cognitive flexibility (Rumbaugh, 1997; Gibson et al. 2001; Reader & Laland, 2002; Deaner et al. 2007), that is, the ability to explore novel tactics when reward contingencies change. It has been suggested that such neural machinery for an enhanced ‘cognitive reserve’ might be required in long-lived species, such as humans, where individuals are likely to face unpredictable socioecological challenges over a long lifespan (Allen et al. 2005).
So, what computational advantage does increasing the absolute size of the brain confer? First, larger brains contain a greater total number of neurons available for data encoding and integration (Roth & Dicke, 2005). In addition, theoretical models and comparative data suggest that neocortical enlargement yields a proliferation of functionally discrete modules, which are involved in specialized information processing (Kaas, 2000; Changizi & Shimogo, 2005) and could be manifest as increased neuroanatomical lateralization (Ringo et al. 1994). In this context, it is interesting that certain characteristics of anatomical brain asymmetry are unique to humans, such as increased neuropil space in the left hemisphere and left-occipital right-frontal petalia torque. Increased anatomical modularity would seem to be congruent with the evolutionary psychology model of human cognitive evolution which posits that new, genetically specified cognitive modules have accumulated in the modern human mind (Cosmides & Tooby, 1994; Buss, 2005; Tooby & Cosmides, 2005). Here, we have discussed several unique psychological specializations in humans, such as the capacity to reason about unobservable causes and the faculty of language. However, as discussed above, because macaque monkey homologues have been described for the majority of human neocortical areas, it seems that the large-scale modular organization of human neocortex is retained from a catarrhine primate ancestor. Although it remains a possibility that improved techniques for cortical mapping may reveal human-specific areas in the future, at the present time, we see no compelling evidence that novel domain-specific cognitive capacities in humans can be readily linked to the addition of genetically specified, specialized processing modules in the neocortex or elsewhere in the brain.
Are there other general features of neuroanatomical organization that change with increasing brain size and might account for our species’ enhanced cognitive powers? One correlate of brain enlargement, at least in anthropoid primates, is an increase in the proportion of neocortical neurons that show molecular adaptations for long-range associational axon projections (Sherwood et al. 2004; Sherwood & Hof, 2007). This suggests that the outputs of local neocortical processing in larger brains are fed-forward to a greater diversity of targets. One unfortunate consequence is that these same neuron classes are especially susceptible to degeneration in aging and Alzheimer's disease in humans (Hof et al. 2002; Hof & Morrison, 2004). Other long-range projecting neuronal types might also be especially vulnerable to degenerative neuropathology. The von Economo neurons, for example, are severely and selectively affected in human frontotemporal dementia cases (Seeley et al. 2006).
Increased numbers of corticocortical association connections might, in part, contribute to the evolution of new functions in human brain regions that have homologues in other species, such as cortical ‘language’ areas. For example, in humans, the cortex of the fronto-operculum, known as Broca's area, mediates phonological and syntactical aspects of speech and language production. In addition to classical language processes, neuroimaging studies in humans have revealed that this cortical region participates in several other functions as well, such as object manipulation and grasping, imagery of motion, imitation of movements, and movement preparation and planning (Nishitani et al. 2005). Many of these non-linguistic functions have also been defined for the ventrolateral prefrontal cortex of macaque monkeys (Petrides, 2005). Thus, this region in both humans and macaques serves as an interface for the perception and orchestration of sensory and motor sequencing essential to planning, observation, understanding, and imitation of actions (Arbib, 2005).
We hypothesize that positively selected alterations in axon guidance and cell adhesion mechanisms of humans (Prabhakar et al. 2006; Uddin et al. 2008b) in combination with the general tendency for long-association projections to form in larger brains provide the inferior frontal cortex of humans with access to a greater diversity of afferents, particularly regions of the temporoparietal cortex containing multimodal semantic and lexical representations. Indeed, new diffusion tensor magnetic resonance imaging data suggest that the arcuate fasciculus, the axon pathway which links temporal cortex to the inferior frontal cortex, has a more prominent projection to middle temporal gyrus semantic processing areas in humans as compared with chimpanzees and macaques (Rilling et al. 2007). Such diversified connectivity might explain a central feature of human language and the thought it expresses – the ‘generalized systematicity’ described above, by virtue of which we can think and express thoughts that systematically combine concepts from diverse domains. This capacity explains the human facility with analogical thinking, e.g. thinking of light as a wave. More generally, enhanced corticocortical associational connectivity might contribute to other distinctive aspects of modern human cognition, such as the capacity of the imitation faculty to incorporate inferences about others’ mental states and intentions.
Shifting the balance
Many genes related to brain development and function show signs of accelerated evolution exclusively in humans (Dorus et al. 2004; Uddin et al. 2008b), suggesting that beyond brain size expansion, many other aspects of neurobiology have been differentially altered in human evolution.
Changes in the balance among brain region sizes that are established early in development might have dramatic implications for the dynamic competitive processes that ultimately result in the connectivity of the adult brain (Deacon, 1997). Shifts in the relative size of cortical and subcortical regions, or the strength of their connections, could contribute to significant alterations in the flow of information during ontogeny, with long-term repercussions for setting biases in perception and learning via modifications to domain-general features such as attention, motivation, working memory, and inhibitory control. One dramatic example of the manner in which early developmental experience can powerfully mold learning and behavior is the ‘enculturation’ experiments in apes (see Tomasello & Carpenter, 2005; but also Bering, 2004 for an alternative perspective). Our model hypothesizes that shifting biases among higher-order processing circuits involving multimodal neocortical areas and their connections in the striatum, thalamus, and cerebellum, could give rise to striking behavioral changes. Furthermore, alterations at the level of histological architecture and gene expression could also determine the shape of activity in networks of neurons.
For example, we reason that dramatic changes during ontogeny in the flow and direction of attention could potentially result in powerful domain-specific mechanisms; that is, mechanisms that perform computation on specific types of stimuli and generate specific types of behaviors. Some of these include edge detection mechanisms, categorical perception, sophisticated types of imitation learning mechanisms (e.g. novel motor and vocal imitation), the ability to make inferences about different unobserved causes (e.g. psychological, physical), and extremely flexible syntactically structured and semantically creative language use. These different cognitive skills, though modularized, are likely to exert an effect on the development and contours of other modules enhancing ‘cognitive self-control’, the ability to inhibit automatic responses based on sophisticated social understanding. For instance, cognitive self-control could help account for inferences about unobservable causes, as such capacities require the ability to inhibit stereotyped responses to superficially similar stimuli, treating them differently if there are reasons to expect different hidden causes. Similarly, some, though not all, of the social learning differences between chimpanzees and humans might amount to a capacity for attentional control – perhaps involving inhibition of stimulus-capture forms of attention that cause distraction (Tomasello & Carpenter, 2005). And our capacity to use language to talk about anything in any circumstances clearly requires sophisticated voluntary control.
In each case, domain-general processes and primary domain-specific mechanisms that are highly encapsulated (i.e. resistant to extraneous information), such as those that mediate the processing of different types of visual and auditory information (e.g. the perception of lines and phonemes), likely result in the generation of other specialized mechanisms, such as those that mediate the identification of letters and spoken words. Other domain-specific cognitive functions are likely responsible for the development of domain-specific skills that mediate the imitation of different types of stimuli and our species’ ability to flexibly and robustly reason about unobservable causes across contexts (Subiaul et al. 2006; Vonk & Povinelli, 2006). These more sophisticated cognitive skills are less encapsulated than the primary domain-specific mechanisms, as they have to be more malleable and responsive to environmental changes in order to effectively solve the species-typical problems that arise in the course of development.
Thus, the dynamic interaction between domain-general and domain-specific mechanisms could result in the proliferation of many specialized cognitive operations that ultimately become highly modularized (e.g. reading, writing). We believe that the neurobiological profile of humans which includes a relatively large neocortex, increased relative size of certain higher-order multimodal cortical areas, specializations of projection neuron classes, and modifications to the rate of postnatal brain growth, makes such a process more powerful in our species than in other primates. Such a framework gives credence to the popular saying among evolutionary psychologists (Cosmides & Tooby, 1994) that humans have more, not fewer, ‘instincts’ than nonhuman animals.
