Abstract
Cdx genes (Cdx1, Cdx2, and Cdx4) encode a family of caudal-related transcription factors that mediate anterior–posterior patterning during embryogenesis through Hox gene regulation. Homologues in the zebrafish have been shown to play key roles in blood formation. To define the role of Cdx genes during embryonic hematopoiesis in a mammalian system, we examined the hematopoietic potential of Cdx-deficient mouse embryonic stem cells (ESCs) in vitro and in vivo. Individual Cdx-deficient ESCs exhibited impaired embryonic hematopoietic progenitor formation and altered Hox gene expression, most notably for Cdx2 deficiency. A more severe hematopoietic defect was observed with compound Cdx deficiency than loss of function of any single Cdx gene. Reduced hematopoietic progenitor formation of ESCs deficient in multiple Cdx genes could be rescued by ectopic expression of Cdx4, concomitant with partially restored Hox gene expression. These results reveal an essential and partially redundant role for multiple Cdx genes during embryonic hematopoiesis in the mouse.
Keywords: Hox genes, embryonic stem cell, A-P patterning, development, embryoid body
Hematopoietic development occurs sequentially at distinct sites during vertebrate embryogenesis. In mice, the first wave of primitive yolk sac (YS) hematopoiesis occurs before 7.5 days postcoitum (dpc), followed by a second wave of definitive hematopoiesis in the aorta–gonad–mesonephros (AGM) region, which then relocates to fetal liver before birth (1). The underlying molecular pathways involved in the initiation and maintenance of hematopoiesis at different stages during embryogenesis are poorly understood.
Over the past several years, accumulating evidence suggests that the master regulators of Hox genes, Cdx family members, are involved in the proliferation and differentiation of hematopoietic cells. Cdx genes are caudal-related transcription factors that mediate anterior–posterior patterning through Hox gene regulation (2–5). Three Cdx genes (Cdx1, Cdx2, and Cdx4) have been identified so far in mammals. A potential role for Cdx genes in embryonic hematopoiesis initially came from studies in zebrafish. Cdx4 mutant zebrafish have severe blood defects and altered hox gene expression during embryogenesis. Cdx1 functions redundantly with cdx4 to promote hematopoiesis in zebrafish (6, 7). In mouse, ectopic expression of Cdx4 enhances hematopoietic mesoderm formation and further promotes blood progenitor specification and hematopoietic engraftment in adult mice from murine embryonic stem cells (ESCs) (8). In addition, Cdx4 overexpression rescues defective blood progenitor formation in mouse ESCs deficient in the Mll, a Hox regulator involved in definitive hematopoiesis (9). During adult hematopoiesis, overexpression of human CDX2 or murine Cdx4 alone results in acute myeloid leukemia in mice (10, 11). Interestingly, despite all of the evidence suggesting an essential role for Cdx genes in hematopoietic development, no significant defects in hematopoiesis have been observed in Cdx single or compound deficient mice (2–5). This led us to hypothesize that Cdx genes may have overlapping or redundant functions during hematopoiesis, and that defects during embryonic stages of hematopoietic development may ultimately be masked by compensatory mechanisms in the adult.
Long-term culture of pluripotent ESCs has been established from a variety of mammalian species, including human and mouse. When induced to differentiate in vitro, ESCs form cystic embryonic bodies (EB) in which hematopoietic cells develop. Collective findings strongly suggest that the ESC/EB in vitro differentiation system recapitulates early hematopoiesis observed in vivo during embryonic development (1). As such, the ESC/EB system provides a powerful and convenient in vitro model to explore the molecular pathways that specify hematopoietic commitment, which otherwise would be difficult to examine in embryos.
In this study, using Cdx-deficient ESCs and murine models, we demonstrate a requirement for the Cdx-Hox gene pathway during primitive embryonic hematopoiesis. Deletion of any single Cdx gene led to impaired embryonic hematopoietic progenitor formation with disturbed Hox expression profiles. In particular, the Cdx2 null state caused the most dramatic hematopoietic deficiency in a cell autonomous manner. In addition, ectopic expression of Cdx4 rescued decreased blood progenitor formation caused by compound Cdx deficiency and partially restored Hox gene expression patterns. Furthermore, HoxB4 transgene activation partially compensated for the impaired hematopoietic defects of Cdx2 deficiency. These results reveal an essential role for the Cdx-Hox pathway during early primitive hematopoiesis in mammals and support our hypothesis that Cdx genes function redundantly in hematopoietic progenitor specification during embryogenesis.
