Abstract
Patterns of transcription factor expression establish a blueprint for the vertebrate forebrain early in embryogenesis. In the future diencephalon, several genes with patterned expression have been identified, yet their specific functions and interactions between them are not well understood. We have uncovered a crucial role for one such gene, zic2a, during formation of the anterior diencephalon in zebrafish. We show that zic2a is required for transcription of the prethalamic markers arx and dlx2a. This function is required during early steps of prethalamic development, soon after its specification. zic genes are evolutionarily related to glis, transcription factors that mediate hedgehog signaling. Intriguingly, the hedgehog signaling pathway also acts to promote development of the prethalamus. We asked if zic2a interacts with hedgehog signaling in the context of forebrain development in zebrafish. Our data show that hedgehog signaling and zic2a function at different times, and therefore act in parallel pathways during forebrain development. Taken together, our results identify Zic2a as a novel regulator of prethalamic development, and show that it functions independently of hedgehog signaling.
Keywords: Zic, arx, dlx2a, forebrain, prethalamus, preoptic area
Introduction
The vertebrate forebrain originates as a sheet of cells in the anterior neural plate during gastrulation. The forebrain gradually acquires its characteristic morphological complexity and cell type diversity, in part through progressive refinement of regional patterns. The genetic mechanisms underlying early forebrain patterning include intercellular communication via secreted growth factors and intracellular events, often involving activation of region-specific transcription factors (Wilson and Houart, 2004; Rhinn et al., 2006). The hedgehog (Hh) family of growth factors and the signaling cascade downstream of it are essential for early forebrain regionalization (Fuccillo et al., 2006; Ingham and Placzek, 2006; Bertrand and Dahmane, 2006). In humans, mutations that disrupt Hh signaling are a major cause of holoprosencephaly (HPE), a birth defect characterized by forebrain abnormalities (Dubourg et al., 2007; Monuki, 2007). Essential roles for Hh signaling in the developing forebrain have also been demonstrated in mouse (Hayhurst et al., 2007; Chiang et al., 1996; Rallu et al., 2002), chick (Kiecker and Lumsden, 2004) and zebrafish (Karlstrom et al., 1999; Tyurina et al., 2005; Scholpp et al., 2006), where Hh signaling promotes formation of the anterior diencephalon (AD).
ZIC2, a zinc-finger transcription factor belonging to the Zic (zinc finger of the cerebellum) gene family (Benedyk et al., 1994; reviewed in Aruga, 2004; Merzdorf, 2007) is among the few genes outside of the Hh pathway also causally linked to HPE. HPE is observed in Zic2 knock-down mice (Nagai et al., 2000), demonstrating a critical role for Zic2 during mouse forebrain development. Mouse Zic2 is also required in more posterior brain subdivisions and in the spinal cord during neural tube closure (Nagai et al., 2000). Similarly, morpholino-mediated knock-down of zebrafish zic2a causes dorsal neural tube defects (Nyholm et al., 2007). Thus, Zic2 function in the forebrain is clearly important and conserved, yet the mechanism of this function remains largely unexplored.
All Zics share a highly conserved DNA binding domain composed of five zinc-finger motifs, as well as N-terminal and C-terminal domains of unknown functions (Aruga, 2004; Merzdorf, 2007). Within the zinc-finger domain, Zics share significant sequence similarity with Glis, components of the Hh pathway. This similarity suggests a common evolutionary origin of Zic- and Gli-encoding genes. DNA binding specificities of Zics and Glis have also been conserved since several Zics are able to bind Gli recognition sites in vitro (Mizugishi et al., 2001). Furthermore, biochemical studies have shown that Zic and Gli may modulate each other’s function through direct protein-protein interactions (Koyabu et al., 2001). Altogether, this evidence suggests that Zics may modulate Hh signaling during vertebrate development, and that Zic2 in particular may do so in the context of the developing forebrain. However, a direct experimental test of such an interaction has not been reported.
The zebrafish genome contains two Zic2 homologs, zic2a and zic2b (Toyama et al., 2004). We present evidence that zic2a plays an early role in the zebrafish forebrain in promoting formation of the prethalamus (PT), a division of the AD. Since Hh signaling plays a similar role in this tissue, we asked if zic2a and components of the Hh pathway genetically interact during PT formation. Our data show that Zic2a and Hh pathway functions are clearly separable in time, with Zic2a acting early in PT development, soon after its initial specification, and Hh signaling playing a later role in PT maturation. Thus, Zic2a acts independently of Hh signaling to promote early formation of the AD.
Methods and Materials
Zebrafish strains and embryo culture
Adult zebrafish were maintained according to established methods (Westerfield, 1995). Embryos were obtained from natural matings and staged according to (Kimmel et al., 1995). The following zebrafish strains were used: wild type AB, smub641 (Varga et al., 2001), syut4 (Odenthal et al., 2000), and Tg(HuC:gfp) (Park et al., 2000).
Mutant genotyping
syut4 homozygous mutant embryos were positively identified either by PCR (forward: 5′-ACAGAAGGCCGTGAAGGAC-3′ and reverse: 5′-GCCACGTTCCCATTTGATAC-3′) after ISH or by lack of shha expression in a double ISH. smub641 homozygous mutant embryos were identified by lack of ptc1 expression in a double ISH.
In situ hybridization (ISH)
Antisense RNA probes were transcribed using the MAXIscript kit (Ambion) from the following plasmid templates:arx (Miura et al., 1997), dbx1a (Hjorth et al., 2002), dlx2a (Amores et al., 1998; (Akimenko et al., 1994)), eomesa (Costagli et al., 2002), emx1 (Kawahara and Dawid, 2002), fezf2 (Jeong et al., 2007), foxg1 (Rohr et al., 2001), gfp (Koster and Fraser, 2001), gli1, gli2a (Karlstrom et al., 2003), gli3 (Tyurina et al., 2005), irx1b (Lecaudey et al., 2005), isl1 (Korzh et al., 1993), itnp (Unger and Glasgow, 2003) lef1 (Dorsky et al., 1999), lhx1a (Toyama and Dawid, 1997), nkx2.2a (Karlstrom et al., 2003), otpb (Eaton and Glasgow, 2007), pax6a (Krauss et al., 1991), ptc1 (Vanderlaan et al., 2005), rx3 (Jeong et al., 2007), shha (Etheridge et al., 2001), sim1 (Serluca and Fishman, 2001) six3b (Seo et al., 1998), titf1a, titf1b (Rohr et al., 2001), and zic2a (Grinblat and Sive, 2001). ISH was carried out as previously described (Gillhouse et al., 2004). The PT domain, delimited by the expression of foxg1 and shha on either side, was measured using the outline tool (AxioVision 3.0) on an Axioskop2 plus (Zeiss).
Proliferation analysis
BrdU incorporation in 10s and 17s embryos was carried out as previously described (Shepard et al., 2004). Embryos were fixed immediately after incorporation. After antibody staining and fluorescent detection, embryos were counterstained with SYTOX green and mounted in DABCO for confocal microscopy. The total cell number and the number of BrdU labelled cells in the approximate prethalamic area were counted manually in four sections per embryo. The prethalamic area was estimated from the arx expression pattern at 10s and the dlx2a expression pattern at 18s. Average total cell number at 10s or 17s were not significantly different between conMOs and zic2aMOs.
Immunohistochemistry and histology
Embryos were fixed in 4% paraformaldehyde in PBS and stained using the following antibodies: anti-human HuC/D (1:500, Molecular Probes, #A-21271), anti-activated caspase-3 (1:200, BD Pharmingen, #559565), anti-BrDU (1:100, Roche, #11170376001) Alexa488-conjugated goat anti-rabbit secondary (1:1000, Molecular Probes), and Alexa568-conjugated goat anti-mouse secondary (1:1000, Molecular Probes). Embryos were embedded in Eponate 12 medium (Ted Pella) and sections (4uM) were cut with a steel blade on an American Optical Company microtome. Nuclei were counterstained with Methyl Red. Confocal images taken with a 25X lens on an Axiovert 100M (Carl Zeiss MicroImaging, Inc.) with Lasersharp Confocal Package (model 1024, Bio-Rad) or with a 40X lens on an Olympus FV1000 with FV10-ASW software (Olympus).
Knockdown assays
Three antisense morpholino oligomers were used to knock down expression of Zic2a in this study: two translation-blocking MOs (zic2a AUG = CGATGAAGTTCAATCCCCGCTCACA, and zic2a PROX = CTCTTTCAAGCAGTCTATTCACGGC), and a splice-blocking MO (zic2aMO = CTCACCTGAGAAGGAAAACATCATA) (Nyholm et al., 2007). conMO = standard control MO (Genetools). MOs were diluted in 1X Danieau buffer (Nasevicius and Ekker, 2000) to 1–2ng/nl (zic2aMO), 4–6ng/nl (Zic2a AUG and PROX), or 3–4ng/nl (conMO).1nl was injected at 1–2 cell stage. Cyclopamine (Sigma #C4116 or Toronto Research Chemicals #C988400) was used at 10μM as previously described (Tyurina et al., 2005).