One of the central puzzles about human cognition is its curious combination of flexibility and efficiency. While the human mind is certainly characterized by an abundance of highly efficient, domain-specific, modular capacities, many of these, like reading, writing, game playing, and musical ability, are clearly recent cultural products, and therefore indicate extraordinary ontogenetic flexibility. In tracing these capacities to ontogenetic effects of evolved, genetically determined modifications to domain-general capacities we share with the LCA, e.g. voluntary control, attention, and perceptual biases, our model accounts for the balance between flexibility and efficiency that characterizes human cognition.
Conclusion
We suggest that ‘descent with modification’ aptly describes the construction of the human mind. The studies we have reviewed demonstrate that, although humans have certainly acquired many novel cognitive and neural specializations in the course of evolution, a large number of features are shared exclusively with our fellow great apes. Our hypothesized model explains how incremental changes in brain development and organization might yield apparent discontinuity in our species-specific behavioral repertoire. Taken together, we consider it inescapable to recognize the continuity in mentality between the LCA and us, despite the significant disparity in phenotypes.
Acknowledgments
The authors thank Drs Bernard Wood and Sarah Elton for inviting us to present this paper at the ‘Human Evolution’ symposium at the 2007 Proceedings of the Anatomical Society of Great Britain and Ireland. We also thank Drs M. A. Raghanti, N. M. Schenker, J. Vonk, J. M. Allman, P. R. Hof, R. L. Holloway, J. K. Rilling, and M. Uddin for providing useful comments on an earlier draft of this manuscript. This work was supported in part by the National Science Foundation (BCS-0515484 and BCS-0549117), the National Institutes of Health (NS-42867), and the James S. McDonnell Foundation (22002078).
References
- Abravanel E, Sigafoos AD. Exploring the presence of imitation during early infancy. Child Dev. 1984;55:381–392. [PubMed] [Google Scholar]
- Aiello LC, Wheeler P. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Current Anthropol. 1995;36:199–211. [Google Scholar]
- Allen JS, Bruss J, Damasio H. The aging brain: the cognitive reserve hypothesis and hominid evolution. Am J Hum Biol. 2005;17:673–689. doi: 10.1002/ajhb.20439. [DOI] [PubMed] [Google Scholar]
- Allman J, McLaughlin T, Hakeem A. Brain weight and life-span in primate species. Proc Natl Acad Sci USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Schleicher A, et al. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schmidt-Passos F, Schleicher A, Zilles K. Postnatal development of interhemispheric asymmetry in the cytoarchitecture of human area 4. Anat Embryol (Berl) 1997;196:393–402. doi: 10.1007/s004290050107. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Ditterich A, Zilles K. Broca's region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Amunts K, Armstrong E, Malikovic A, et al. Gender-specific left-right asymmetries in human visual cortex. J Neurosci. 2007;27:1356–1364. doi: 10.1523/JNEUROSCI.4753-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Sallaberry P, Barbier H. Use of experimenter-given cues during object-choice tasks by capuchin monkeys. Animal Behav. 1995;49:201–208. [Google Scholar]
- Anderson JR, Montant M, Schmitt D. Rhesus monkeys fail to use gaze direction as an experimenter-given cue in an object-choice task. Behav Processes. 1996;37:47–55. doi: 10.1016/0376-6357(95)00074-7. [DOI] [PubMed] [Google Scholar]
- Arbib M. From monkey-like action recognition to human language: an evolutionary framework for neurolinguistics. Behav Brain Sci. 2005;28:105–124. doi: 10.1017/s0140525x05000038. [DOI] [PubMed] [Google Scholar]
- Armstrong E. A quantitative comparison of the hominoid thalamus. IV. Posterior association nuclei – the pulvinar and lateral posterior nucleus. Am J Phys Anthropol. 1981;55:369–383. doi: 10.1002/ajpa.1330550311. [DOI] [PubMed] [Google Scholar]
- Armstrong E. Mosaic evolution in the primate brain: differences and similarities in the hominoid thalamus. In: Armstrong E, Falk D, editors. Primate Brain Evolution: Methods and Concepts. New York: Plenum Press; 1982. pp. 131–162. [Google Scholar]
- Armstrong E. Evolution of the brain. In: Paxinos G, editor. The Human Nervous System. New York: Academic Press; 1990. pp. 1–16. [Google Scholar]
- Asfaw B, White T, Lovejoy O, Latimer B, Simpson S, Suwa G. Australopithecus garhi: a new species of early hominid from Ethiopia. Science. 1999;284:629–635. doi: 10.1126/science.284.5414.629. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Baker JF, Haas K, Lima R. Neurochemical organization of the nucleus paramedianus dorsalis in the human. Brain Res. 2007;1176:45–52. doi: 10.1016/j.brainres.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger N, Stefanacci L, Semendeferi K. A comparative volumetric analysis of the amygdaloid complex and basolateral division in the human and ape brain. Am J Phys Anthropol. doi: 10.1002/ajpa.20684. in press. [DOI] [PubMed] [Google Scholar]
- Barrickman NL, Bastian ML, Isler K, van Schaik CP. Life history costs and benefits of encephalization: a comparative test using data from long-term studies of primates in the wild. J Hum Evol. doi: 10.1016/j.jhevol.2007.08.012. in press. [DOI] [PubMed] [Google Scholar]
- Begun DR. Enhanced cognitive capacity as a contingent fact of hominid phylogeny. In: Russon AE, Begun DR, editors. The Evolution of Thought: Evolutionary Origins of Great Ape Intelligence. Cambridge: Cambridge University Press; 2004. pp. 15–27. [Google Scholar]
- Beran MJ, Evans TA. Maintenance of delay of gratification by four chimpanzees (Pan troglodytes): the effects of delayed reward visibility, experimenter presence, and extended delay intervals. Behav Processes. 2006;73:315–324. doi: 10.1016/j.beproc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Summation and numerousness judgments of sequentially presented sets of items by chimpanzees (Pan troglodytes. J Comp Psychol. 2001;115:181–191. doi: 10.1037/0735-7036.115.2.181. [DOI] [PubMed] [Google Scholar]
- Bering JM. A critical review of the ‘enculturation hypothesis’: the effects of human rearing on great ape social cognition. Anim Cogn. 2004;7:201–212. doi: 10.1007/s10071-004-0210-6. [DOI] [PubMed] [Google Scholar]
- Bickerton D. Language and Species. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Bickerton D. Catastrophic evolution: the case for a single step from protolanguage to full human language. In: Hurford JR, Studdert-Kennedy M, Knight C, editors. Approaches to the Evolution of Language: Social and Cognitive Bases. Cambridge: Cambridge University Press; 1998. pp. 341–358. [Google Scholar]
- Bickerton D. How protolanguage became language. In: Knight C, Hurford JR, Studdert-Kennedy M, editors. The Evolutionary Emergence of Language: Social Function and the Origins of Linguistic Form. Cambridge: Cambridge University Press; 2000. pp. 264–284. [Google Scholar]
- Bickerton D. Symbol and structure: a comprehensive framework for language evolution. In: Christiansen MS, Kirby S, editors. Language Evolution: The States of the Art. Oxford: Oxford University Press; 2003. pp. 77–93. [Google Scholar]
- Blinkov SM, Glezer II. The Human Brain in Figures and Tables: A Quantitative Handbook. New York: Plenum Press; 1968. [Google Scholar]
- Boesch C. Cooperative hunting roles among Taï chimpanzees. Hum Nat. 2002;13:27–46. doi: 10.1007/s12110-002-1013-6. [DOI] [PubMed] [Google Scholar]
- Boesch C, Boesch H. Tool use and tool making in wild chimpanzees. Folia Primatol. 1990;54:86–89. doi: 10.1159/000156428. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Bernston GG, Hannan MB, Cacioppo JT. Quantity-based interference and symbolic representations in chimpanzees (Pan troglodytes. J Exp Psychol Anim Behav Process. 1996;22:76–86. [PubMed] [Google Scholar]
