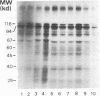
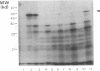
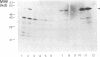
Abstract
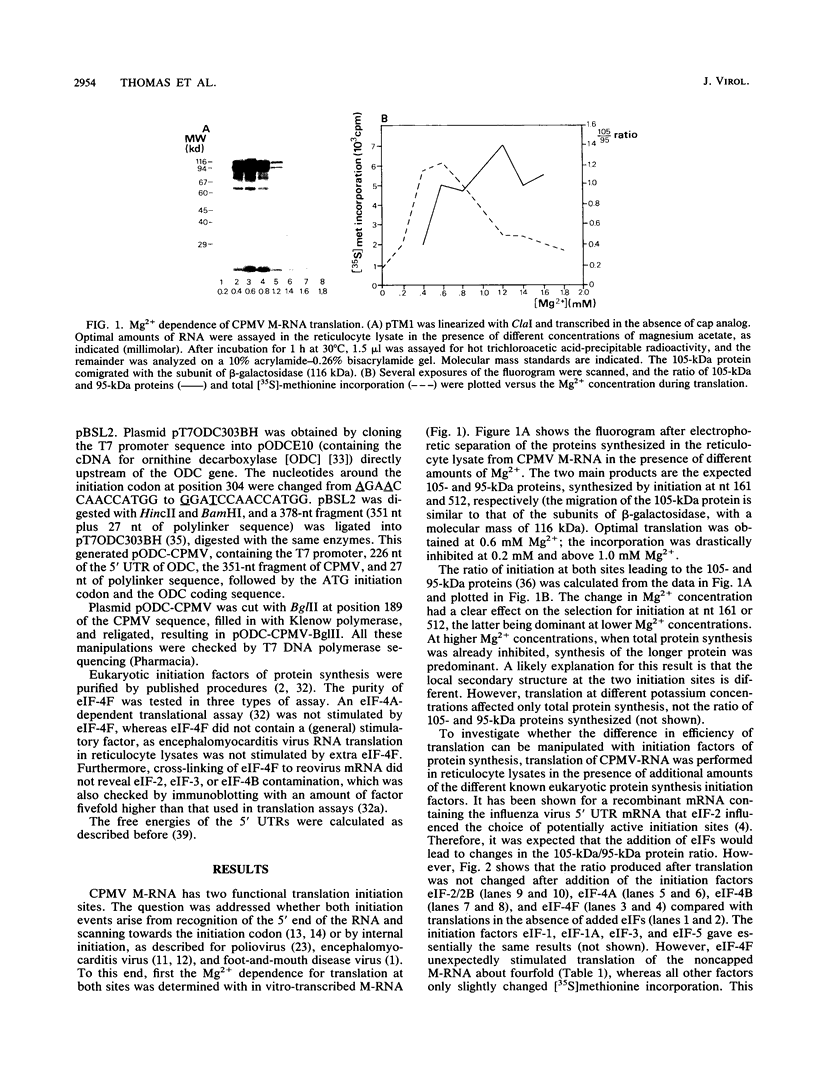
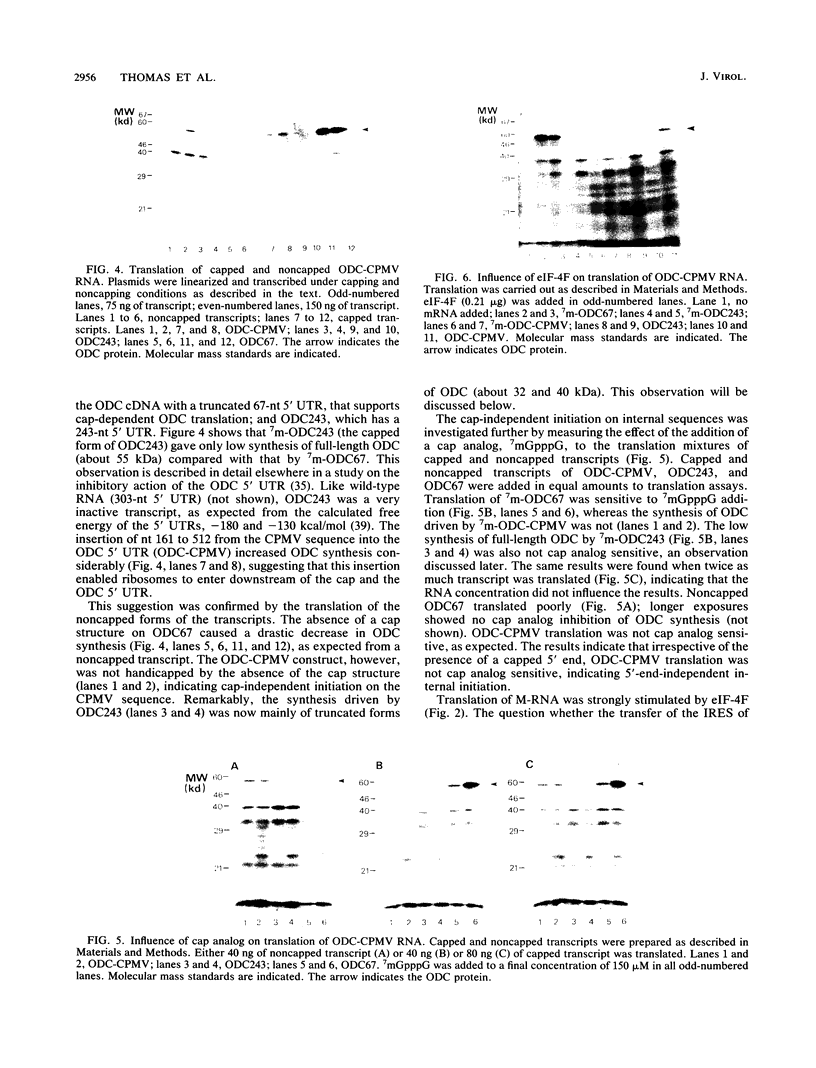
Cowpea mosaic virus (CPMV) middle component RNA (M-RNA) encodes two proteins of 105 and 95 kDa, of which translation starts at nucleotide (nt) 161 and nt 512, respectively. In vitro translation of both proteins directed by T7 transcripts of M-RNA was stimulated fourfold by eukaryotic initiation factor 4F (eIF-4F), the cap-binding protein complex. The ratio of the synthesis of both proteins after translation was not influenced by eIF-4F or by any known eIF. Part of the CPMV 5' sequence was cloned downstream of the 5' untranslated region of ornithine decarboxylase (ODC); the latter untranslated sequence has a highly stable secondary structure, preventing efficient translation of ODC. Insertion of nt 161 to 512 of CPMV M-RNA upstream of the ODC initiation codon resulted in a marked increase in ODC translation, which indicates that the CPMV sequence contains an internal ribosome-binding site. The insertion conferred stimulation by eIF-4F on ODC translation, showing that eIF-4F is able to stimulate internal initiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belsham G. J., Brangwyn J. K. A region of the 5' noncoding region of foot-and-mouth disease virus RNA directs efficient internal initiation of protein synthesis within cells: involvement with the role of L protease in translational control. J Virol. 1990 Nov;64(11):5389–5395. doi: 10.1128/jvi.64.11.5389-5395.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley B., Ehrenfeld E. The cap-binding protein complex in uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1987 Oct 5;262(28):13599–13606. [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D. Cap-independent enhancement of translation by a plant potyvirus 5' nontranslated region. J Virol. 1990 Apr;64(4):1590–1597. doi: 10.1128/jvi.64.4.1590-1597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M. C., Milburn S. C., Hershey J. W., Jackson R. J. Selection of the 5'-proximal translation initiation site is influenced by mRNA and eIF-2 concentrations. Eur J Biochem. 1990 Jan 26;187(2):361–371. doi: 10.1111/j.1432-1033.1990.tb15313.x. [DOI] [PubMed] [Google Scholar]
- Edery I., Lee K. A., Sonenberg N. Functional characterization of eukaryotic mRNA cap binding protein complex: effects on translation of capped and naturally uncapped RNAs. Biochemistry. 1984 May 22;23(11):2456–2462. doi: 10.1021/bi00306a021. [DOI] [PubMed] [Google Scholar]