Results
Zic2a functions in the prethalamus during forebrain development
zic2a is expressed broadly in the anterior neural plate starting at mid-gastrulation (Grinblat and Sive, 2001; Toyama et al., 2004; Nyholm et al., 2007). By early somitogenesis (4s), zic2a transcription was restricted to several subdivisions of the forebrain primordium, including the prospective telencephalon, retina, and a domain fated to give rise to the PT (Staudt and Houart, 2007; arrow in Fig. 1A). zic2a expression in the PT primordium was transient, since it was not detected before the 4s stage (not shown) or after the 8s stage (Fig. 1B, C). Starting at 8s, zic2a was expressed in the thalamus, pretectum, and in part of the retina. Expression of arx, a marker of the early PT (Staudt and Houart, 2007; Miura et al., 1997), overlapped the medial portion of the zic2a expression domain at 4s (Fig. 1D and data not shown). Subsequently arx was found in a domain adjacent to zic2a at 8s (Fig. 1E) and 12s (Fig. 1F).
Fig. 1. Zic2a is required for maintenance, but not for initiation of arx expression in the PT primordium.
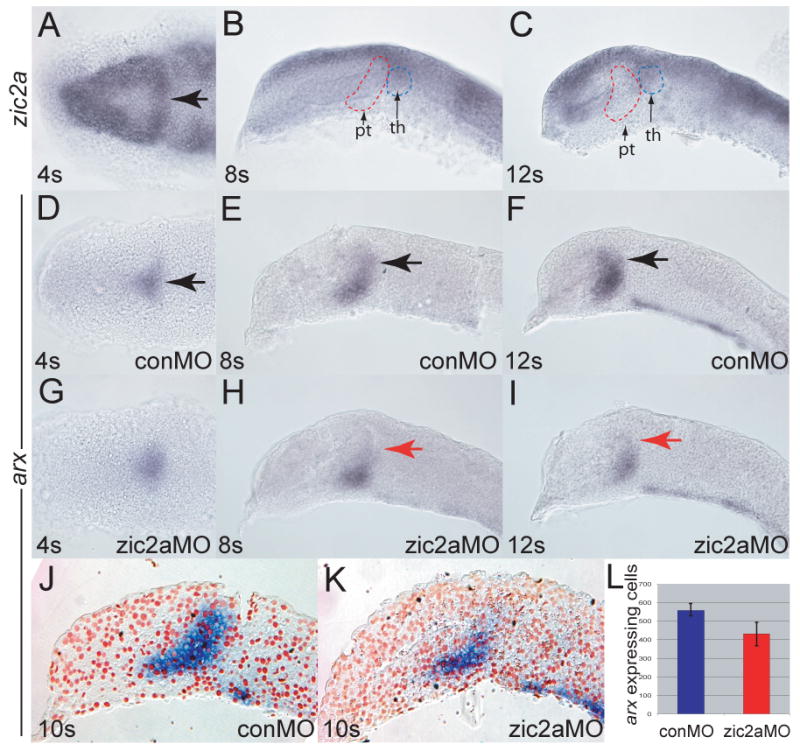
Embryos were stained by ISH for expression of zic2a (A-C) or arx (D-I). (A) Uninjected embryos express zic2a transiently in the early PT at 4 somites. (B, C) zic2a is not expressed in the PT at 8 and 12 somites. Prethalamus (pt) and thalamus (th) are outlined for reference. (D-E) Normal arx expression in the PT primordium of control MO injected embryos at 4, 8 and 12 somites. (G-H) arx expression in zic2a morphants. (G) arx is expressed normally at 4s (44/50 embryos, 3 exp.). (H) arx is mildly reduced at 8 somites (8/23 embryos, 2 exp.). (I) arx is drastically reduced at 12 somites (28/33 embryos, 2 exp.). (J, K) Representative parasagittal sections of conMO-and zic2aMO-injected embryos stained for arx by ISH at 10s. (L) Graph of average number of arx expressing cells in conMO (n = 3) and zic2aMO-injected (n = 3) embryos (results significant at p = 0.05). All embryos are shown with anterior to the left. A, D and G are dorsal views, all others are lateral views. Arrows mark the PT primordium.
The early and widespread expression of zic2a in the forebrain primordium suggested an early role for Zic2a. To test this hypothesis, Zic2a was knocked down using a splice-blocking antisense morpholino oligonucleotide specific for zic2a (zic2aMO), described previously (Nyholm et al., 2007). The overall telencephalic and diencephalic pattern formed correctly in zic2a morphants, as indicated by correct expression of telencephalic markers (six3b and fezf2), eye field markers (six3b and rx3), as well as thalamic (irx1b) and hypothalamic (fezf2) markers (Supplementary Fig. 1). In contrast, expression of the PT marker arx was initiated correctly in zic2a morphants (4s, Fig. 1G), but was not maintained, becoming mildly reduced by 8s (Fig. 1H), and strongly reduced by 10s (Fig. 1I-L). arx expression was similarly reduced using non-overlapping translation-blocking MOs against zic2a (Supplementary Fig. 2). Interestingly, fezf2 expression in the PT primordium was not affected in Zic2a-depleted embryos (Supplementary Fig. 1). These data suggest that initiation of arx transcription occurs independently of Zic2a, but its maintenance requires Zic2a function.
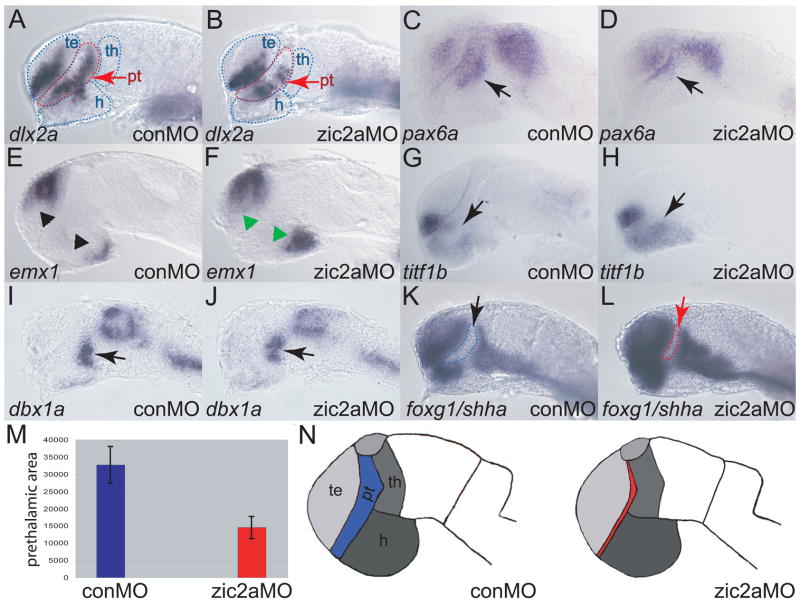
We next asked if Zic2a also functioned later in the developing forebrain, where it continues to be expressed. Forebrain pattern in zic2a morphants was assayed at the end of somitogenesis using a panel of markers (Supplemental Table 1). Several markers of the AD (PT and preoptic area), had reduced expression domains in zic2a morphants. These markers included dlx2a (Fig. 2A, B), pax6a (Fig. 2C, D), and eomesa (Supplementary Fig. 3). We confirmed the specificity of the AD morphant defect using non-overlapping translation-blocking MOs against zic2a, and found them to cause a similar dlx2a reduction (Supplementary Fig. 2). dlx2a reduction in the PT was evident by 17–18s (Supplementary Fig. 4), soon after it is first expressed there (Akimenko et al., 1994). The telencephalon and hypothalamus of Zic2a-depleted embryos showed a mild expansion of posterior markers emx1 (Fig. 2E, F), titf1b (Fig. 2G, H), and titf1a (Supplementary Fig. 3). The thalamus, marked by dbx1a, was patterned normally (Fig. 2I, J).
Fig. 2. Zic2a functions primarily in the PT during forebrain development.
The effect of Zic2a depletion on overall pattern in the forebrain at Prim-5 was examined using ISH with several markers of forebrain subdivisions (see Supplemental Table 1 for numbers). B) dlx2a expression is reduced in the PT, but not affected in the telencephalon of zic2a morphants. (C, D) pax6a expression is reduced in the PT region of Zic2a depleted embryos (arrows), but not affected in the telencephalon. (E, F) Expression of emx1, a marker of posterior telencephalon and posterior hypothalamus is expanded in both domains. (G, H) titf1b expression in the hypothalamus is expanded anteriorly inzic2a morphants. (I, J) The thalamus, marked by dbx1a, is formed normally. (K, L) Expression of foxg1 in the telencephalon and shha in the ZLI are normal in zic2a morphants. Note that the AD area, bordered by expression of foxg1 anteriorly and shha posteriorly, is reduced in zic2a morphants. (M) The AD area was measured in pixels2 using Axiovision software (Zeiss). The bar graphs represent average AD areas calculated from 12 conMO injected embryos and 18 zic2aMO injected embryos. Standard error bars shown, results significant at p = .001. (N) Summary of the effects of Zic2a depletion on forebrain regionalization. The strongest defect is observed in the AD, indicated in red. All embryos are at Prim-5 stage and are shown in lateral views with anterior to the left. Arrows mark the PT and arrowheads mark expanded domains in the telencephalon and hypothalamus. te = telencephalon, th = thalamus, pt = prethalamus, h = hypothalamus.
