Abstract
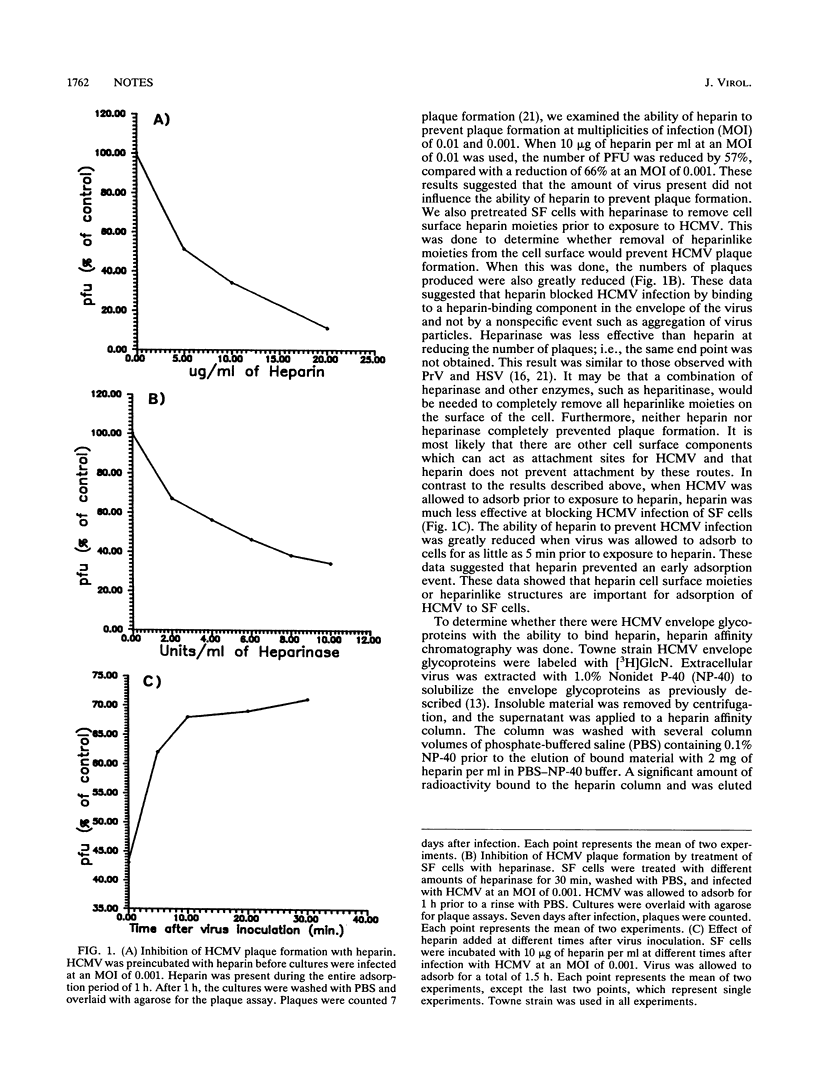
The purposes of this study were to determine whether heparin would block human cytomegalovirus (HCMV) infection of skin fibroblast (SF) cells and to identify HCMV envelope glycoproteins which might have affinity for heparin. It was determined that soluble heparin in concentrations of 5 to 20 micrograms/ml was capable of blocking HCMV infection of SF cells. However, after virus had adsorbed to the SF cells, heparin lost its ability to block infection. It was also determined that treatment of SF cells with heparinase to remove cell surface heparinlike moieties prevented HCMV infection of SF cells. These data showed that HCMV, like other herpesviruses, adsorbed to cells by binding cell surface heparin. Heparin affinity chromatography was done to determine which HCMV envelope glycoproteins bound heparin. HCMV envelope glycoproteins were solubilized in a nonionic detergent and applied to a heparin affinity column. An HCMV glycoprotein complex designated gC-II was the major component to bind to immobilized heparin and elute in the presence of soluble heparin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dall'Olio F., Malagolini N., Speziali V., Campadelli-Fiume G., Serafini-Cessi F. Sialylated oligosaccharides O-glycosidically linked to glycoprotein C from herpes simplex virus type 1. J Virol. 1985 Oct;56(1):127–134. doi: 10.1128/jvi.56.1.127-134.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Babiuk L. A., Zamb T. J. Nucleotide sequence of bovine herpesvirus type 1 glycoprotein gIII, a structural model for gIII as a new member of the immunoglobulin superfamily, and implications for the homologous glycoproteins of other herpesviruses. Virology. 1989 Nov;173(1):46–57. doi: 10.1016/0042-6822(89)90220-1. [DOI] [PubMed] [Google Scholar]
- Gretch D. R., Kari B., Gehrz R. C., Stinski M. F. A multigene family encodes the human cytomegalovirus glycoprotein complex gcII (gp47-52 complex). J Virol. 1988 Jun;62(6):1956–1962. doi: 10.1128/jvi.62.6.1956-1962.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretch D. R., Kari B., Rasmussen L., Gehrz R. C., Stinski M. F. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J Virol. 1988 Mar;62(3):875–881. doi: 10.1128/jvi.62.3.875-881.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretch D. R., Stinski M. F. Transcription of the human cytomegalovirus glycoprotein gene family in the short unique component of the viral genome. Virology. 1990 Feb;174(2):522–532. doi: 10.1016/0042-6822(90)90106-2. [DOI] [PubMed] [Google Scholar]