- Boysen ST, Berntson GG, Shreyer TA, Quigley KS. Processing of ordinality and transitivity by chimpanzees (Pan troglodytes. J Comp Psychol. 1993;107:208–215. doi: 10.1037/0735-7036.107.2.208. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Berntson GG. Responses to quantity: perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes. J Exp Psychol Anim Behav Process. 1995;21:82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- Bradley BJ. Reconstructing phylogenies and phenotypes: A molecular view of human evolution. J Anat. 2008;212:337–353. doi: 10.1111/j.1469-7580.2007.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandom RB. Making It Explicit. Cambridge, MA: Harvard University Press; 1994. [Google Scholar]
- Brannon EM. The representation of numerical magnitude. Curr Opin Neurobiol. 2006;16:222–229. doi: 10.1016/j.conb.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Neue Ergebnisse über die vergleichende histologische Lokalisation der Grosshirnrinde mit besonderer Berücksichtigung des Stirnhirns. Erg Heft Anat Anz. 1912;41:157–216. [Google Scholar]
- Brosnan SF, Schiff HC, de Waal FB. Tolerance for inequity may increase with social closeness in chimpanzees. Proc Biol Sci. 2005;272:253–258. doi: 10.1098/rspb.2004.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Ngan E, Liotti M. A larynx area in the human motor cortex. Cereb Cortex. doi: 10.1093/cercor/bhm131. in press. doi: 10.1093/cercor/bhm131. [DOI] [PubMed] [Google Scholar]
- Bruner E. Geometric morphometrics and paleoneurology: brain shape evolution in the genus Homo. J Hum Evol. 2004;47:279–303. doi: 10.1016/j.jhevol.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Burki F, Kaessmann H. Birth and adaptive evolution of a hominoid gene that supports high neurotransmitter flux. Nat Genet. 2004;36:1061–1063. doi: 10.1038/ng1431. [DOI] [PubMed] [Google Scholar]
- Bush EC, Allman JM. The scaling of frontal cortex in primates and carnivores. Proc Natl Acad Sci USA. 2004;101:3962–3966. doi: 10.1073/pnas.0305760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss DM. The Handbook of Evolutionary Psychology. New York: Wiley; 2005. [Google Scholar]
- Buxhoeveden DP, Switala AE, Litaker M, Roy E, Casanova MF. Lateralization of minicolumns in human planum temporale is absent in nonhuman primate cortex. Brain Behav Evol. 2001;57:349–358. doi: 10.1159/000047253. [DOI] [PubMed] [Google Scholar]
- Cáceres M, Lachuer J, Zapala MA, et al. Elevated gene expression levels distinguish human from non-human primate brains. Proc Natl Acad Sci USA. 2003;100:13030–13035. doi: 10.1073/pnas.2135499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres M, Suwyn C, Maddox M, Thomas JW, Preuss TM. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex. 2007;17:2312–232. doi: 10.1093/cercor/bhl140. [DOI] [PubMed] [Google Scholar]
- Call J. Past and present challenges in theory of mind research in nonhuman primates. Prog Brain Res. 2007;164:341–353. doi: 10.1016/S0079-6123(07)64019-9. [DOI] [PubMed] [Google Scholar]
- Call J, Tomasello M. Use of social information in the problem solving of orangutans (Pongo pygmaeus) and human children (Homo sapiens. J Comp Pyschol. 1995;109:308–320. doi: 10.1037/0735-7036.109.3.308. [DOI] [PubMed] [Google Scholar]
- Calvin WH. The emergence of intelligence: language, foresight, musical skills and other hallmarks of intelligence are connected through an underlying facility that enhances rapid movements. Sci Am. 1994;271:100–107. doi: 10.1038/scientificamerican1094-100. [DOI] [PubMed] [Google Scholar]
- Camp E. The generality constraint and categorial restrictions. Philos Q. 2004;54:209–231. [Google Scholar]
- Cantalupo C, Hopkins WD. Asymmetric Broca's area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM. Shared system for ordering small and large numbers in monkeys and humans. Psychol Sci. 2006;17:401–406. doi: 10.1111/j.1467-9280.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM. Basic math in monkeys and college students. PLoS Bioloy. 2007;5:2912–2919. doi: 10.1371/journal.pbio.0050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monogr Soc Res Child Dev. 1998;63:1–143. [PubMed] [Google Scholar]
- Carruthers P. On Fodor's problem. Mind Lang. 2003;18:502–523. [Google Scholar]
- Cartmill M. New views on primate origins. Evol Anthropol. 1992;1:105–111. [Google Scholar]
- Chalfin BP, Cheung DT, Muniz JA, de Lima Silveira LC, Finlay BL. Scaling of neuron number and volume of the pulvinar complex in New World primates: comparisons with humans, other primates, and mammals. J Comp Neurol. 2007;504:264–274. doi: 10.1002/cne.21406. [DOI] [PubMed] [Google Scholar]
- Chance MRA. Attention structure as a basis of primate rank orders. Man. 1967;2:503–518. [Google Scholar]
- Changizi MA, Shimojo S. Parcellation and area-area connectivity as a function of neocortex size. Brain Behav Evol. 2005;66:88–98. doi: 10.1159/000085942. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. How Monkeys See the World: Inside the Mind of Another Species. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Cheney DL, Seyfarth RM. Baboon Metaphysics: The Evolution of a Social Mind. Chicago: University of Chicago Press; 2007. [Google Scholar]
- Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci USA. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsky N. Syntactic Structures. The Hague: Mouton; 1957. [Google Scholar]
- Christiansen M, Kirby S. Language Evolution: The States of the Art. Oxford: Oxford University Press; 2003a. [Google Scholar]
- Christiansen M, Kirby S. Language evolution: consensus and controversies. Trends Cogn Sci. 2003b;7:300–307. doi: 10.1016/s1364-6613(03)00136-0. [DOI] [PubMed] [Google Scholar]
- Connolly CJ. The External Morphology of the Primate Brain. Springfield, IL: C.C. Thomas; 1950. [Google Scholar]
- Corballis MC. The Lopsided Ape. Oxford: Oxford University Press; 1991. [Google Scholar]
- Corballis MC. Did language evolve from manual gestures? In: Wray A, editor. The Transition to Language. Oxford: Oxford University Press; 2002. pp. 161–179. [Google Scholar]
- Cosmides L, Tooby J. Origins of domain-specificity: the evolution of functional organization. In: Hirschfeld L, Gelman S, editors. Mapping the Mind: Domain-specificity in Cognition and Culture. New York: Cambridge University Press; 1994. pp. 85–116. [Google Scholar]
- Darwin C. The Descent of Man and Selection in Relation to Sex. London: J. Murray; 1871. [Google Scholar]
- Deacon TW. The Symbolic Species: The Co-evolution of Language and the Brain. New York: W.W. Norton & Company, Inc; 1997. [Google Scholar]
- Deacon T. Universal grammar and semiotic constraints. In: Christiansen MH, Kirby S, editors. Language Evolution: The States of the Art. Oxford: Oxford University Press; 2003. pp. 111–139. [Google Scholar]
- Deaner RO, Barton RA, van Schaik CP. Primate brains and life histories: renewing the connection. In: Kappeler PM, Pereira ME, editors. Primate Life Histories and Socioecology. Chicago: University of Chicago Press; 2002. pp. 3–265. [Google Scholar]
- Deaner RO, Isler K, Burkart J, van Schaik C. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav Evol. 2007;70:115–124. doi: 10.1159/000102973. [DOI] [PubMed] [Google Scholar]
- Dobson SD. Comparative Study of Facial Mobility in Anthropoid Primates. Saint Louis: Washington University; 2006. [Google Scholar]
- Dorus S, Vallender EJ, Evans PD, et al. Accelerated evolution of nervous system genes in the origin of Homo sapiens. Cell. 2004;119:1027–1040. doi: 10.1016/j.cell.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. Grooming, Gossip, and the Evolution of Language. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, et al. Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec. 2006;288A:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI. Gaze following and joint attention in rhesus monkeys (Macaca mulatta. J Comp Pyschol. 1997;111:286–293. doi: 10.1037/0735-7036.111.3.286. [DOI] [PubMed] [Google Scholar]
- Enard W, Khaitovich P, Klose J, et al. Intra- and interspecific variation in primate gene expression patterns. Science. 2002a;296:340–343. doi: 10.1126/science.1068996. [DOI] [PubMed] [Google Scholar]
- Enard W, Przeworski M, Fisher SE, et al. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002b;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- van Essen DC. Cerebral cortical folding patterns in primates: why they vary and what they signify. In: Preuss TM, Kaas JH, editors. Evolution of Nervous Systems. Vol. 4. The Evolution of Primate Nervous Systems. Oxford: Academic Press; 2007. pp. 267–276. [Google Scholar]
- Evans G. The Varieties of Reference. Oxford: Oxford University Press; 1982. [Google Scholar]