We extended our analysis of the AD reduction observed in zic2a morphants by asking if it was smaller, or if it was correctly sized but mispatterned at the end of somitogenesis. The AD area was demarcated by telencephalic expression of foxg1 anteriorly and expression of shha, a ZLI marker, posteriorly (Fig. 2K, L). The area between the foxg1 and shha expression domains was significantly smaller in zic2a morphants compared to conMO injected embryos at the end of somitogenesis (p=0.001, Fig. 2M). Together, these data show that AD is significantly reduced in size, while the adjacent forebrain subdivisions develop correctly in Zic2a-depleted embryos (Fig. 2N).
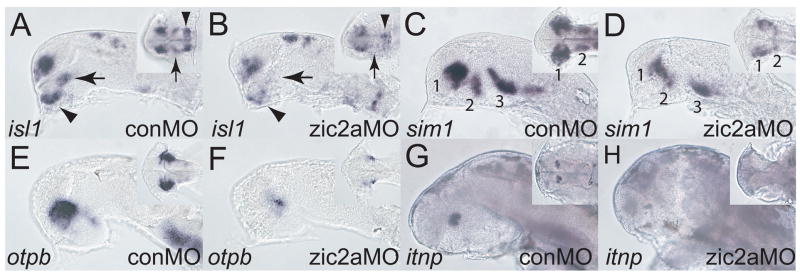
We further examined Zic2a-depleted embryos for persistent patterning defects using markers of diencephalic neurons. isl1 marks two primary neuron clusters in the diencephalon of prim-5 stage embryos: the dorsorostral cluster (DRC), formed in the preoptic area of the hypothalamus, and the ventrorostral cluster (VRC), located in the PT (Fig. 3A). In zic2a morphants, the DRC and VRC were strongly reduced or absent (Fig. 3B). In contrast, the telencephalic isl1-expressing cluster was only mildly affected. Expression domains of sim1 and otpb, transcription factors that mark overlapping clusters of neurons in the preoptic area, were dramatically reduced in Zic2a-depleted embryos (Fig. 3D, F). sim1 and otpb are required cell-autonomously for the formation of isotocin producing neurons marked by itnp (Eaton and Glasgow, 2006; Eaton and Glasgow, 2007). At 2 dpf, expression of itnp was lost in zic2a morphants (Fig. 3G, H). Diencephalic lhx1a expression in zic2a morphants remained normal (Supplementary Fig. 3), showing that not all neurogenesis in the diencephalon was affected.
Fig. 3. Zic2a is required for diencephalic neurogenesis.
The effect of Zic2a depletion on neurogenesis in the forebrain was examined using ISH with neuronal markers. (A, B) isl1 expression in zic2a morphants at Prim-5 shows loss of the ventrorostral cluster (VRC, arrow), and a fusion of the dorsorostral cluster (DRC, arrowhead) (35/39 embryos, 2 exp.). (C, D) sim1 expression in the preoptic area is strongly reduced (27/36 embryos, 2 exp.) at Prim-5. (E, F) otpb expression in the preoptic area is also strongly reduced (30/30 embryos, 2 exp.). (G, H) Expression of itnp, a marker of differentiated neurons at 2dpf, is lost in zic2aMO injected embryos (28/34 embryos, 2 exp.). Embryos are shown in lateral view with anterior to the left. Insets are ventral views of the same embryos, except in A and B, which are anterio-ventral views. Numbers in panels C and D mark different neural clusters.
Together, results of Zic2a knock-down experiments suggest that Zic2a is required between 4s and 12s to activate arx transcription in the newly formed PT primordium. Since zic2a transcripts were not detected in the PT primordium or the adjacent preoptic area after the 8s stage, it is likely that the growth and neuronal differentation defects observed at later stages were an indirect consequence of the early role Zic2a plays during somitigenesis.
Reduced proliferation, but not apoptosis or premature differentiation, contributes to the early anterior diencephalic defect in zic2a morphants
We have shown that the PT primordium is sized correctly by 12s, but becomes reduced by late somitogenesis stages. This reduction may be due to increased apoptosis, premature neuronal differentiation, or failure to proliferate sufficiently. To test apoptosis rates in zic2a morphants, we examined the distribution of apoptotic cell markers, acridine orange (Hill et al., 2003) and activated caspase-3 (Ryu et al., 2005). Neither method revealed an increase in the number of apoptotic cells in zic2a morphants at 10s and 14s (data not shown). We also used an antisense morpholino against p53 (p53MO, Robu et al., 2007) to block the apoptotic pathway in zic2a morphants. If Zic2a normally functions to prevent apoptosis, then in Zic2a depleted embryos apoptotic cell death should lead to the characteristic reduction in PT size, and in zic2a/p53 double morphants this defect should be alleviated (rescued). We did not observe rescue of the zic2aMO-induced defect in the presence of p53MO (Fig. 4). At the 15s stage, the arx expressing domain was reduced similarly in both zic2a morphants and in zic2a/p53 double morphants compared to p53MO injected controls (Fig. 4A–C). At the end of somitogenesis, dlx2a expression in zic2a morphants and in zic2a/p53 double morphantsalso showed equivalent reduction of the PT (Fig. 4D–F). Together, these data show that Zic2a promotes PT development independently of regulating apoptosis.
Fig. 4. Zic2a regulates proliferation, but not apoptosis or differentiation of PT precursors.
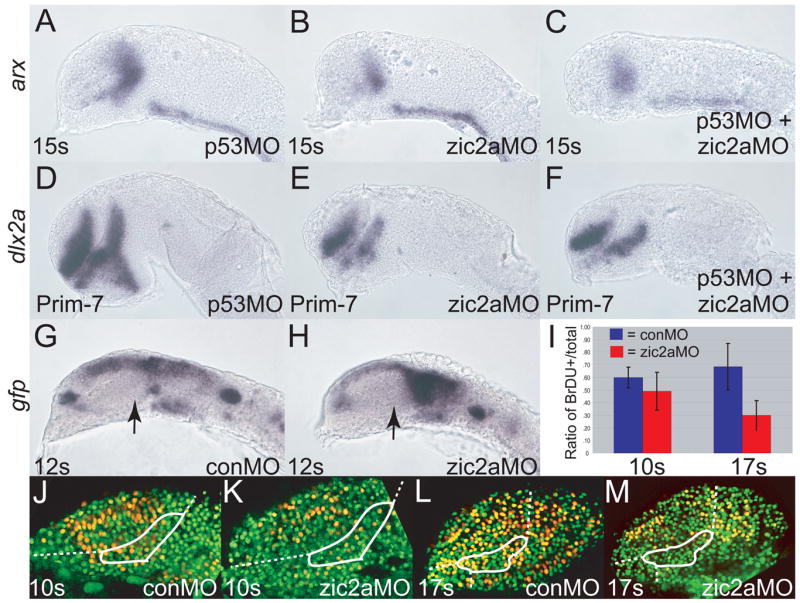
Embryos were injected singly or co-injected with zic2aMO and p53MO and stained out for expression of arx, an early PT marker at 15 somites (A-C), dlx2a, a late PT marker, at Prim-7 (D-F), or gfp (G, H). (A) p53 morphants show normal expression of arx (26/26 embryos, 2 exp.). (B) Embryos injected with zic2aMO alone show a strong reduction of arx expression at 15s (13/17 embryos, 2 exp.). (C) Co-injection of zic2aMO and p53MO leads to a similar reduction of arx expression (11/16 embryos, 2 exp.). (D) p53MO-injected embryos show no patterning defect at Prim-7 (29/30 embryos, 2 exp.). (E) zic2aMO-injected, or (F) zic2aMO and p53MO co-injected embryos show equivalent loss of dlx2a expression at Prim-7 (19/26 embryos, 2 exp. and 33/45 embryos, 2 exp. respectively). (G, H) Transgenic Tg(HuC:gfp) embryos express gfp in post-mitotic neurons. (G) conMO-injected transgenic embryos show no evidence of gfp-positive post-mitotic cells in the PT at 12s. (H) zic2aMO morphants do not contain prematurely differentiating cells in the PT at 12s (17/17 embryos, 3 exp.). (I) Ratios of BrdU positive cells/total cells in conMOs and zic2aMOs at 10s and 17s.10s analysis revealed no significant difference between conMO-injected (n = 5) and zic2aMO-injected (n = 4) embryos.17s analysis showed a significant difference (p = .005) between conMOs (n = 5) and zic2aMOs (n = 4). (J-M) Representative confocal sections of MO-injected embryos, showing BrdU-positive cells in yellow and BrdU-negative nuclei in green. White outlines the approximate prethalamic area determined by arx expression at the same stages. Embryos are shown in lateral view, anterior to the left. Arrows mark the PT.
Another plausible explanation for the reduced size of the PT primordium in zic2a morphants is premature cell-cycle exit and differentiation of neuronal precursors. To test this hypothesis we used Tg(HuC:gfp), a transgenic line that expresses Gfp in post-mitotic neuronal precursors (Park et al., 2000). Tg(HuC:gfp) embryos were injected with conMO or zic2aMO and examined for expression of Gfp by fluorescence at 10s and 14s (data not shown) and for expression of gfp by WISH at 8s and 12s (Fig. 4G, H). We found noevidence of increased gfp RNA or protein, suggesting that Zic2a does not regulate the timing of cell cycle exit and differentiation of neuronal precursors in the PT.