Results
Cdx4 Deficiency Causes Modest Defects in Embryonic Hematopoiesis.
To investigate the physiological function of Cdx4 during embryonic hematopoiesis in mammals, we created Cdx4-deficient ESC lines and mice by replacing the first exon of Cdx4 on the X-chromosome with a cDNA encoding GFP [supporting information (SI) Fig. S1 A–D].
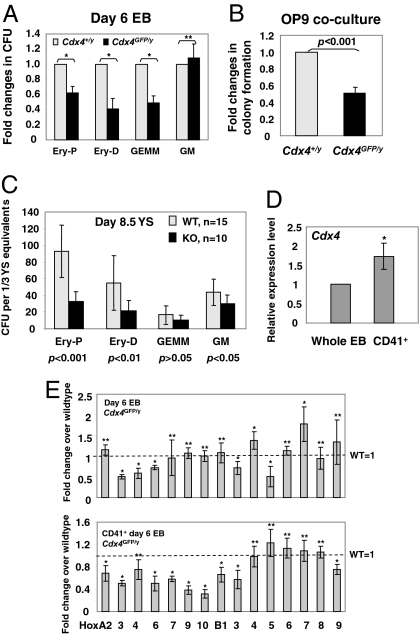
We first evaluated the ability of Cdx4-deficient (Cdx4GFP/y) ESCs to undergo hematopoietic differentiation in vitro. As shown in Fig. 1A, Cdx4GFP/y ESCs demonstrated a modest reduction in number of blood progenitors, primarily in erythroid and mixed progenitor colonies, when assayed for hematopoietic progenitor colony formation. In addition, day 6 Cdx4GFP/y EB-derived cells showed decreased hematopoietic expansion on OP9 stroma (Fig. 1B), a coculture system that allows the expansion of multipotential hematopoietic progenitors (12). The reduced hematopoietic progenitor formation in vitro caused by loss of function of Cdx4 was further confirmed by analyzing hematopoietic tissues in Cdx4-deficient mice (Cdx4GFP/y and Cdx4GFP/GFP). In this study, YS-derived primitive erythroid colony formation was reduced in Cdx4-deficient embryos from 7.5 to 9 dpc compared with their WT littermates (Fig. 1C, Fig. S1F). Taken together, these data suggest that primitive hematopoiesis is impaired, although modestly, upon Cdx4 deficiency.
Fig. 1.
Cdx4 deficiency leads to reduced hematopoietic progenitor formation and altered Hox gene expression profiles. (A) Hematopoietic progenitor and (B) OP9 colony formation of day 6 EB-derived cells. Day 6 EB cells from Cdx4+/y or Cdx4GFP/y ESCs were plated into M3434 methylcult (A) or onto OP9 stromal cells (B), and colony number was assessed. CFU, colony forming unit; Ery-P, primitive erythroid; Ery-D, definitive erythroid; GEMM, multilineage progenitor of granulocyte, erythroid, macrophage, megakaryocyte; GM, multilineage progenitor of granulocyte and macrophage. (C) Hematopoietic colony formation of YS at 8.5 dpc from either Cdx4-deficient (Cdx4GFP/GFP and Cdx4GFP/y: KO) embryos or their WT littermates (Cdx4+/+ or Cdx4+/y: WT). Data represent an average of five litters from two independent experiments. (D) Relative expression levels of Cdx4 in CD41+ or whole day 6 EBs, measured by real-time RT-PCR. (E) Expression levels of Hox genes in CD41+ or whole day 6 EBs, assessed by real-time RT-PCR. (A and B, D and E) Data represent averaged fold changes relative to WT control from replicates in two to three independent experiments. Each data point denotes six biological replicates. *, P value <0.01; **, P value >0.05. For all experiments, error bars represent ± 1 SD.