- Herold B. C., WuDunn D., Soltys N., Spear P. G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991 Mar;65(3):1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Muzithras V. P. Fine mapping of transcripts expressed from the US6 gene family of human cytomegalovirus strain AD169. J Virol. 1991 Apr;65(4):2024–2036. doi: 10.1128/jvi.65.4.2024-2036.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari B., Gehrz R. Analysis of human antibody responses to human cytomegalovirus envelope glycoproteins found in two families of disulfide linked glycoprotein complexes designated gC-I and gC-II. Arch Virol. 1990;114(3-4):213–228. doi: 10.1007/BF01310750. [DOI] [PubMed] [Google Scholar]
- Kari B., Gehrz R. Isolation and characterization of a human cytomegalovirus glycoprotein containing a high content of O-linked oligosaccharides. Arch Virol. 1988;98(3-4):171–188. doi: 10.1007/BF01322167. [DOI] [PubMed] [Google Scholar]
- Kari B., Goertz R., Gehrz R. Characterization of cytomegalovirus glycoproteins in a family of complexes designated gC-II with murine monoclonal antibodies. Arch Virol. 1990;112(1-2):55–65. doi: 10.1007/BF01348985. [DOI] [PubMed] [Google Scholar]
- Kari B., Liu Y. N., Goertz R., Lussenhop N., Stinski M. F., Gehrz R. Structure and composition of a family of human cytomegalovirus glycoprotein complexes designated gC-I (gB). J Gen Virol. 1990 Nov;71(Pt 11):2673–2680. doi: 10.1099/0022-1317-71-11-2673. [DOI] [PubMed] [Google Scholar]
- Kari B., Lussenhop N., Goertz R., Wabuke-Bunoti M., Radeke R., Gehrz R. Characterization of monoclonal antibodies reactive to several biochemically distinct human cytomegalovirus glycoprotein complexes. J Virol. 1986 Nov;60(2):345–352. doi: 10.1128/jvi.60.2.345-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollert-Jöns A., Bogner E., Radsak K. A 15-kilobase-pair region of the human cytomegalovirus genome which includes US1 through US13 is dispensable for growth in cell culture. J Virol. 1991 Oct;65(10):5184–5189. doi: 10.1128/jvi.65.10.5184-5189.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Zuckermann F., Sugg N., Kern H., Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990 Jan;64(1):278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Matsuzaki T., Sugahara Y., Okada J., Hasebe M., Iwamura Y., Ohnishi M., Kanno T., Shimizu M., Honda E. BHV-1 adsorption is mediated by the interaction of glycoprotein gIII with heparinlike moiety on the cell surface. Virology. 1991 Apr;181(2):666–670. doi: 10.1016/0042-6822(91)90900-v. [DOI] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K., Barrell B. G. Sequence of the short unique region, short repeats, and part of the long repeats of human cytomegalovirus. J Mol Biol. 1986 Nov 20;192(2):177–208. doi: 10.1016/0022-2836(86)90359-1. [DOI] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. Pseudorabies virus glycoprotein gIII is required for efficient virus growth in tissue culture. J Virol. 1988 Jul;62(7):2512–2515. doi: 10.1128/jvi.62.7.2512-2515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