The remaining possible explanation for the smaller PT primordium in morphants is reduced proliferation. Wetested this possibility by examining BrdU incorporation, a method for marking cells in the S phase of the cell cycle. At 10s, the proportion of BrdU-labelled cells was somewhat reduced in zic2aMOs compared to conMOs, but this reduction was not statistically significant (Fig. 4I–K). However, by 17s the ratio of BrdU positive/total cells was significantly reduced in zic2aMOs (Fig. 4I, L–M, p = .005). These data show that Zic2a is required to promote the mitotic cell cycle, but that the PT patterning requirement (activation of arx expression) precedes the mitogenic requirement.
Zic2a cooperates with Hh signaling to promote anterior diencephalic formation
Hh signaling plays an important role in promoting AD development in zebrafish embryos. We asked if zic2a genetically interacts with the Hh pathway. We first examined expression of dlx2a in the forebrains of sonic hedgehog (syut4, Odenthal et al., 2000) and smoothened (smub641, Varga et al., 2001) mutant embryos. dlx2a expression was reduced in the PT of both mutants, while expression in the telencephalon remained relatively normal (Fig. 5C, E), as previously observed (Scholpp et al., 2006). To generate embryos depleted for both Zic2a and Hh signaling, zic2aMO was injected into progeny from a syut4/+ incross. While most embryos showed a dlx2a reduction similar to zic2a morphants (77% of 74 total), some of the zic2aMO/syut4 mutant clutch exhibited a greater loss of dlx2a expression in the PT (Fig. 5D, 23% of 74 total) than in either the homozygous mutant or zic2a morphant alone. In a separate experiment, we confirmed that these strongly affected embryos were genotypically syut4/syut4 (9/9 embryos, see Materials and Methods for details). Similarly, when progeny from a smub641/+ incross were injected with zic2aMO, dlx2a expression was completely lost in 33% of the injected embryos (Fig. 5F, 60/195). Genotyping confirmed that all embryos exhibiting complete loss of dlx2a were smub641/smub641 (17/17 embryos, see Materials and Methods). These data indicate that Zic2a acts in parallel with, but not epistatically to the Hh signaling pathway in the PT primordium.
Fig. 5. Zic2a promotes PT formation in cooperation with components of the hedgehog signaling pathway.
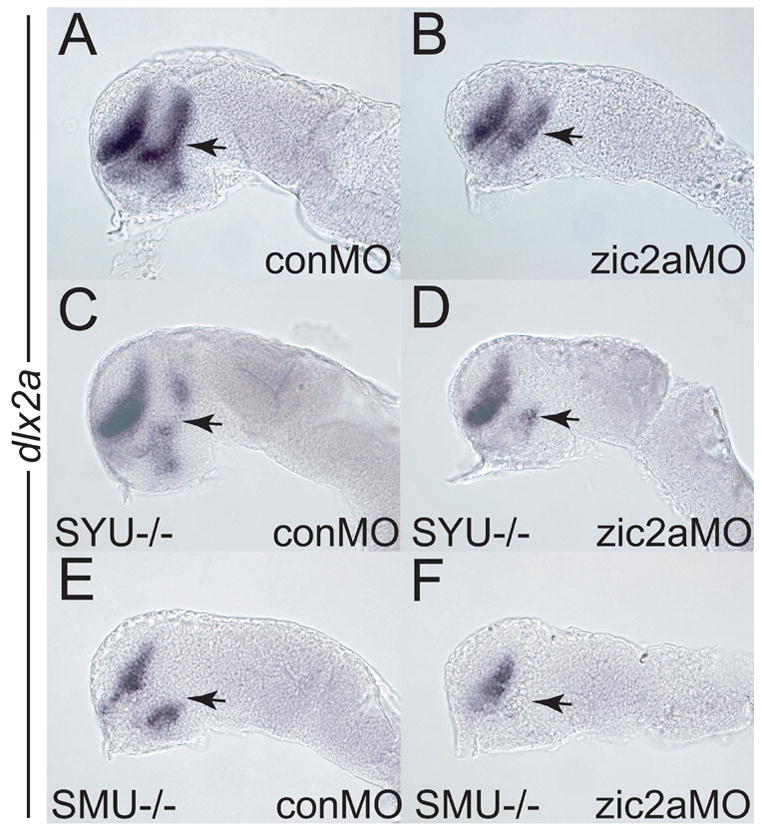
Embryos of different genetic backgrounds were injected with a conMO (A, C, E) or a zic2aMO (B, D, F) and stained for dlx2a expression by ISH at the prim-5 stage. (A, B) Wild type embryos depleted of Zic2a (B) show a typical reduction of PT relative to control morphants (A). (C) Homozygous syut4 mutant embryos show reduction of PT dlx2a (9/35 embryos, 2 exp.). (D) syu14 mutants depleted of Zic2a exhibit an almost complete loss of dlx2a in the PT (17/74 embryos, 3 exp.). (E) homozygous smob641 embryos show reduced dlx2a expression in the PT (25/83 embryos, 3 exp.). (F) smob641 mutants depleted of Zic2a show complete loss of dlx2a in the PT (60/195 embryos, 4 exp.). Embryos are shown in lateral view, anterior to the left. Arrows point to the PT.
Zic2a acts before Hh signaling to promote maturation of the prethalamic primordium
Since Zic2a function is required for arx transcription by 8s (Fig. 1), we next asked if Hh signaling is required at the same time in the PT primordium. Progeny from smub641/+ in crosses were analyzed for expression of arx at mid-somitogenesis. smub641/b641 embryos, identified by the absence of ptc1 expression (Varga et al., 2001), showed normal arx expression at both 8s and 12s (Fig, 6, A–D). However, by 18s dlx2a expression in the PT was strongly reduced in smub641/smub641 embryos (Fig. 6E, F). We next asked if Hh signaling and Zic2a may be playing synergistic roles in the early PT primordium. To test this hypothesis, we examined arx expression in embryos simultaneously depleted for Zic2a using zic2aMO, and for Hh signaling using exposure to an alkaloid inhibitor of Hh signaling, cyclopamine (Tyurina et al., 2005). arx expression in conMO-injected, vehicle treated embryos was indistinguishable from arx expression in conMO-injected, cyclopamine-treated embryos (Fig. 6G, I), confirming our conclusion that Hh signaling does not play arole in early PT patterning. zic2amorphants treated with vehicle showed the typical reduction of the arx expression domain that was indistinguishable from the defect seen in cyclopamine treated zic2a morphants (Fig. 6H, J). Together, these results argue that Hh signaling acts after 12s in the developing PT.
Fig. 6. Zic2a acts before hedgehog signaling to promote maturation of the PT primordium.
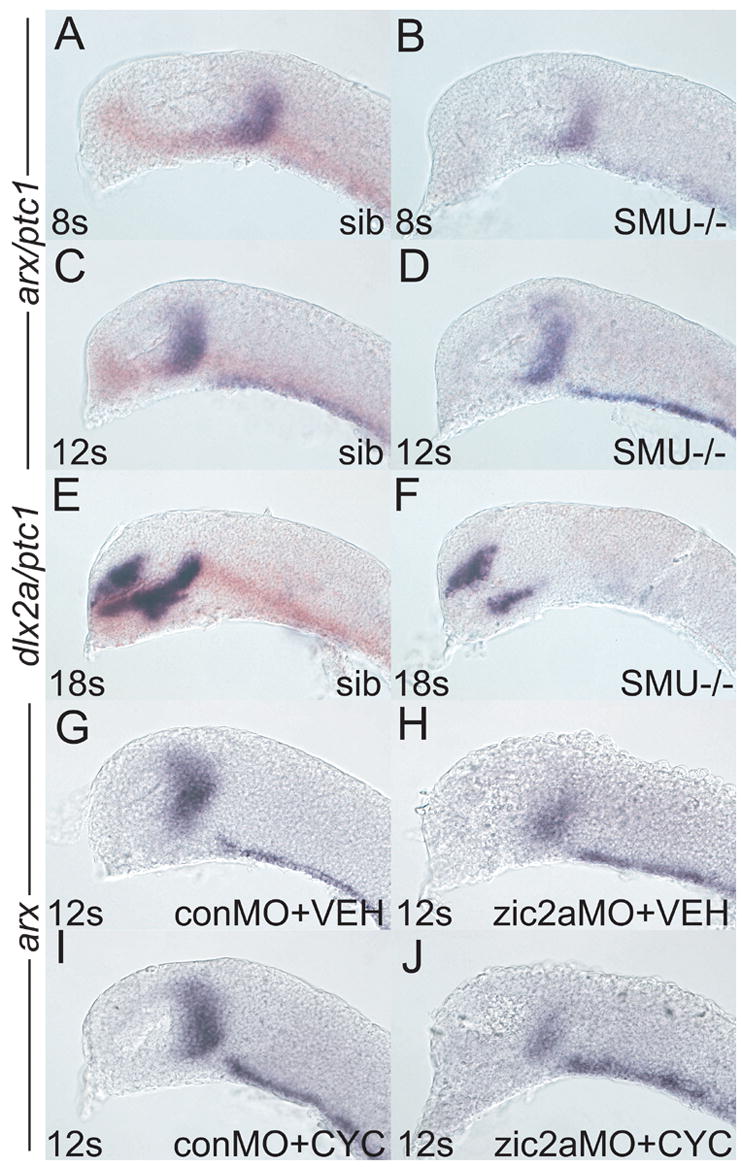
Embryos of different genetic backgrounds were injected with conMO or zic2aMO and stained for arx expression by ISH, except in E, F, which were stained for dlx2a. (A-F) Embryos were derived from a smob641/+ incross. Wildtype and heterozygous siblings were identified by the presence of ptc1 expression (orange), while mutant embryos lacked any ptc1 expression. (A) Wildtype sibling embryos (84/117) have very similar arx expression as (B) mutant siblings (33/107 embryos) at 8s. (C) Wildtype embryos at 12s (70/95 embryos) are indistinguishable from (D) mutant siblings (25/95 embryos). (E) At 18s, dlx2a is strongly expressed in the PT of wildtype embryos (109/143 embryos). (F) Mutant embryos display a dramatic reduction of dlx2a expression by 18s (23/132 embryos). (G, H) Embryos were injected with either conMO (G, 13/13 embryos) or zic2aMO (H, 7/7 embryos), treated with vehicle at 50–60% epiboly and fixed at 12s. (I, J) Embryos were injected with conMO (I, 12/12 embryos) or zic2aMO (J, 8/8 embryos), treated with 10μM cyclopamine at 50–60% epiboly and fixed at 12s. Embryos are shown in lateral view, anterior to the left.