Because Cdx4 is a master regulator of Hox genes, and altered hox gene expression patterns due to cdx4 deficiency is associated with blood formation defects in zebrafish (6), we next measured Hox gene expression levels by real-time RT-PCR from either CD41+ or whole day 6 EBs derived from Cdx4GFP/y or Cdx4+/y ESCs. CD41+ cells are enriched with early hematopoietic progenitors (9, 13), and expression of Cdx4 was higher in CD41-sorted cells than whole day 6 EBs (Fig. 1D). Although Hox gene levels were not profoundly altered in whole EBs, the expression of posterior A cluster genes was notably reduced in Cdx4GFP/y CD41+ cells (Fig. 1E). Taken together, these data suggest that Cdx4 affects Hox gene expression patterns specifically in CD41+ hematopoietic cells, which in turn affect hematopoietic colony forming potential and/or proliferation.
Cdx1 Deficiency Reduces Hematopoietic Formation from ESCs.
Cdx1-deficient (Cdx1−/−) mice were established (4). Analysis of the early YS hematopoiesis at 7.5–9.5 dpc or adult hematopoiesis from Cdx1−/− mice did not reveal any obvious blood defects compared with Cdx1+/+ controls (Fig. S2).
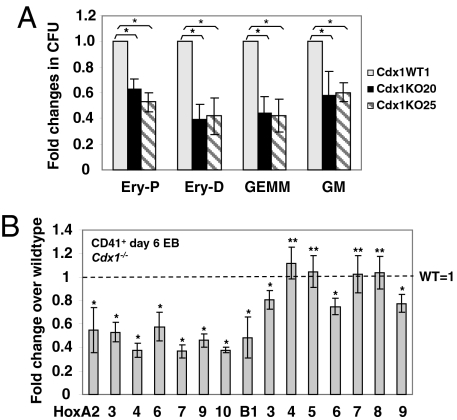
To investigate whether subtle defects in hematopoietic specification could be detected with the ESC/EB in vitro system, we generated Cdx1−/− and Cdx1+/+ ESC lines from these mice (Fig. S2A). The growth and appearance of Cdx1−/− EBs were indistinguishable from WT controls (data not shown). However, hematopoietic progenitor formation from day 6 Cdx1−/− EB cells was notably reduced compared with Cdx1+/+, as measured by progenitor colony-forming assay (Fig. 2A). These results indicate that Cdx1 may play a role in early hematopoiesis, but compensatory mechanisms in vivo may mask subtle phenotypes caused by Cdx1 deficiency. The expression of several posterior Hox A cluster genes was down-regulated in both CD41+ hematopoietic cells and whole day 6 EBs from Cdx1−/− ESCs (Fig. 2B, Fig. S2C). These findings correlate altered Hox expression patterns with decreased blood formation in Cdx1−/− ESCs.
Fig. 2.
Cdx1 deficiency causes reduced hematopoietic progenitor formation and altered Hox gene expression profiles from ESCs. (A) Hematopoietic colony formation of day 6 EB cells from either WT or two Cdx1−/− ESC lines (KO20 and KO25). Data represent averaged fold changes (±1 SD) in CFU relative to WT control from triplicates in two independent experiments. (B) Expression levels of Hox genes in day 6 CD41+ Cdx1−/− EBs, measured by real-time RT-PCR analysis and presented as Cdx1−/− relative to Cdx1+/+ (WT). Data represent the averaged fold changes (±1 SD) from triplicates in two independent experiments. *, P value <0.01; **, P value >0.01.
Cdx2 Deficiency Results in Severe Blood Defects During Embryonic Hematopoiesis.
Cdx2 null embryos die between 3.5 and 5.5 dpc because of defects in trophectoderm differentiation (2, 14). Analysis of in vitro differentiation of Cdx2-deficient ESCs (3) provides an opportunity to survey potentially later stages of embryonic development in hematopoiesis.