- Evans PD, Anderson JR, Vallender EJ, et al. Adaptive evolution of ASPM, a major determinant of cerebral cortical size in humans. Hum Mol Genet. 2004;13:489–494. doi: 10.1093/hmg/ddh055. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Delay of gratification and delay maintenance by rhesus macaques (Macaca mulatta. J Gen Psychol. 2007;134:199–216. doi: 10.3200/GENP.134.2.199-216. [DOI] [PubMed] [Google Scholar]
- Falk D, Redmond JC, Jr, Guyer J, et al. Early hominid brain evolution: a new look at old endocasts. J Hum Evol. 2000;38:695–717. doi: 10.1006/jhev.1999.0378. [DOI] [PubMed] [Google Scholar]
- Ferland RJ, Eyaid W, Collura RV, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–1013. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4:1501–1508. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Marcus GF. The eloquent ape: genes, brains and the evolution of language. Nat Rev Genet. 2006;7:9–20. doi: 10.1038/nrg1747. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Hauser MD. Computational constraints on syntactive processing in a nonhuman primate. Science. 2004;303:377–80. doi: 10.1126/science.1089401. [DOI] [PubMed] [Google Scholar]
- Fitch WT. Kin selection and ‘mother tongues’: a neglected component in language evolution. In: Oller DK, Griebel U, editors. Evolution of Communication Systems. Cambridge, MA: MIT Press; 2004. pp. 275–296. [Google Scholar]
- Fitch WT. The evolution of language: a comparative review. Biology and Philosophy. 2005;20:193–230. [Google Scholar]
- Fitch WT, Hauser MD, Chomsky N. The evolution of the language faculty: clarifications and implications. Cognition. 2005;97:179–210. doi: 10.1016/j.cognition.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Flombaum JI, Santos LR. Rhesus monkeys attribute perceptions to others. Curr Biol. 2005;15:447–452. doi: 10.1016/j.cub.2004.12.076. [DOI] [PubMed] [Google Scholar]
- Fodor J, Pylyshyn Z. Connectionism and cognitive architecture: a critical analysis. In: Pinker S, Mehler J, editors. Connections and Symbols. Cambridge, MA: MIT Press; 1988. pp. 1–71. [DOI] [PubMed] [Google Scholar]
- Foley RA, Lee PC. Finite social space, evolutionary pathways, and reconstructing hominid behavior. Science. 1989;243:901. doi: 10.1126/science.2493158. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Perry S. The Biology of Traditions. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- von Frisch K. The Dance Language and Orientation of Bees. Cambridge, MA: Harvard University Press; 1967. [Google Scholar]
- Fujita K, Kuroshima H, Asai S. How do tufted capuchin monkeys (Cebus apella) understand causality involved in tool use? J Exp Psychol Anim Behav Processes. 2003;19:233–242. doi: 10.1037/0097-7403.29.3.233. [DOI] [PubMed] [Google Scholar]
- Gallup GG. Chimpanzees: self-recognition. Science. 1970;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke's brain language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Kheck N, Hof PR. Leftward interhemispheric asymmetry of macaque monkey temporal lobe language area homolog is evident at the cytoarchitectural, but not gross anatomic level. Brain Res. 2008;1199:62–73. doi: 10.1016/j.brainres.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Gibson KR, Rumbaugh G, Beran M. Bigger is better: primate brain size in relationship to cognition. In: Falk D, Gibson KR, editors. Evolutionary Anatomy of the Primate Cerebral Cortex. Cambridge: Cambridge University Press; 2001. pp. 79–97. [Google Scholar]
- Gilbert SL, Dobyns WB, Lahn BT. Genetic links between brain development and brain evolution. Nat Rev Genet. 2005;6:581–590. doi: 10.1038/nrg1634. [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Martin A, Lopes MA, Munoz M, Fritz JB, Braun AR. Species-specific calls activate homologs of Broca's and Wernicke's areas in the macaque. Nat Neurosci. 2006;9:1064–1070. doi: 10.1038/nn1741. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman LI, Wildman DE, Schmidt TR, Goodman M. Accelerated evolution of the electron transport chain in anthropoid primates. Trends Genet. 2004;20:578–585. doi: 10.1016/j.tig.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Hare B. Can competitive paradigms increase the validity of experiments on primate social cognition? Anim Cogn. 2001;4:269–280. doi: 10.1007/s100710100084. [DOI] [PubMed] [Google Scholar]
- Hare B, Tomasello M. Chimpanzees are more skilful in competitive than in cooperative tasks. Anim Behav. 2004;68:571–81. [Google Scholar]
- Hare B, Call J, Tomasello M. Chimpanzees know what conspecifics do and do not see. Anim Behav. 2000;59:771–785. doi: 10.1006/anbe.1999.1377. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know? Anim Behav. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Chimpanzees deceive a human competitor by hiding. Cognition. 2006;101:495–514. doi: 10.1016/j.cognition.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr Biol. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Hauser MD. Artifactual kinds and functional design features: What a primate understands without language. Cognition. 1997a;64:285–308. doi: 10.1016/s0010-0277(97)00028-0. [DOI] [PubMed] [Google Scholar]
- Hauser MD. The Evolution of Communication. Cambridge, MA: MIT Press; 1997b. [Google Scholar]
- Hauser MD. Knowing about knowing: dissociations between perception and action systems over evolution and during development. Ann N Y Acad Sci. 2003;1001:79–103. doi: 10.1196/annals.1279.006. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Kralik J, Botto-Mahan C. Problem solving and functional design features: experiments on cotton-top tamarins (Saguinus oedipus. Anim Behav. 1999;57:565–582. doi: 10.1006/anbe.1998.1032. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Miller CT, Liu K, Gubta R. Cotton-top tamarins (Saguinus oedipus) fail to show mirror-guided self-exploration. Am J Primatol. 2001;53:131–137. doi: 10.1002/1098-2345(200103)53:3<131::AID-AJP4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Chomsky N, Fitch WT. The faculty of language: what is it, who has it, and how did it evolve? Science. 2002a;298:1569–1579. doi: 10.1126/science.298.5598.1569. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Pearson HM, Seelig D. Ontogeny of tool-use in cotton-top tamarins (Saguinus oedipus): innate recognition of functionally relevant features. Anim Behav. 2002b;64:299–311. [Google Scholar]
- Hauser MD, Santos LR, Spaepen GM, Pearson HM. Problem solving, inhibition and domain-specific experience: experiments on cotton-top tamarins (Saguinus oedipus. Anim Behav. 2002c;64:387–396. [Google Scholar]
- Haygood R, Fedrigo O, Hanson B, Yokoyama KD, Wray GA. Promoter regions of many neural-and nutrition-related genes have experiences positive selection during human evolution. Nat Genet. 2007;39:1140–1144. doi: 10.1038/ng2104. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science. 2007;317:1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Tomasello M. Apes’ and children's understanding of cooperative and competitive motives in a communicative situation. Dev Sci. 2006;9:518–512. doi: 10.1111/j.1467-7687.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- Herve PY, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. NeuroImage. 2006;29:1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hockett CF. The origin of speech. Sci Am. 1960;203:89–97. [PubMed] [Google Scholar]
- Hof PR, van der Gucht E. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae(Cetacea, Mysticeti, Balaenopteridae) Anat Rec. 2007;290:1–31. doi: 10.1002/ar.20407. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JM. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hof PR, Gilissen EP, Sherwood CC. Comparative neuropathology of brain aging in primates. In: Erwin JM, Hof PR, et al., editors. Aging in Nonhuman Primates. Basel: Karger; 2002. pp. 130–154. [Google Scholar]
- Holloway RL. The evolution of the human brain: some notes toward a synthesis between neural structure and the evolution of complex behavior. Gen Syst. 1967;12:3–19. [Google Scholar]
- Holloway RL. The evolution of the primate brain: some aspects of quantitative relations. Brain Res. 1968;7:121–172. doi: 10.1016/0006-8993(68)90094-2. [DOI] [PubMed] [Google Scholar]
- Holloway RL. Culture: a human domain. Curr Anthropol. 1969;10:395–412. [Google Scholar]
- Holloway RL. Human paleontological evidence relevant to language behavior. Hum Neurobiol. 1983;2:105–114. [PubMed] [Google Scholar]