Zic2a and Hh signaling both promote development of the AD. Since Zic2a acts prior to Hh signaling, it is possible that Zic2a modulates the Hh pathway by controlling transcription of its genetic components. To address this possibility we asked if Zic2a regulates transcription of several members of the Hh signaling pathway. At mid-somitigenesis, ptc1 (Fig. 7A, B) and gli1 (Fig. 7C, D) were transcribed correctly in Zic2a-depleted embryos. The ZLI, the main Hh source in the diencephalon, was established normally (Fig. 7E, F). At prim-5 stage, Hh signaling was also unaffected in zic2a morphants as evidenced by correct expression of Hh targets nkx2.2a, ptc1 and gli1 (Cohen, 2003; Barth and Wilson, 1995; Fig. 7G–L). gli2 and gli3 were also expressed correctly(Fig. 7M–P). Conversely, zic2a expression was not affected in smub641/smub641 mutants (Supp. Fig. 5). Together these results suggest that Zic2a and Hh signaling carry out similar, but independent functions in the PT primordium.
Fig. 7. Zic2a controls PT patterning independent of hedgehog signaling.
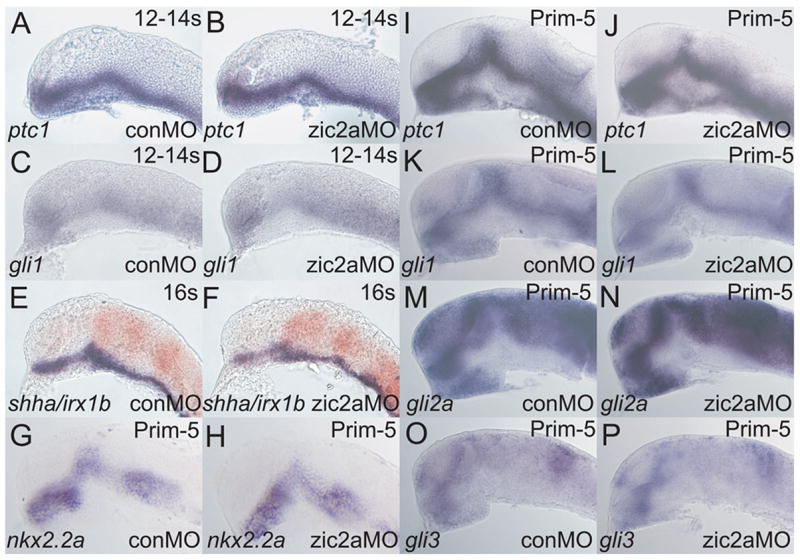
Embryos were injected with conMO or zic2aMO, and examined by ISH for expression of the following markers, which were expressed correctly in zic2a moprhants. (A, B) ptc1 at 12–14s (20/20 morphants, 2 exp.). (C, D) gli1 at 12–14s (9/9 morphants, 2 exp.). (E, F)shha at 16s (38/38 morphants, 2 exp.). (G-P) Embryos are at Prim-5 (see Supplemental Table 1 for numbers). (G, H) nkx2.2a. (I, J) ptc1. (K, L) gli1. (M, N) gli2a. (O, P) gli3. Embryos are shown in lateral views, anterior to the left.
Discussion
Zic2 is essential for correct forebrain development in mammals, yet the mechanism of this function is not understood. We have characterized a novel role for zic2a during forebrain development of the zebrafish. We show that Zic2a is required for the correct formation of the AD (prethalamus and preoptic area), and identify an early requirement for Zic2a in the PT primordium that involves maintenance of arx transcription. We further demonstrate that, while Zic2a and Hh signaling function similarly in the AD, they act independently. This study is the first demonstration of a role for zic2 in non-mammalian forebrain development.
Zic2a plays a patterning role in the forming prethalamus
While the mechanism of Zic2a function in the forming PT has yet to be fully elucidated, our current data argue in support of a primary patterning role for Zic2a, rather than a role in modifying cell cycle progression. The relative timing of zic2a expression in the PT primordium and its role in regulating transcription of arx suggest that arx is a proximal transcriptional target of Zic2a. arx, a homeobox transcription factor, is required to activate transcription of dlx genes in the prethalamus of mammals (Seufert et al., 2005; Kitamura et al., 2002).
zic2a is expressed in the PT primordium for a brief period during early somitogenesis, but continues to be expressed in the adjacent thalamus throughout somitogenesis (Grinblat and Sive, 2001). Our data are consistent with the hypothesis that Zic2a is functioning in the PT shortly after its brief pulse of expression there. Alternatively, Zic2a may function in the thalamus, and the thalamus in turn may signal to the adjacent prethalamus to promote its growth. Correct formation of the thalamus and ZLI in zic2a morphants (Figs 2 and 7, respectively) argue against the latter explanation. Furthermore, recent studies show that thalamus and prethalamus are specified and maintained independently (Jeong et al., 2007; Scholpp et al., 2007).
High levels of zic2a transcript in the zebrafish telencephalon (Toyama et al., 2004; Grinblat and Sive, 2001), together with the prevalence of telencephalic defects in human HPE patients with mutations in ZIC2, predict a role for Zic2 in the telencephalon. Absence of significant telencephalic defects in zic2a morphants is likely due to functional redundancy with other Zic family members (Nyholm et al., 2007; Aruga et al., 2002; Ogura et al., 2001; Inoue et al., 2007). zic2b, zic1, zic4, and zic5 are co-expressed with zic2a in the telencephalic primordium and may play partially redundant roles there (Nagai et al., 1997; Toyama et al., 2004; Grinblat and Sive, 2001).
Zic2a functions independently of Hh signaling to pattern the diencephalon
The hypothesis that Zic and Gli proteins interact in vivo is supported by several lines of evidence. Zic family members interact directly in vitro and bind to the same binding site sequence (Mizugishi et al., 2001). Moreover, co-overexpression of Zic1 and Gli1 in cultured mammalian cells results in Gli protein relocalizing from the cytoplasm into the nucleus (Koyabu et al., 2001). In our study, a careful temporal dissection allowed us to determine that a direct functional interaction between Zic2 and Gli proteins was not likely in the AD. However, Zic and Gli proteins may interact to pattern other developing tissues. Zic1 and Gli3 double-knockout mice show synergistic phenotypes in the vertebral arches, suggesting that these proteins may interact there (Aruga et al., 1999). The relative temporal requirements of these two factors have not been resolved. Likewise, Gli3 is involved in development of optic stalk and retina (Tyurina et al., 2005; Furimsky and Wallace, 2006). Analysis of a potential role for Zic2a in these tissues is in progress
Our finding that Zic2a and Hh pathway components do not cross-regulate at the level of transcription is in contrast to reports of aberrant Zic2 expression in Hh-depleted mouse embryos (Hayhurst et al., 2007, Brown et al., 2003). Hayhurst et al. (2007) further show that Hh signaling activates forebrain Zic2 expression indirectly, likely through regulating FGF8. Cross-regulation between the Hh and Fgf signaling pathways is an important conserved mechanism of forebrain formation in vertebrates, including zebrafish (reviewed in Bertrand and Dahmane, 2006). In this study, we have begun to ask what role, if any, zebrafish zic2a plays in this interaction. In future studies, we will continue this examination by focusing on FGF signaling as a candidate regulator of zic2a transcription.