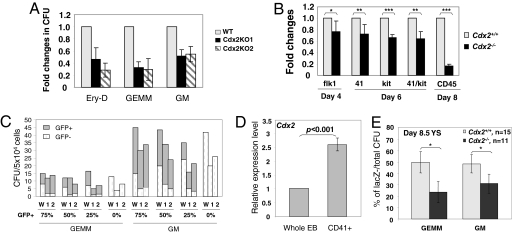
As shown in Fig. 3A, the number of multipotential blood progenitor colonies was reduced in Cdx2−/− day 6 EBs, suggesting that hematopoietic development was impaired in the absence of Cdx2 activity. We next explored hematopoietic differentiation in Cdx2−/− EBs by conducting flow cytometry analysis on surface antigens that are enriched for hematopoietic precursors. Flk1 is a marker for multipotential mesodermal progenitors, which develop into hematopoietic and endothelial lineages (15). As for CD41, c-kit+ cells are also enriched for early hematopoietic precursors, and the majority of EB-derived hematopoietic progenitors are CD41+/c-kit+ (9, 13). CD45 is a panhematopoietic marker. As shown in Fig. 3B, the overall hematopoietic compartment was reduced in Cdx2−/− day 6 EBs, as reflected by surface antigen analysis. Notably, the frequency of CD41+, c-kit+, or CD41+/c-kit+ cells showed only a moderate decrease, in contrast with more significant reductions in CD45+ from Cdx2−/− EBs, suggesting that Cdx2 may largely affect the differentiation or proliferation of hematopoietic progenitors. Taken together, these data support an essential role for Cdx2 in early embryonic hematopoiesis.
Fig. 3.
Cdx2 deficiency results in severe blood defects during embryonic hematopoiesis. (A) Hematopoietic colony formation of day 6 EB cells from Cdx2+/+ (WT) or two Cdx2−/− ESC lines, KO1 and KO2. P value <0.01 for all comparison of Cdx2−/− to WT ESC lines. (B) Surface antigens analyzed by flow cytometry at different time points during EB development. *, P value >0.1; **, P value = 0.01–0.04; ***, P value <0.01. (C) Hematopoietic colony formation of day 6 EB cells from mixed population of either Cdx2+/+ (W) or two Cdx2−/− ESC lines (1 and 2) with normal GFP+ ESCs. Percentage of GFP+ ESC composition is indicated below the x axis. Data are averaged CFU from duplicates in one representative experiment and reproduced from two independent experiments. (D) Relative expression level of Cdx2 in CD41+ or whole day 6 EBs, measured by real-time RT-PCR. (A, B, and D) Data are averaged fold changes (±1 SD) relative to WT control from replicates in two or three independent experiments. Each data point denotes six to eight biological replicates. (E) Progenitor colony activity of YS from chimeric embryos at 8.5 dpc generated with either Cdx2−/− or Cdx2+/+ ESCs. Donor blastocysts are lacZ+. The percentage of lacZ− (ESC-contributed)/total CFU was measured. Data represent average ± 1 SD. * P values <0.001.
To examine whether the requirement of Cdx2 for hematopoiesis was cell autonomous, we conducted an in vitro chimera study. In this analysis, Cdx2+/+ or Cdx2−/− ESCs were mixed with WT GFP+ ESCs at different ratios, and the hematopoietic potential of these mixed populations was measured. Because GFP was not well expressed in erythroid colonies, only mixed progenitor colonies were counted. As shown in Fig. 3C, Cdx2−/− ESCs generated a reduced number of blood progenitors, indicating that the blood defects could not be rescued by mixing with normal GFP+ ESCs. These results suggest an intrinsic requirement for Cdx2 in hematopoiesis. Consistent with this finding, higher Cdx2 expression was observed in the CD41+ hematopoietic fraction compared with whole day 6 EBs (Fig. 3D).
We further investigated whether Cdx2 was required for embryonic hematopoiesis in mice by an in vivo chimera study. Cdx2−/− or Cdx2+/+ ESCs were injected into normal lacZ+ blastocysts. The overall contribution of ESCs was then determined by X-Gal staining. Because a high contribution of Cdx2−/− ESCs resulted in embryonic lethality, we limited our analysis to embryos with overall contribution of 40–60% of Cdx2−/− or Cdx2+/+ ESCs. In this case, the appearance of YS was indistinguishable in chimeras with Cdx2−/− ESCs compared to WT control. The hematopoietic activity of YS at 8.5 and 9 dpc was measured, and the ratio of lacZ− (Cdx2−/−) progenitor colonies was determined. Consistent with our in vitro results, the percentage of lacZ− hematopoietic colonies was reduced in chimeric mice, indicating a reduced contribution of Cdx2−/− ESCs to YS-derived blood progenitors (Fig. 3E, Fig. S3C). In summary, our results demonstrate an intrinsic requirement for Cdx2 during primitive embryonic hematopoiesis in vitro and in vivo.
Expression Analysis Implicates the Involvement of Cdx2 in Embryonic Hematopoiesis.