- Eggen R., Verver J., Wellink J., De Jong A., Goldbach R., van Kammen A. Improvements of the infectivity of in vitro transcripts from cloned cowpea mosaic virus cDNA: impact of terminal nucleotide sequences. Virology. 1989 Dec;173(2):447–455. doi: 10.1016/0042-6822(89)90557-6. [DOI] [PubMed] [Google Scholar]
- Fletcher L., Corbin S. D., Browning K. S., Ravel J. M. The absence of a m7G cap on beta-globin mRNA and alfalfa mosaic virus RNA 4 increases the amounts of initiation factor 4F required for translation. J Biol Chem. 1990 Nov 15;265(32):19582–19587. [PubMed] [Google Scholar]
- Franssen H., Leunissen J., Goldbach R., Lomonossoff G., Zimmern D. Homologous sequences in non-structural proteins from cowpea mosaic virus and picornaviruses. EMBO J. 1984 Apr;3(4):855–861. doi: 10.1002/j.1460-2075.1984.tb01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grens A., Scheffler I. E. The 5'- and 3'-untranslated regions of ornithine decarboxylase mRNA affect the translational efficiency. J Biol Chem. 1990 Jul 15;265(20):11810–11816. [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. A profusion of controls. J Cell Biol. 1988 Jul;107(1):1–7. doi: 10.1083/jcb.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. G., Lee K. A., Maimone M. M., Abramson R. D., Dever T. E., Merrick W. C., Thach R. E. Dissociation of double-stranded polynucleotide helical structures by eukaryotic initiation factors, as revealed by a novel assay. Biochemistry. 1989 May 30;28(11):4729–4734. doi: 10.1021/bi00437a033. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Sonenberg N. Inactivation of cap-binding proteins accompanies the shut-off of host protein synthesis by poliovirus. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3447–3451. doi: 10.1073/pnas.79.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Shanks M. The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 1983;2(12):2253–2258. doi: 10.1002/j.1460-2075.1983.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzella J. M., Blackshear P. J. Regulation of rat ornithine decarboxylase mRNA translation by its 5'-untranslated region. J Biol Chem. 1990 Jul 15;265(20):11817–11822. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5' noncoding region. Mol Cell Biol. 1988 Mar;8(3):1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Ray B. K., Brendler T. G., Adya S., Daniels-McQueen S., Miller J. K., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc Natl Acad Sci U S A. 1983 Feb;80(3):663–667. doi: 10.1073/pnas.80.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads R. E. Cap recognition and the entry of mRNA into the protein synthesis initiation cycle. Trends Biochem Sci. 1988 Feb;13(2):52–56. doi: 10.1016/0968-0004(88)90028-x. [DOI] [PubMed] [Google Scholar]