- Holloway RL. Evolution of the human brain. In: Lock A, Peters CR, editors. Handbook of Human Symbolic Evolution. Oxford: Oxford University Press; 1996. pp. 74–114. [Google Scholar]
- Holloway RL. Brief communication: how much larger is the relative volume of area 10 of the prefrontal cortex in humans? Am J Phys Anthropol. 2002;118:399–401. doi: 10.1002/ajpa.10090. [DOI] [PubMed] [Google Scholar]
- Holloway RL, De La Coste-Lareymondie MC. Brain endocast asymmetry in pongids and hominids: some preliminary findings on the paleontology of cerebral dominance. Am J Phys Anthropol. 1982;58:101–110. doi: 10.1002/ajpa.1330580111. [DOI] [PubMed] [Google Scholar]
- Holloway RL, Post DG. The relativity of relative brain measures and hominid mosaic evolution. In: Armstrong E, Falk D, editors. Primate Brain Evolution: Methods and Concepts. New York: Plenum Publishing Co; 1982. pp. 57–76. [Google Scholar]
- Holloway RL, Broadfield DC, Yuan MS. In: The Human Fossil Record. Volume 3. Brain Endocasts-The Paleoneurological Evidence. Schwartz JH, Tattersal I), editors. New York: Wiley; 2004. [Google Scholar]
- Hopkins WD, Marino L, Rilling JK, MacGregor LA. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Stoinski TS, Lukas KE, Ross SR, Wesley MJ. Comparative assessment of handedness for a coordinated bimanual task in chimpanzees (Pan troglodytes), gorillas (Gorilla gorilla) and orangutans (Pongo pygmaeus. J Comp Psychol. 2003;117:302–308. doi: 10.1037/0735-7036.117.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees (Pan troglodytes) are predominantly right-handed: replication in three populations of apes. Behav Neurosci. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner V, Whiten A. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens. Animal Cogn. 2005;8:164–181. doi: 10.1007/s10071-004-0239-6. [DOI] [PubMed] [Google Scholar]
- Horner V, Whiten A, Flynn E, de Waal FBM. Faithful replication of foraging techniques along cultural transmission chains by chimpanzees and children. Proc Natl Acad Sci USA. 2006;103:13878–13883. doi: 10.1073/pnas.0606015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz AC. Do humans ape? Or do apes human? Imitation and intention in humans (Homo sapiens) and other animals. J Comp Pyschol. 2003;117:325–336. doi: 10.1037/0735-7036.117.3.325. [DOI] [PubMed] [Google Scholar]
- Hostetter AB, Russell JL, Freeman H, Hopkins WD. Now you see me, now you don't: evidence that chimpanzees understand the role of the eyes in attention. Anim Cogn. 2007;10:55–62. doi: 10.1007/s10071-006-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Charman T. Gradations of emulation learning in infants’ imitation of actions on objects. J Exp Child Psychol. 2005;92:276–302. doi: 10.1016/j.jecp.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Hurford J. The language mosaic and its evolution. In: Christiansen MH, Kirby S, editors. Language Evolution: The States of the Art. Oxford: University Press; 2003. pp. 38–57. [Google Scholar]
- Hurley S. Animal action in the space of reasons. Mind and Language. 2003;18:231–256. [Google Scholar]
- Itakura S. An exploratory study of gaze-monitoring in non-human primates. Jpn Psychol Res. 1996;38:174–180. [Google Scholar]
- Itakura S, Anderson JR. Learning to use experimenter-given cues during an object-choice task by a capuchin monkey. Cahiers de Psychologie Cognitive. 1996;15:103–112. [Google Scholar]
- Itakura S, Tanaka M. Use of experimenter-given cues during object-choice tasks by chimpanzees (Pan troglodytes), and orangutan (Pongo pygmaeus), and human infants (Homo sapiens. J Comp Psychol. 1998;112:119–126. doi: 10.1037/0735-7036.112.2.119. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Kuzuhara S, Kanemitsu A, Shimada H, Toyokura Y. Corticofugal projections to the motor nuclei of the brainstem and spinal cord in humans. Neurology. 1990;40:309–312. doi: 10.1212/wnl.40.2.309. [DOI] [PubMed] [Google Scholar]
- Jackendoff R, Pinker S. The nature of the language faculty and its implications for evolution of language (reply to Fitch, Hauser, and Chomsky) Cognition. 2005;97:211–225. [Google Scholar]
- Jerison HJ. Evolution of the Brain and Intelligence. New York: Academic Press; 1973. [Google Scholar]
- Johnson JI, Kirsch JA, Switzer RC. Brain traits through phylogeny: evolution of neural characters. Brain Behav Evol. 1984;24:169–176. doi: 10.1159/000121314. [DOI] [PubMed] [Google Scholar]
- Jordan KE, Brannon EM, Logothetis NK, Ghazanfar AA. Monkeys match the number of voices they hear to the number of faces they see. Curr Biol. 2005;15:1034–1038. doi: 10.1016/j.cub.2005.04.056. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Why is brain size so important: design problems and solutions as neocortex gets bigger or smaller. Brain and Mind. 2000;1:7–23. [Google Scholar]
- Kawai M. Newly-acquired pre-cultural behavior of the natural troop of Japanese monkeys on Koshima islet. Primates. 1965;6:1–30. [Google Scholar]
- Kelley J. Life history and cognitive evolution in the apes. In: Russon AE, Begun DR, editors. The Evolution of Thought: Evolutionary Origins of Great Ape Intelligence. Cambridge: Cambridge University Press; 2004. pp. 280–297. [Google Scholar]
- Kouprina N, Pavlicek A, Mochida GH, et al. Accelerated evolution of the ASPM gene controlling brain size begins prior to human brain expansion. PLoS Biol. 2004;2:E126. doi: 10.1371/journal.pbio.0020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Lalueza-Fox C, Orlando L, et al. The derived FOXP2 variant of modern humans was shared with Neandertals. Curr Biol. 2007;17:1908–1912. doi: 10.1016/j.cub.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Krubitzer L. The magnificent compromise: cortical field evolution in mammals. Neuron. 2007;56:201–208. doi: 10.1016/j.neuron.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Corticobulbar connections to the pons and lower brainstem in man. Brain. 1958a;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Some projections from the peri-central cortex to the pons and lower brainstem in monkey and chimpanzee. J Comp Neurol. 1958b;110:221–251. doi: 10.1002/cne.901100205. [DOI] [PubMed] [Google Scholar]
- Ledbetter DH, Basen JA. Failure to demonstrate self-recognition in gorillas. Am J Primatol. 1982;2:307–310. doi: 10.1002/ajp.1350020309. [DOI] [PubMed] [Google Scholar]
- Leigh SR. Brain growth, life history, and cognition in primate and human evolution. Am J Primatol. 2004;62:139–164. doi: 10.1002/ajp.20012. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Lethmate J, Dücker G. Untersuchungen zum Selbsterkennen im Spiegel bei Orang-Utans und einigen anderen Affenarten (Studies on mirror self-recognition by orangutans and some other primate species) Zeitschrift für Tierpsychologie. 1973;33:248–269. [PubMed] [Google Scholar]
- Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci. 2001;4:931–936. doi: 10.1038/nn0901-931. [DOI] [PubMed] [Google Scholar]
- Limongelli L, Boysen ST, Visalberghi E. Comprehension of cause-effect relations in a tool-using task by chimpanzees (Pan troglodytes. J Comp Psychol. 1995;109:18–26. doi: 10.1037/0735-7036.109.1.18. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO. The natural history of human gait and posture. Part 1. Spine and pelvis. Gait Posture. 2005;21:95–112. doi: 10.1016/j.gaitpost.2004.01.001. [DOI] [PubMed] [Google Scholar]
- MacLeod CE. What's in a brain? The question of a distinctive brain anatomy in great apes. In: Russon AE, Begun DR, editors. The Evolution of Thought: Evolutionary Origins of Great Ape Intelligence. Cambridge: Cambridge University Press; 2004. pp. 105–121. [Google Scholar]
- MacLeod CE, Zilles K, Schleicher A, Rilling JK, Gibson KR. Expansion of the neocerebellum in Hominoidea. J Hum Evol. 2003;44:401–429. doi: 10.1016/s0047-2484(03)00028-9. [DOI] [PubMed] [Google Scholar]
- Martin RD. Human Brain Evolution in an Ecological Context. New York: American Museum of Natural History; 1983. [Google Scholar]
- Martin RD. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton: Princeton University Press; 1990. [Google Scholar]
- Matano S. Brief communication: proportions of the ventral half of the cerebellar dentate nucleus in humans and great apes. Am J Phys Anthropol. 2001;114:163–165. doi: 10.1002/1096-8644(200102)114:2<163::AID-AJPA1016>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Ybk Phys Anthropol. 1997;40:201–232. [Google Scholar]