Zic2a and Wnt signaling in of anterior diencephalic development
We have previously shown that canonical Wnt signaling directly activates zic2a transcription in the midbrain and forebrain (Nyholm et al., 2007). Wnt signaling plays a major role in patterning the forebrain along the A-P axis (Houart et al., 2002), promoting posterior and repressing anterior forebrain fates. Since zic2a is activated by Wnt signaling but functions to promote anterior diencephalic development, we speculate that Zic2a may be part of a feedback loop that limits the inhibitory action of Wnt signaling in the AD. A similar role was recently demonstrated for fezf2, a zinc-finger transcription factor that promotes PT formation by attenuating the posteriorizing effects of Wnt signaling (Jeong et al., 2007). We show that Zic2a does not regulate fezf2 transcription, suggesting that zic2a likely acts downstream or in parallel with fezf2 in the PT primordium.
The HPE connection
While the classical defining trait of HPE is the failure of cerebral hemispheres to separate (Sarnat and Flores-Sarnat, 2001), deletion of diencephalic structures is also frequently associated with HPE. The prethalamic and preoptic areas are diencephalic structures that are strongly reduced in zic2a morphants. In humans, the subthalamus (human equivalent of the PT) is important mainly for controlling skeletal muscle coordination (Colnat-Coulbois et al., 2005), and the preoptic area plays a major role in thermoregulation (Blatteis, 2007). While defects in the subthalamus or the preoptic area have not been described specifically in humans with HPE, spasticity and temperature dysregulation are symptoms commonly associated with HPE. Therefore, similar areas of the diencephalon may be impaired in zebrafish lacking Zic2a and in humans with reduced ZIC2 levels. Another disorder frequently associated with HPE is diabetes insipidus (Hahn et al., 2005, Dubourg et al., 2007) although this association has not been examined specifically in ZIC2-linked HPE cases. Diabetes insipidus can be caused by the loss of specific neuronal clusters which secrete oxytocin (Burbach et al., 2001). Zic2a-depleted embryos fail to express itnp, which encodes isotocin, the functional zebrafish analog of oxytocin. zic2a morphants also show reduced expression of otpb and sim1, transcription factors required for development of itnp-expressing neurons (Eaton and Glasgow, 2007). These data further suggest that Zic2a-depleted zebrafish may accurately recapitulate some aspects of human HPE, although they do not exhibit the full HPE phenotype, possibly due to functional redundancy with zic2b.
arx, a proximal target of zic2a according to this study, has not been associated with HPE. In humans, mutations in ARX are causally linked to XLAG (X-linked lissencephaly with abnormal genitalia). XLAG is characterized by many symptoms, including agenesis of the corpus callosum and poor temperature regulation (Kitamura et al., 2002). Interestingly both of these symptoms are also found in HPE patients. These findings taken together strongly suggest that we are uncovering aspects of forebrain development and Zic2a function that have been conserved during vertebrate evolution. Future zebrafish studies are likely to provide valuable insights into the genetic nature of human HPE, despite the overt differences in their forebrain morphogenesis (Wullimann and Rink, 2002).
A model for zic2a function in the developing diencephalon
Our findings show that zebrafish Zic2a acts to maintain the PT soon after it is specified, at least in part through transcriptional control of arx. arx in turn is likely to play an essential role in promoting growth and/or differentiation of the PT primoridum through regulation of other PT transcription factors such as dlx2a (Kitamura et al., 2002). These data place Zic2a in a key early position in the regulatory cascade of transcription factors that control development of the PT (Fig. 8). While Zic2a and Hh signaling carry out similar roles in the forming PT, they function consecutively rather than concomitantly, and therefore do not directly interact. Parallels between humans with HPE caused by Zic2 mutations and zic2a-depleted zebrafish suggest that we are uncovering conserved regulatory mechanisms that govern forebrain development in vertebrates.
Fig. 8. A proposed model for Zic2a function in the developing prethalamus.
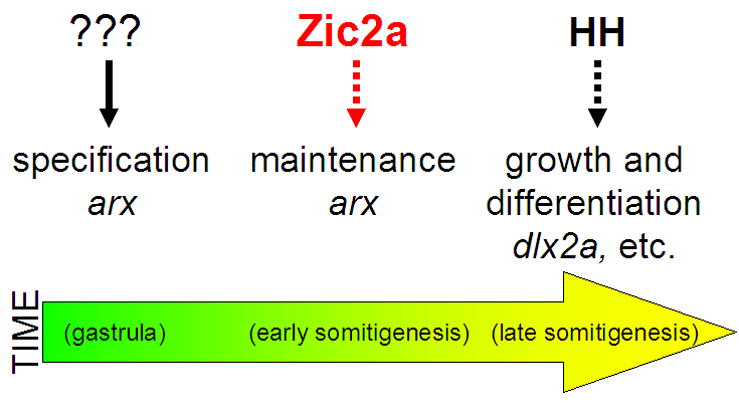
zic2a is expressed transiently in the PT primordium during early somitogenesis and acts there to maintain transcription of arx. arx, and possibly other PT-specific transcription factors, are in turn required to promote growth of the PT primordium and correct neurogenesis in the PT and the adjacent preoptic area.
Supplementary Material
Forebrain patterning in zic2a morphants at 4s-12s. (A, B) six3b, which marks the eye field and irx1b, which marks the future thalamus, are not affected at 4s (46/47 morphants, 3 exp.). (C, D) rx3, another marker of the eye field and the anterior hypothalamus is not affected at 8s (31/32 morphants, 2 exp.). (E, F) fezf2 marks the forming prethalamus and is not affected at any time between 4s and 12s (12s stage shown, 24/25 morphants, 2 exp.). Embryos are shown in lateral views, anterior to the left.
Non-overlapping zic2aMOs cause similar patterning defects. (A, B)Embryos co-injected with translation-blocking MOs designed against the proximal promoter (zic2a P MO) and the first AUG (zic2a A MO) show similar arx reductions at 12s (4/20, 1 exp.) as embryos injected with splice-blocking zic2aMO (see Fig. 1). (C, D)Prim-5 stage embryos injected with zic2a A+P MO also show reduced dlx2a expression (14/45, 2 exp.) similar to zic2aMO-injected embryos (see Fig. 2).
Forebrain patterning in zic2a morphants. (A, B) foxg1 expression in the telencephalon is not affected in zic2a morphants. (C, D) eomesa expression is normal in the telencephalon, but is reduced in anterior diencephalon (dashed red lines demarcate border between telencephalon and diencephalon). (E, F) lef1 expression is reduced in the PT region of Zic2a depleted embryos. (G, H) rx3 expression is not affected in the anterior hypothalamus, however (I, J) titf1a is weakly expanded anteriorly. (K, L) lhx1a expression is not affected in zic2a morphants at 2 dpf. Embryos are shown in lateral views, anterior to the left. All numbers for these experiments can be found in Supplementary Table 1.
PT defects in zic2a morphants at 17–18s. (A, B) dlx2a expression in the PT is reduced in Zic2a-depleted embryos (49/80 embryos, 3 exp.). (C)Graph showing average numbers of dlx2a-expressing cells in the telencephalon and diencephalon of conMO injected (n = 5) and zic2aMO injected (n = 9) embryos stained for dlx2a at 17–18s. Embryos were sectioned (see methods) to obtain accurate counts of dlx2a-expressing cells. There was no difference in number of telencephalic dlx2a-expressing cells between control and Zic2a morphants. However, there were significantly fewer diencephalic dlx2a-expressing cells in zic2aMOs compared to conMOs (p = .01). (D, E) foxg1 and shha expression was used to deliniate the prethalamic area at 17–18s. The dotted lines in D and E outline the prethalamic area. (F) Graph representing the average prethalamic area (pixels2) in conMOs (n = 10) and zic2aMOs (n = 8). Results are significant at p = .001. Embryos are shown in lateral views, anterior to the left.
Zic2a expression is unaffected by loss of hedgehog signaling. (A, B) Double ISH for zic2a and ptc1 expression in smob641/+ incross progeny at tail-bud stage (TB). (B) Homozygous null embryos were identified by lack of ptc1 expression. (C, D)ISH for zic2a expression in smob641/+ incross progeny at Prim-5 stage. Homozygous null embryos were identified morphologically.
Summary of zic2a morphant analysis at prim-5 and later stages.