- Rozen F., Edery I., Meerovitch K., Dever T. E., Merrick W. C., Sonenberg N. Bidirectional RNA helicase activity of eucaryotic translation initiation factors 4A and 4F. Mol Cell Biol. 1990 Mar;10(3):1134–1144. doi: 10.1128/mcb.10.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Pelletier J. Poliovirus translation: a paradigm for a novel initiation mechanism. Bioessays. 1989 Nov;11(5):128–132. doi: 10.1002/bies.950110504. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V., Pestova T. V., Maslova S. V., Agol V. I. Point mutations modify the response of poliovirus RNA to a translation initiation factor: a comparison of neurovirulent and attenuated strains. Virology. 1988 Oct;166(2):394–404. doi: 10.1016/0042-6822(88)90510-7. [DOI] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Thomas A., Goumans H., Amesz H., Benne R., Voorma H. O. A comparison of the initiation factors of eukaryotic protein synthesis from ribosomes and from the postribosomal supernatant. Eur J Biochem. 1979 Aug 1;98(2):329–337. doi: 10.1111/j.1432-1033.1979.tb13192.x. [DOI] [PubMed] [Google Scholar]
- Van Steeg H., Van Oostrom C. T., Hodemaekers H. M., Peters L., Thomas A. A. The translation in vitro of rat ornithine decarboxylase mRNA is blocked by its 5' untranslated region in a polyamine-independent way. Biochem J. 1991 Mar 1;274(Pt 2):521–526. doi: 10.1042/bj2740521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P., Verver J., Jaegle M., Wellink J., van Kammen A., Goldbach R. Two viral proteins involved in the proteolytic processing of the cowpea mosaic virus polyproteins. Nucleic Acids Res. 1988 Mar 25;16(5):1967–1985. doi: 10.1093/nar/16.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P., Verver J., van Wezenbeek P., van Kammen A., Goldbach R. Study of the genetic organisation of a plant viral RNA genome by in vitro expression of a full-length DNA copy. EMBO J. 1984 Dec 20;3(13):3049–3053. doi: 10.1002/j.1460-2075.1984.tb02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kranen H. J., van de Zande L., van Kreijl C. F., Bisschop A., Wieringa B. Cloning and nucleotide sequence of rat ornithine decarboxylase cDNA. Gene. 1987;60(2-3):145–155. doi: 10.1016/0378-1119(87)90222-8. [DOI] [PubMed] [Google Scholar]
- van Steeg H., van Oostrom C. T., Hodemaekers H. M., van Kreyl C. F. Cloning and functional analysis of the rat ornithine decarboxylase-encoding gene. Gene. 1990 Sep 14;93(2):249–256. doi: 10.1016/0378-1119(90)90232-g. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]