- Meltzoff AN, Moore KM. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Menzel EW, Halperin S. Purposive behavior as a basis for objective communication between chimpanzees. Science. 1975;189:652–654. doi: 10.1126/science.1162352. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Hersh LB, Mash DC, Geula C. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J Comp Neurol. 1992;318:316–328. doi: 10.1002/cne.903180308. [DOI] [PubMed] [Google Scholar]
- Mitani JC. Demographic influences on the behavior of chimpanzees. Primates. 2006;47:6–13. doi: 10.1007/s10329-005-0139-7. [DOI] [PubMed] [Google Scholar]
- Mithen S. The Prehistory of the Mind. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Moore JK. The primate cochlear nuclei: loss of lamination as a phylogenetic process. J Comp Neurol. 1980;193:609–629. doi: 10.1002/cne.901930303. [DOI] [PubMed] [Google Scholar]
- Myowa-Yamakoshi M, Tomonaga M, Tanaka M, Matsuzawa T. Imitation in neonatal chimpanzees (Pan troglodytes. Dev Sci. 2004;7:437–442. doi: 10.1111/j.1467-7687.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- Nagell K, Olguin R, Tomasello M. Processes of social learning in the tool use of chimpanzees (Pan troglodytes) and human children (Homo sapiens. J Comp Psychol. 1993;107:174–186. doi: 10.1037/0735-7036.107.2.174. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Vogt BA, Morrison JH, Hof PR. Spindle neurons of the human anterior cingulate cortex. J Comp Neurol. 1995;355:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Schürmann M, Amunts K, Hari R. Broca's region: from action to language. Physiology. 2005;20:60–69. doi: 10.1152/physiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Kaas JH. The emergence and evolution of mammalian neocortex. Trends Neurosci. 1995;18:373–379. doi: 10.1016/0166-2236(95)93932-n. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Tomonaga M, Ishii K, Kawai N, Tanaka M, Matsuzawa T. An infant chimpanzee (Pan troglodytes) follows human gaze. Anim Cogn. 2002;5:107–114. doi: 10.1007/s10071-002-0133-z. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Tanaka M, Tomonaga M. Looking back: the ‘representational mechanism’ of joint attention in an infant chimpanzee (Pan troglodytes. Japanese Psychol Res. 2004;46:236–245. [Google Scholar]
- Okamoto-Barth S, Call J, Tomasello M. Great apes’ understanding of other individual's line of sight. Psychol Sci. 2007;18:462–468. doi: 10.1111/j.1467-9280.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- Okanoya K. Sexual display as a syntactical vehicle: the evolution of syntax in birdsong and human language through sexual selection. In. In: Wray A, editor. The Transition to Language. New York: Oxford University Press; 2002. pp. 46–63. [Google Scholar]
- Orban GA, Claeys K, Nelissen K, et al. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Panger M, Perry S, Rose LM, Gros-Louis J, Vogel E, Mackinnon KC, Baker M. Cross-site differences in foraging behavior of white-faced capuchins (Cebus capucinus. Am J Phys Anthropol. 2002;119:52–66. doi: 10.1002/ajpa.10103. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Anatomical differences between the neocortex of man and the other primates. Brain Behav Evol. 1973;7:337–359. doi: 10.1159/000124422. [DOI] [PubMed] [Google Scholar]
- Passingham RE. The brain and intelligence. Brain Behav Evol. 1975a;11:1–15. doi: 10.1159/000123620. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Changes in the size and organisation of the brain in man and his ancestors. Brain Behav Evol. 1975b;11:73–90. doi: 10.1159/000123626. [DOI] [PubMed] [Google Scholar]
- Patterson FGP, Cohn RH. Self-recognition and self-awareness in lowland gorillas. In: Parker ST, Mitchell RW, Boccia ML, editors. Self-awareness in Animals and Humans: Developmental Perspectives. New York: Cambridge University Press; 1994. pp. 273–290. [Google Scholar]
- Perry S, Panger M, Rose LM, Baker M, Gros-Louis J, Jack K, Mackinnon KC, Manson J, Fedian L, Pyle K. Traditions in wild white-faced capuchin monkeys. In: Fragaszy D, Perry S, editors. The Biology of Traditions. Cambridge: Cambridge University Press; 2003. pp. 391–425. [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Phil Trans R Soc B. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Amsterdam: Elsevier; 1994. pp. 17–58. [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque homologue of Broca's area. Nature. 2005;435:1235–1238. doi: 10.1038/nature03628. [DOI] [PubMed] [Google Scholar]
- Pinker S, Jackendoff R. The faculty of language: what's special about it? Cognition. 2005;95:201–236. doi: 10.1016/j.cognition.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Plotnik JM, de Waal FBM, Reiss D. Self-recognition in an Asian elephant. Proc Natl Acad Sci USA. 2006;103:17053–17057. doi: 10.1073/pnas.0608062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Salama SR, Lambert N, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–172. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]
- Pollick AS, de Waal FB. Ape gestures and language evolution. Proc Natl Acad Sci USA. 2007;104:8184–8189. doi: 10.1073/pnas.0702624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popesco MC, Maclaren EJ, Hopkins J, et al. Human lineage-specific amplification, selection, and neuronal expression of DUF1220 domains. Science. 2006;313:1304–1307. doi: 10.1126/science.1127980. [DOI] [PubMed] [Google Scholar]
- Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P, Mishkin M. Species-specific calls evoke asymmetric activity in the monkey's temporal poles. Nature. 2004;427:448–451. doi: 10.1038/nature02268. [DOI] [PubMed] [Google Scholar]
- Potts R. Paleoenvironmental basis of cognitive evolution in great apes. Am J Primatol. 2004;62:209–228. doi: 10.1002/ajp.20016. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ. Failure to find self-recognition in Asian elephants (Elephas maximus) in contrast to their use of mirror cues to discover hidden food. J Comp Pyschol. 1989;103:122–131. [Google Scholar]
- Povinelli DJ. Folk Physics for Apes: The Chimpanzee's Theory of How the World Works. Oxford: Oxford University Press; 2000. [Google Scholar]
- Povinelli DJ, Cant JGH. Arboreal clambering and the evolution of self-conception. Q Rev Biol. 1995;70:393–421. doi: 10.1086/419170. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Eddy TJ. Chimpanzees: joint visual attention. Psychol Sci. 1996a;7:129–135. [Google Scholar]
- Povinelli DJ, Eddy TJ. Factors influencing young chimpanzee's (Pan troglodytes) recognition of attention. J Comp Psychol. 1996b;110:336–345. doi: 10.1037/0735-7036.110.4.336. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Eddy TJ. What young chimpanzees know about seeing. Monogr Soc Res Child Dev. 1996c;61:i–vi. 1–152, 153–191. [PubMed] [Google Scholar]
- Povinelli DJ, Eddy TJ. Specificity of gaze-following in young chimpanzees. Br J Dev Psychol. 1997;15:213–222. [Google Scholar]
- Povinelli DJ, Vonk J. Chimpanzee minds: suspiciously human? Trends Cogn Sci. 2003;7:157–160. doi: 10.1016/s1364-6613(03)00053-6. [DOI] [PubMed] [Google Scholar]
- Prabhakar S, Noonan JP, Pääbo S, Rubin EM. Accelerated evolution of conserved noncoding sequences in humans. Science. 2006;314:786. doi: 10.1126/science.1130738. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind. Behav Brain Sci. 1978;1:515–526. [Google Scholar]
- Preuss TM. What is it like to be a human? In: Gazzaniga MS, editor. The Cognitive Neurosciences III. Cambridge, MA: MIT Press; 2004. pp. 5–22. [Google Scholar]
- Preuss TM, Coleman GQ. Human-specific organization of primary visual cortex: alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A. Cereb Cortex. 2002;12:671–691. doi: 10.1093/cercor/12.7.671. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J Comp Neurol. 1991;310:475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Qi H, Kaas JH. Distinctive compartmental organization of human primary visual cortex. Proc Natl Acad Sci USA. 1999;96:11601–11606. doi: 10.1073/pnas.96.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM, Cáceres M, Oldham MC, Geschwind DH. Human brain evolution: insights from microarrays. Nat Rev Genet. 2004;5:850–860. doi: 10.1038/nrg1469. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, et al. Cholinergic innervation of the frontal cortex: differences among humans, chimpanzees, and macaque monkeys. J Comp Neurol. 2008a;506:409–424. doi: 10.1002/cne.21546. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, et al. Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cereb Cortex. 2008b;18:584–597. doi: 10.1093/cercor/bhm089. [DOI] [PubMed] [Google Scholar]