Acknowledgments
We thank Rolf Karlstrom, Victoria Prince, Mary Ellen Lane, Klaus Rohr, Reiko Toyama, Marina Mione, Eric Glasgow, Sylvie Schneider-Maunoury, Rich Dorsky and the Zebrafish International Resource Center for providing plasmids and zebrafish lines. We are grateful to Andrea Gallagher and Nathan Holman for expert technical help, and Molly Nyholm and Aaron Taylor for valuable discussions throughout the course of this work. We would also like to thank Mary Ellen Lane and Tobias Langenberg for critical comments during manuscript preparation. This work was funded by an NIH RO1 grant to Y. G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimenko MA, Ekker M, Wegner J, Lin W, Westerfield M. Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J Neurosci. 1994;14:3475–86. doi: 10.1523/JNEUROSCI.14-06-03475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–4. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Aruga J. The role of Zic genes in neural development. Mol Cell Neurosci. 2004;26:205–21. doi: 10.1016/j.mcn.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Aruga J, Inoue T, Hoshino J, Mikoshiba K. Zic2 controls cerebellar development in cooperation with Zic1. J Neurosci. 2002;22:218–25. doi: 10.1523/JNEUROSCI.22-01-00218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruga J, Mizugishi K, Koseki H, Imai K, Balling R, Noda T, Mikoshiba K. Zic1 regulates the patterning of vertebral arches in cooperation with Gli3. Mech Dev. 1999;89:141–50. doi: 10.1016/s0925-4773(99)00220-8. [DOI] [PubMed] [Google Scholar]
- Barth KA, Wilson SW. Expression of zebrafish nk2.2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development. 1995;121:1755–68. doi: 10.1242/dev.121.6.1755. [DOI] [PubMed] [Google Scholar]
- Benedyk MJ, Mullen JR, DiNardo S. odd-paired: a zinc finger pair-rule protein required for the timely activation of engrailed and wingless in Drosophila embryos. Genes Dev. 1994;8:105–17. doi: 10.1101/gad.8.1.105. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Dahmane N. Sonic hedgehog signaling in forebrain development and its interactions with pathways that modify its effects. Trends Cell Biol. 2006;16:597–605. doi: 10.1016/j.tcb.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. The onset of fever: new insights into its mechanism. Prog Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81:1197–267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr The hedgehog signaling network. Am J Med Genet A. 2003;123:5–28. doi: 10.1002/ajmg.a.20495. [DOI] [PubMed] [Google Scholar]
- Colnat-Coulbois S, Gauchard GC, Maillard L, Barroche G, Vespignani H, Auque J, Perrin PP. Bilateral subthalamic nucleus stimulation improves balance control in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76:780–7. doi: 10.1136/jnnp.2004.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagli A, Kapsimali M, Wilson SW, Mione M. Conserved and divergent patterns of Reelin expression in the zebrafish central nervous system. J Comp Neurol. 2002;450:73–93. doi: 10.1002/cne.10292. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Snyder A, Cretekos CJ, Grunwald DJ, Geisler R, Haffter P, Moon RT, Raible DW. Maternal and embryonic expression of zebrafish lef1. Mech Dev. 1999;86:147–50. doi: 10.1016/s0925-4773(99)00101-x. [DOI] [PubMed] [Google Scholar]
- Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V. Holoprosencephaly. Orphanet J Rare Dis. 2007;2:8. doi: 10.1186/1750-1172-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JL, Glasgow E. The zebrafish bHLH PAS transcriptional regulator, single-minded 1 (sim1), is required for isotocin cell development. Dev Dyn. 2006;235:2071–82. doi: 10.1002/dvdy.20848. [DOI] [PubMed] [Google Scholar]
- Eaton JL, Glasgow E. Zebrafish orthopedia (otp) is required for isotocin cell development. Dev Genes Evol. 2007;217:149–58. doi: 10.1007/s00427-006-0123-2. [DOI] [PubMed] [Google Scholar]
- Etheridge LA, Wu T, Liang JO, Ekker SC, Halpern ME. Floor plate develops upon depletion of tiggy-winkle and sonic hedgehog. Genesis. 2001;30:164–9. doi: 10.1002/gene.1056. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–83. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Furimsky M, Wallace VA. Complementary Gli activity mediates early patterning of the mouse visual system. Dev Dyn. 2006;235:594–605. doi: 10.1002/dvdy.20658. [DOI] [PubMed] [Google Scholar]
- Gillhouse M, Wagner Nyholm M, Hikasa H, Sokol SY, Grinblat Y. Two Frodo/Dapper homologs are expressed in the developing brain and mesoderm of zebrafish. Dev Dyn. 2004;230:403–9. doi: 10.1002/dvdy.20060. [DOI] [PubMed] [Google Scholar]
- Grinblat Y, Gamse J, Patel M, Sive H. Determination of the zebrafish forebrain: induction and patterning. Development. 1998;125:4403–16. doi: 10.1242/dev.125.22.4403. [DOI] [PubMed] [Google Scholar]
- Grinblat Y, Sive H. zic Gene expression marks anteroposterior pattern in the presumptive neurectoderm of the zebrafish gastrula. Dev Dyn. 2001;222:688–93. doi: 10.1002/dvdy.1221. [DOI] [PubMed] [Google Scholar]
- Hahn JS, Hahn SM, Kammann H, Barkovich AJ, Clegg NJ, Delgado MR, Levey E. Endocrine disorders associated with holoprosencephaly. J Pediatr Endocrinol Metab. 2005;18:935–41. doi: 10.1515/jpem.2005.18.10.935. [DOI] [PubMed] [Google Scholar]
- Hayhurst M, Gore BB, Tessier-Lavigne M, McConnell SK. Ongoing sonic hedgehog signaling is required for dorsal midline formation in the developing forebrain. Dev Neurobiol. 2007 doi: 10.1002/dneu.20576. [DOI] [PubMed] [Google Scholar]
- Hill A, Howard CV, Strahle U, Cossins A. Neurodevelopmental defects in zebrafish (Danio rerio) at environmentally relevant dioxin (TCDD) concentrations. Toxicol Sci. 2003;76:392–9. doi: 10.1093/toxsci/kfg241. [DOI] [PubMed] [Google Scholar]
- Hjorth JT, Connor RM, Key B. Role of hlx1 in zebrafish brain morphogenesis. Int J Dev Biol. 2002;46:583–96. [PubMed] [Google Scholar]
- Houart C, Caneparo L, Heisenberg C, Barth K, Take-Uchi M, Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–65. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–50. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- Inoue T, Ota M, Ogawa M, Mikoshiba K, Aruga J. Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J Neurosci. 2007;27:5461–73. doi: 10.1523/JNEUROSCI.4046-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JY, Einhorn Z, Mathur P, Chen L, Lee S, Kawakami K, Guo S. Patterning the zebrafish diencephalon by the conserved zinc-finger protein Fezl. Development. 2007;134:127–36. doi: 10.1242/dev.02705. [DOI] [PubMed] [Google Scholar]
- Karlstrom RO, Trowe T, Klostermann S, Baier H, Brand M, Crawford AD, Grunewald B, Haffter P, Hoffmann H, Meyer SU, Muller BK, Richter S, van Eeden FJ, Nusslein-Volhard C, Bonhoeffer F. Zebrafish mutations affecting retinotectal axon pathfinding. Development. 1996;123:427–38. doi: 10.1242/dev.123.1.427. [DOI] [PubMed] [Google Scholar]
- Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999;13:388–93. doi: 10.1101/gad.13.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstrom RO, Tyurina OV, Kawakami A, Nishioka N, Talbot WS, Sasaki H, Schier AF. Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development. 2003;130:1549–64. doi: 10.1242/dev.00364. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Dawid IB. Developmental expression of zebrafish emx1 during early embryogenesis. Gene Expr Patterns. 2002;2:201–6. doi: 10.1016/s1567-133x(02)00062-5. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7:1242–9. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, Kasahara M, Yoshioka H, Ogata T, Fukuda T, Kondo I, Kato M, Dobyns WB, Yokoyama M, Morohashi K. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–69. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Korzh V, Edlund T, Thor S. Zebrafish primary neurons initiate expression of the LIM homeodomain protein Isl-1 at the end of gastrulation. Development. 1993;118:417–25. doi: 10.1242/dev.118.2.417. [DOI] [PubMed] [Google Scholar]
- Koster RW, Fraser SE. Tracing transgene expression in living zebrafish embryos. Dev Biol. 2001;233:329–46. doi: 10.1006/dbio.2001.0242. [DOI] [PubMed] [Google Scholar]
- Koyabu Y, Nakata K, Mizugishi K, Aruga J, Mikoshiba K. Physical and functional interactions between Zic and Gli proteins. J Biol Chem. 2001;276:6889–92. doi: 10.1074/jbc.C000773200. [DOI] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Moens U, Ericson JU, Fjose A. Zebrafish pax[zf-a]: a paired box-containing gene expressed in the neural tube. Embo J. 1991;10:3609–19. doi: 10.1002/j.1460-2075.1991.tb04927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecaudey V, Anselme I, Dildrop R, Ruther U, Schneider-Maunoury S. Expression of the zebrafish Iroquois genes during early nervous system formation and patterning. J Comp Neurol. 2005;492:289–302. doi: 10.1002/cne.20765. [DOI] [PubMed] [Google Scholar]
- Merzdorf CS. Emerging roles for zic genes in early development. Dev Dyn. 2007;236:922–40. doi: 10.1002/dvdy.21098. [DOI] [PubMed] [Google Scholar]
- Miura H, Yanazawa M, Kato K, Kitamura K. Expression of a novel aristaless related homeobox gene ‘Arx’ in the vertebrate telencephalon, diencephalon and floor plate. Mech Dev. 1997;65:99–109. doi: 10.1016/s0925-4773(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Aruga J, Nakata K, Mikoshiba K. Molecular properties of Zic proteins as transcriptional regulators and their relationship to GLI proteins. J Biol Chem. 2001;276:2180–8. doi: 10.1074/jbc.M004430200. [DOI] [PubMed] [Google Scholar]