- Reader SM, Laland KN. Social intelligence, innovation, and enhanced brain size in primates. Proc Natl Acad Sci USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Marino L. Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proc Natl Acad Sci USA. 2001;98:5937–5942. doi: 10.1073/pnas.101086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell L, Whitehead H. Culture in whales and dolphins. Behav Brain Sci. 2001;24:309–382. doi: 10.1017/s0140525x0100396x. [DOI] [PubMed] [Google Scholar]
- Rilling JK. Human and nonhuman primate brains: are they allometrically scaled versions of the same design? Evol Anthropol. 2006;15:65–77. [Google Scholar]
- Rilling JK, Insel TR. Evolution of the cerebellum in primates: differences in relative volume among monkeys, apes and humans. Brain Behav Evol. 1998;52:308–314. doi: 10.1159/000006575. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J Hum Evol. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Seligman RA. A quantitative morphometric comparative analysis of the primate temporal lobe. J Hum Evol. 2002;42:505–533. doi: 10.1006/jhev.2001.0537. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, et al. A comparative diffusion tensor imaging (DTI) study of the arcuate fasciculus language pathway in humans, chimpanzees and rhesus macaques. Am J Phys Anthropol. 2007;44(Suppl):199. [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V. From mirror neurons to imitation: facts and speculations. In: Prinz W, Meltzoff AN, editors. The Imitative Mind: Development, Evolution and Brain Bases. Cambridge: Cambridge University Press; 2002. pp. 247–266. [Google Scholar]
- Robson SL, Wood B. Hominin life history: reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat P. The Infant's World. Cambridge, MA: Harvard University Press; 2001. [Google Scholar]
- Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9:250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Rumbaugh DM. Competence, cortex, and primate models: a comparative primate perspective. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of the Prefrontal Cortex: Evolution, Neurobiology, and Behavior. Baltimore, MD: Paul H. Brookes; 1997. pp. 117–139. [Google Scholar]
- Rumbaugh DM, Savage-Rumbaugh S, Hegel MT. Summation in the chimpanzee (Pan troglodytes. J Exp Psychol Anim Behav Process. 1987;13:107–101. [PubMed] [Google Scholar]
- Sacher GA. The role of brain maturation in the evolution of the primates. In: Armstrong E, Falk D, editors. Primate Brain Evolution: Methods and Concepts. New York: Plenum Press; 1982. pp. 97–112. [Google Scholar]
- Santos LR, Hauser MD. A non-human primate's understanding of solidity: dissociations between seeing and acting. Dev Sci. 2002;5:F1–F7. [Google Scholar]
- Santos LR, Pearson HM, Spaepen GM, Tsao F, Hauser MD. Probing the limits of tool competence: experiments with two non-tool-using species (Cercopithecus aethiops and Saguinus oedipus. Anim Cogn. 2006;9:94–109. doi: 10.1007/s10071-005-0001-8. [DOI] [PubMed] [Google Scholar]
- Santos LR, Rosati A, Sproul C, Spaulding B, Hauser MD. Means-means-end tool choice in cotton-top tamarins (Saguinus oedipus): finding the limits on primates’ knowledge of tools. Anim Cogn. 2005;8:236–246. doi: 10.1007/s10071-004-0246-7. [DOI] [PubMed] [Google Scholar]
- Savage-Rumbaugh S, Shanker SG, Taylor TJ. Apes, Language, and the Human Mind. New York: Oxford University Press; 1998. [Google Scholar]
- van Schaik CP, Deaner RO, Merrill MY. The conditions for tool use in primates: implications for the evolution of material culture. J Hum Evol. 1999;36:719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- Schenker NM, Desgouttes AM, Semendeferi K. Neural connectivity and cortical substrates of cognition in hominoids. J Hum Evol. 2005;49:547–569. doi: 10.1016/j.jhevol.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat Neurosci. 2005;8:242–252. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Carlin DA, Allman JM, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H. The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. J Hum Evol. 2000;38:317–332. doi: 10.1006/jhev.1999.0381. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat Neurosci. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- Shariff GA. Cell counts in the primate cerebral cortex. J Comp Neurol. 1953;98:381–400. doi: 10.1002/cne.900980302. [DOI] [PubMed] [Google Scholar]
- Sherwood CC. Comparative anatomy of the facial motor nucleus in mammals, with an analysis of neuron numbers in primates. Anat Rec. 2005;287A:1067–1079. doi: 10.1002/ar.a.20259. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Hof PR. The evolution of neuron types and cortical histology in apes and humans. In: Preuss TM, Kaas JH, editors. Evolution of Nervous Systems. Vol. 4. The Evolution of Primate Nervous Systems. Oxford: Academic Press; 2007. pp. 355–378. [Google Scholar]
- Sherwood CC, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca's area homologue in African great apes: implications for language evolution. Anat Rec. 2003;271A:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Holloway RL, Erwin JM, Hof PR. Cortical orofacial motor representation in Old World monkeys, great apes, and humans. II. Stereologic analysis of chemoarchitecture. Brain Behav Evol. 2004;63:82–106. doi: 10.1159/000075673. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Hof PR, Holloway RL, et al. Evolution of the brainstem orofacial motor system in primates: a comparative study of trigeminal, facial, and hypoglossal nuclei. J Hum Evol. 2005a;48:45–84. doi: 10.1016/j.jhevol.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Holloway RL, Semendeferi K, Hof PR. Is prefrontal white matter enlargement a human evolutionary specialization? Nat Neurosci. 2005b;8:537–538. doi: 10.1038/nn0505-537. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, et al. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA. 2006;103:13606–11311. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Wahl E, Erwin JM, Hof PR, Hopkins WD. Histological asymmetries of primary motor cortex predict handedness in chimpanzees (Pan troglodytes. J Comp Neurol. 2007;503:525–537. doi: 10.1002/cne.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Ioannides AA. Contribution of the human superior parietal lobule to spatial selection process: an MEG study. Brain Res. 2001;897:164–168. doi: 10.1016/s0006-8993(01)02088-1. [DOI] [PubMed] [Google Scholar]
- Shillito DJ, Gallup GG, Jr, Beck BB. Factors affecting mirror behaviour in western lowland gorillas, Gorilla gorilla. Anim Behav. 1999;57:999–1004. doi: 10.1006/anbe.1998.1062. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- Singleton M. Fossil hominoid diets, extractive foraging, and the origins of great ape intelligence. In: Russon AE, Begun DR, editors. The Evolution of Thought: Evolutionary Origins of Great Ape Intelligence. Cambridge: Cambridge University Press; 2004. pp. 298–319. [Google Scholar]
- Slobin D. Learning to think for speaking: native language, cognition, and rhetorical style. Pragmatics. 1991;1:7–25. [Google Scholar]
- Smith BH. Dental development as a measure of life history in primates. Evolution. 1989;43:683–688. doi: 10.1111/j.1558-5646.1989.tb04266.x. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Stout D, Chaminade T. The evolutionary neuroscience of tool making. Neuropsychologia. 2007;45:1091–1100. doi: 10.1016/j.neuropsychologia.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Stout D, Toth N, Schick K, Chaminade T. Neural correlates of Early Stone Age toolmaking: technology, language and cognition in human evolution. Philos Trans R Soc Lond B Biol Sci. doi: 10.1098/rstb.2008.0001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. Principles of Brain Evolution. Sunderland, MA: Sinauer Associates, Inc; 2005. [Google Scholar]
- Suarez SD, Gallup GG. Self-recognition in chimpanzees and orangutans, but not gorillas. J Hum Evol. 1981;10:175–188. [Google Scholar]
- Subiaul F. The imitation faculty in monkeys: evaluating its features, distribution and evolution. J Anthropol Sci. 2007;85:35–62. [Google Scholar]
- Subiaul F, Cantlon J, Holloway R, Terrace H. Cognitive imitation in rhesus macaques. Science. 2004;305:407–410. doi: 10.1126/science.1099136. [DOI] [PubMed] [Google Scholar]
- Subiaul F, Okamoto-Barth S, Barth J, Povinelli DJ. Human cognitive specializations. In: Preuss TM, Kaas JH, editors. Evolution of Nervous Systems. Vol. 4: The Evolution of Primate Nervous Systems. Oxford: Academic Press; 2006. pp. 509–528. [Google Scholar]
- Subiaul F, Romansky K, Cantlon J, Klein T, Terrace H. Cognitive imitation in 2-year old human children (Homo sapiens): a comparison with rhesus monkeys (Macaca mulatta. Anim Cogn. 2007;10:369–375. doi: 10.1007/s10071-006-0070-3. [DOI] [PubMed] [Google Scholar]
- Sulkowski GM, Hauser MD. Can rhesus monkeys spontaneously subtract? Cognition. 2001;79:239–262. doi: 10.1016/s0010-0277(00)00112-8. [DOI] [PubMed] [Google Scholar]
- Tilney F. The Brain from Ape to Man. New York: Hoeber; 1928. [Google Scholar]
- Thompson D, Russell J. The ghost condition: imitation versus emulation in young children's observational learning. Dev Psychol. 2004;40:882–889. doi: 10.1037/0012-1649.40.5.882. [DOI] [PubMed] [Google Scholar]
- Tomasello M. The Cultural Origins of Human Cognition. Cambridge, MA: Harvard University Press; 1999. [Google Scholar]
- Tomasello M. On the different origins of symbols and grammar. In: Christiansen MH, Kirby S, editors. Language Evolution: The States of the Art. Oxford: Oxford University Press; 2003. pp. 94–110. [Google Scholar]
- Tomasello M, Call J. Primate Cognition. Oxford: Oxford University Press; 1997. [Google Scholar]
- Tomasello M, Carpenter M. The emergence of social cognition in three young chimpanzees. Monogr Soc Res Child Dev. 2005;70:vii–132. doi: 10.1111/j.1540-5834.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Call J, Hare B. Five primate species follow the visual gaze of conspecifics. Anim Behav. 1998;55:1063–1069. doi: 10.1006/anbe.1997.0636. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Fogleman T. The ontogeny of gaze following in chimpanzees, Pan troglodytes, and rhesus macaques, Macaca mulatta. Anim Behav. 2001;61:335–343. [Google Scholar]
- Tomasello M, Savage-Rumbaugh ES, Kruger AC. Imitative learning of objects by chimpanzees, enculturated chimpanzees, and human children. Child Dev. 1993;64:1688–1705. [PubMed] [Google Scholar]
- Tomonaga M. Attending to the others’ attention in macaques’ joint attention or not? Primate Res. 1999;15:425. [Google Scholar]
- Tooby J, Cosmides L. Conceptual foundations of evolutionary psychology. In: Buss DM, editor. The Handbook of Evolutionary Psychology. New York: Wiley; 2005. pp. 5–67. [Google Scholar]
- Uddin M, Wildman DE, Liu G, et al. Sister grouping of chimpanzees and humans as revealed by genome-wide phylogenetic analysis of brain gene expression profiles. Proc Natl Acad Sci USA. 2004;101:2957–2962. doi: 10.1073/pnas.0308725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Opazo JC, Wildman DE, et al. Molecular evolution of the cytochrome c oxidase subunit 5A gene in primates. BMC Evol Biol. 2008a;8:8. doi: 10.1186/1471-2148-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Goodman M, Erez O, et al. Distinct genomic signatures of adaptation in pre-and postnatal environments in human evolution. Proc Natl Acad Sci USA. 2008b;105:3215–3320. doi: 10.1073/pnas.0712400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HBM, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rats and primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Vallender EJ, Lahn BT. A primate-specific acceleration in the evolution of the caspase-dependent apoptosis pathway. Hum Mol Genet. 2006;15:3034–3040. doi: 10.1093/hmg/ddl245. [DOI] [PubMed] [Google Scholar]
- Visalberghi E. Tool use in Cebus. Folia Primatol. 1990;54:146–54. doi: 10.1159/000156438. [DOI] [PubMed] [Google Scholar]
- Visalberghi E, Fragszy DM, Savage-Rumbaugh ES. Performance in a tool-using task by common chimpanzees (Pan troglodytes), bonobos (Pan paniscus), an orangutan (Pongo pygmaeus), and capuchin monkeys (Cebus apella. J Comp Psychol. 1995;109:52–60. doi: 10.1037/0735-7036.109.1.52. [DOI] [PubMed] [Google Scholar]
- Vonk J, Povinelli DJ. Similarity and difference in the conceptual systems of primates: the unobservability hypothesis. In. In: Wasserman E, Zentall T, editors. Comparative Cognition: Experimental Explorations of Animal Intelligence. Oxford: Oxford University Press; 2006. pp. 363–387. [Google Scholar]
- de Waal FBM, Dindo M, Freeman CA, Hall MJ. The monkey in the mirror: hardly a stranger. Proc Natl Acad Sci USA. 2005;102:11140–11147. doi: 10.1073/pnas.0503935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Su B. Molecular evolution of microcephalin, a gene determining human brain size. Hum Mol Genet. 2004;13:1131–1137. doi: 10.1093/hmg/ddh127. [DOI] [PubMed] [Google Scholar]
- Want SC, Harris PL. Learning from other people's mistakes: causal understanding in learning to use a tool. Child Dev. 2001;72:431–443. doi: 10.1111/1467-8624.00288. [DOI] [PubMed] [Google Scholar]
- Watson KK, Jones TK, Allman JM. Dendritic architecture of the von Economo neurons. Neuroscience. 2006;141:1107–1112. doi: 10.1016/j.neuroscience.2006.04.084. [DOI] [PubMed] [Google Scholar]
- Westergaard GC. Structural analysis of tool-use by tufted capuchins (Cebus apella) and chimpanzees (Pan troglodytes. Anim Cogn. 1999;2:141–145. [Google Scholar]
- Whiten A. The second inheritance system of chimpanzees and humans. Nature. 2005;437:52–55. doi: 10.1038/nature04023. [DOI] [PubMed] [Google Scholar]
- Whiten A, van Schaik CP. The evolution of animal ‘cultures’ and social intelligence. Philos Trans R Soc Lond B Biol Sci. 2007;362:603–620. doi: 10.1098/rstb.2006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A, Byrne RW. The manipulation of attention in primate tactile deception. In: Byrne RW, Whiten A, editors. Machavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes and Humans. Oxford: Oxford University Press; 1988. pp. 211–223. [Google Scholar]
- Whiten A, Custance DM, Gomez JC, Teixidor P, Bard KA. Imitative learning of artificial fruit processing in children (Homo sapiens) and chimpanzees (Pan troglodytes. J Comp Psychol. 1996;110:3–14. doi: 10.1037/0735-7036.110.1.3. [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Charting cultural variation in chimpanzees. Behaviour. 2001;138:1489–1525. [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Cultures in chimpanzee. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Whiting BA, Barton RA. The evolution of the cortico-cerebellar complex in primates: anatomical connections predict patterns of correlated evolution. J Hum Evol. 2003;44:3–10. doi: 10.1016/s0047-2484(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Wildman DE, Uddin M, Liu G, Grossman LI, Goodman M. Implications of natural selection in shaping 99.4% nonsynonymous DNA identity between humans and chimpanzees: enlarging genus Homo. Proc Natl Acad Sci USA. 2003;100:7181–7188. doi: 10.1073/pnas.1232172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Britton NF, Franks NR. Chimpanzees and the mathematics of battle. Proc Biol Sci. 2002;269:1107–1112. doi: 10.1098/rspb.2001.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Freimer NB, De Young JA, et al. Normal variants of microcephalin and ASPM do not account for brain size variability. Hum Mol Gen. 2006;15:2025–2029. doi: 10.1093/hmg/ddl126. [DOI] [PubMed] [Google Scholar]
- Wrangham R, Peterson D. Demonic Males: Apes and the Origins of Human Violence. New York: Houghton Mifflin Co; 1996. [Google Scholar]
- Wray A. Holistic utterances in protolanguage: the link from primates to humans. In: Knight C, Hurford JR, Studdert-Kennedy M, editors. The Evolutionary Emergence of Language: Social Function and the Origins of Linguistic Form. Cambridge: Cambridge University Press; 2000. pp. 285–302. [Google Scholar]
- Zawidzki T. Sexual selection for syntax and kin selection for semantics: problems and prospects. Biology and Philosophy. 2006;21:453–470. [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain Behav Evol. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]
- Zuberbühler K. Alarm calls – Evolutionary and cognitive mechanisms. In: Brown K, editor. Encyclopedia of Language and Linguistics. Vol. 2. Oxford: Elsevier; 2006. pp. 143–155. [Google Scholar]