- Monuki ES. The morphogen signaling network in forebrain development and holoprosencephaly. J Neuropathol Exp Neurol. 2007;66:566–75. doi: 10.1097/nen.0b013e3180986e1b. [DOI] [PubMed] [Google Scholar]
- Nagai T, Aruga J, Minowa O, Sugimoto T, Ohno Y, Noda T, Mikoshiba K. Zic2 regulates the kinetics of neurulation. Proc Natl Acad Sci U S A. 2000;97:1618–23. doi: 10.1073/pnas.97.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Aruga J, Takada S, Gunther T, Sporle R, Schughart K, Mikoshiba K. The expression of the mouse Zic1, Zic2, and Zic3 gene suggests an essential role for Zic genes in body pattern formation. Dev Biol. 1997;182:299–313. doi: 10.1006/dbio.1996.8449. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nyholm MK, Wu SF, Dorsky RI, Grinblat Y. The zebrafish zic2a-zic5 gene pair acts downstream of canonical Wnt signaling to control cell proliferation in the developing tectum. Development. 2007;134:735–46. doi: 10.1242/dev.02756. [DOI] [PubMed] [Google Scholar]
- Odenthal J, van Eeden FJ, Haffter P, Ingham PW, Nusslein-Volhard C. Two distinct cell populations in the floor plate of the zebrafish are induced by different pathways. Dev Biol. 2000;219:350–63. doi: 10.1006/dbio.1999.9589. [DOI] [PubMed] [Google Scholar]
- Ogura H, Aruga J, Mikoshiba K. Behavioral abnormalities of Zic1 and Zic2 mutant mice: implications as models for human neurological disorders. Behav Genet. 2001;31:317–24. doi: 10.1023/a:1012235510600. [DOI] [PubMed] [Google Scholar]
- Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, Hong SK, Shin J, Yoo KW, Hibi M, Hirano T, Miki N, Chitnis AB, Huh TL. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol. 2000;227:279–93. doi: 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–74. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Picker A, Brand M. Global and local mechanisms of forebrain and midbrain patterning. Curr Opin Neurobiol. 2006;16:5–12. doi: 10.1016/j.conb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr KB, Barth KA, Varga ZM, Wilson SW. The nodal pathway acts upstream of hedgehog signaling to specify ventral telencephalic identity. Neuron. 2001;29:341–51. doi: 10.1016/s0896-6273(01)00210-0. [DOI] [PubMed] [Google Scholar]
- Ryu S, Holzschuh J, Erhardt S, Ettl AK, Driever W. Depletion of minichromosome maintenance protein 5 in the zebrafish retina causes cell-cycle defect and apoptosis. Proc Natl Acad Sci U S A. 2005;102:18467–72. doi: 10.1073/pnas.0506187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat HB, Flores-Sarnat L. Neuropathologic research strategies in holoprosencephaly. J Child Neurol. 2001;16:918–31. doi: 10.1177/088307380101601211. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Foucher I, Staudt N, Peukert D, Lumsden A, Houart C. Otx1l, Otx2 and Irx1b establish and position the ZLI in the diencephalon. Development. 2007;134:3167–76. doi: 10.1242/dev.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpp S, Wolf O, Brand M, Lumsden A. Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon. Development. 2006;133:855–64. doi: 10.1242/dev.02248. [DOI] [PubMed] [Google Scholar]
- Serluca FC, Fishman MC. Pre-pattern in the pronephric kidney field of zebrafish. Development. 2001;128:2233–41. doi: 10.1242/dev.128.12.2233. [DOI] [PubMed] [Google Scholar]
- Seo HC, Drivenes O, Ellingsen S, Fjose A. Transient expression of a novel Six3-related zebrafish gene during gastrulation and eye formation. Gene. 1998;216:39–46. doi: 10.1016/s0378-1119(98)00328-x. [DOI] [PubMed] [Google Scholar]
- Seufert DW, Prescott NL, El-Hodiri HM. Xenopus aristaless-related homeobox (xARX) gene product functions as both a transcriptional activator and repressor in forebrain development. Dev Dyn. 2005;232:313–24. doi: 10.1002/dvdy.20234. [DOI] [PubMed] [Google Scholar]
- Shepard JL, Stern HM, Pfaff KL, Amatruda JF. Analysis of the cell cycle in zebrafish embryos. Methods Cell Biol. 2004;76:109–25. doi: 10.1016/s0091-679x(04)76007-0. [DOI] [PubMed] [Google Scholar]
- Staudt N, Houart C. The prethalamus is established during gastrulation and influences diencephalic regionalization. PLoS Biol. 2007;5:e69. doi: 10.1371/journal.pbio.0050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama R, Dawid IB. lim6, a novel LIM homeobox gene in the zebrafish: comparison of its expression pattern with lim1. Dev Dyn. 1997;209:406–17. doi: 10.1002/(SICI)1097-0177(199708)209:4<406::AID-AJA8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Toyama R, Gomez DM, Mana MD, Dawid IB. Sequence relationships and expression patterns of zebrafish zic2 and zic5 genes. Gene Expr Patterns. 2004;4:345–50. doi: 10.1016/j.modgep.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Tyurina OV, Guner B, Popova E, Feng J, Schier AF, Kohtz JD, Karlstrom RO. Zebrafish Gli3 functions as both an activator and a repressor in Hedgehog signaling. Dev Biol. 2005;277:537–56. doi: 10.1016/j.ydbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Unger JL, Glasgow E. Expression of isotocin-neurophysin mRNA in developing zebrafish. Gene Expr Patterns. 2003;3:105–8. doi: 10.1016/s1567-133x(02)00064-9. [DOI] [PubMed] [Google Scholar]
- Vanderlaan G, Tyurina OV, Karlstrom RO, Chandrasekhar A. Gli function is essential for motor neuron induction in zebrafish. Dev Biol. 2005;282:550–70. doi: 10.1016/j.ydbio.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga ZM, Amores A, Lewis KE, Yan YL, Postlethwait JH, Eisen JS, Westerfield M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene: University of Oregon Press; 1995. [Google Scholar]
- Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–81. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullimann MF, Rink E. The teleostean forebrain: a comparative and developmental view based on early proliferation, Pax6 activity and catecholaminergic organization. Brain Res Bull. 2002;57:363–70. doi: 10.1016/s0361-9230(01)00666-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forebrain patterning in zic2a morphants at 4s-12s. (A, B) six3b, which marks the eye field and irx1b, which marks the future thalamus, are not affected at 4s (46/47 morphants, 3 exp.). (C, D) rx3, another marker of the eye field and the anterior hypothalamus is not affected at 8s (31/32 morphants, 2 exp.). (E, F) fezf2 marks the forming prethalamus and is not affected at any time between 4s and 12s (12s stage shown, 24/25 morphants, 2 exp.). Embryos are shown in lateral views, anterior to the left.
Non-overlapping zic2aMOs cause similar patterning defects. (A, B)Embryos co-injected with translation-blocking MOs designed against the proximal promoter (zic2a P MO) and the first AUG (zic2a A MO) show similar arx reductions at 12s (4/20, 1 exp.) as embryos injected with splice-blocking zic2aMO (see Fig. 1). (C, D)Prim-5 stage embryos injected with zic2a A+P MO also show reduced dlx2a expression (14/45, 2 exp.) similar to zic2aMO-injected embryos (see Fig. 2).
Forebrain patterning in zic2a morphants. (A, B) foxg1 expression in the telencephalon is not affected in zic2a morphants. (C, D) eomesa expression is normal in the telencephalon, but is reduced in anterior diencephalon (dashed red lines demarcate border between telencephalon and diencephalon). (E, F) lef1 expression is reduced in the PT region of Zic2a depleted embryos. (G, H) rx3 expression is not affected in the anterior hypothalamus, however (I, J) titf1a is weakly expanded anteriorly. (K, L) lhx1a expression is not affected in zic2a morphants at 2 dpf. Embryos are shown in lateral views, anterior to the left. All numbers for these experiments can be found in Supplementary Table 1.
PT defects in zic2a morphants at 17–18s. (A, B) dlx2a expression in the PT is reduced in Zic2a-depleted embryos (49/80 embryos, 3 exp.). (C)Graph showing average numbers of dlx2a-expressing cells in the telencephalon and diencephalon of conMO injected (n = 5) and zic2aMO injected (n = 9) embryos stained for dlx2a at 17–18s. Embryos were sectioned (see methods) to obtain accurate counts of dlx2a-expressing cells. There was no difference in number of telencephalic dlx2a-expressing cells between control and Zic2a morphants. However, there were significantly fewer diencephalic dlx2a-expressing cells in zic2aMOs compared to conMOs (p = .01). (D, E) foxg1 and shha expression was used to deliniate the prethalamic area at 17–18s. The dotted lines in D and E outline the prethalamic area. (F) Graph representing the average prethalamic area (pixels2) in conMOs (n = 10) and zic2aMOs (n = 8). Results are significant at p = .001. Embryos are shown in lateral views, anterior to the left.
Zic2a expression is unaffected by loss of hedgehog signaling. (A, B) Double ISH for zic2a and ptc1 expression in smob641/+ incross progeny at tail-bud stage (TB). (B) Homozygous null embryos were identified by lack of ptc1 expression. (C, D)ISH for zic2a expression in smob641/+ incross progeny at Prim-5 stage. Homozygous null embryos were identified morphologically.
Summary of zic2a morphant analysis at prim-5 and later stages.