The biological functions of Cdx2 during later embryonic development have not been understood completely because of the early lethality of Cdx2 null embryos. To identify the target genes of Cdx2 during embryogenesis and to explore the molecular mechanism of reduced hematopoiesis caused by Cdx2 deficiency, we performed a genome-wide microarray expression analysis on total RNA isolated from Cdx2−/− or Cdx2+/+ day 6 EBs.
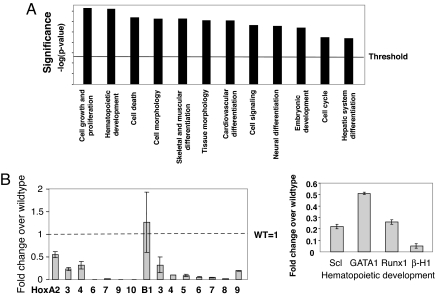
Using a 2-fold expression difference from WT control as a threshold, ingenuity pathway analysis (IPA) revealed >10 altered biological functions upon Cdx2 deficiency. Among them, “cell growth and proliferation” and “hematopoietic development” scored as the most significant annotated function groups (Fig. 4A). As expected, the expression of Hox genes was significantly altered, and most of them displayed dramatic down-regulation upon Cdx2 deficiency (Fig. 4B, Table 1). This observation was consistent with the role of Cdx2 as a Hox gene regulator. In addition, the expression of several hematopoiesis-specific genes was decreased 2- to 20-fold, including Scl, Gata1, Runx1, and β-H1 globin (Fig. 4B). Notably, Scl, Gata1, and β-H1 are markers for primitive hematopoietic development (16). Dramatic decrease in their expression because of Cdx2 deficiency thus supports our previous results that Cdx2 is required for primitive hematopoiesis. Interestingly, the expression of several components of canonical signal transduction pathways was altered in Cdx2−/− EBs (Table 1, Fig. S3 F and G). Several of these pathways, such as the Jak/Stat and sonic hedgehog pathways, have been implicated in hematopoietic development through protein network interactions (17–19), suggesting that Cdx2 deficiency may impair embryonic hematopoiesis indirectly through broad signal transduction networks. Taken together, our genome-wide expression analysis reveals that Cdx2 deficiency perturbs expression profiles of Hox genes and genes involved in signal transduction and hematopoiesis, thus indicating a role for Cdx2 during hematopoietic development.
Fig. 4.
Microarray expression analysis of day 6 EBs from Cdx2−/− or Cdx2+/+ ESCs. (A) Functional analysis with IPA software. Genes included in this analysis all had P values <0.001 and a >2-fold expression alteration upon Cdx2 deficiency compared with WT control. These genes were associated with biological functions through IPA analysis. Significance (−log P value) is reverse to the P value to demonstrate the most relevant biological functions beyond the threshold. (B) Partial verification of the results from microarray analysis by real-time RT-PCR in day 6 EBs from Cdx2-deficient ESCs. Data represent fold changes (±1 SD) in Cdx−/− EBs, relative to WT control. All results were reproduced from two independent experiments.
Table 1.
Gene expression changes in Cdx2 null EBs in microarray analysis
| Gene class | Up-regulated | Down-regulated |
|---|---|---|
| Hox genes | B1 | A7, 9–11, 13, B2–5, 7–9, C6, 8, 10, D1, 3, 4, 8–11. 13 |
| Hematopoiesis | Scl, GATA1, Runx1, Vav1, pu.1, β-H1, β-major, Fcrh3, Ddx3y, Tox, Tgtp, Gimap3, ifi204 | |
| Cell signaling | Socs2, Edil3, Gbp1, Shh, Fzd2, Cacna1d, Ppp2r2b, PDGFD | Jak3, Stat1–3, Stat5a, 5b, Stat6, Gpr123, FGFR1 |
Functional Redundancy Among Cdx Genes During Embryonic Hematopoiesis.
Although impaired hematopoietic progenitor activity was observed upon single Cdx gene deficiency, Cdx mutants are not totally bloodless, and Cdx1 or Cdx4 null mutations caused only modest defects in blood formation. Given the functional redundancy known to exist among Cdx family members during vertebral patterning and axis elongation (20), it is likely they also function redundantly during embryonic hematopoiesis. To examine this hypothesis, we investigated the effect of compound Cdx deficiency using RNA interference technology to knock down Cdx1 or Cdx2 in the Cdx4 null background.
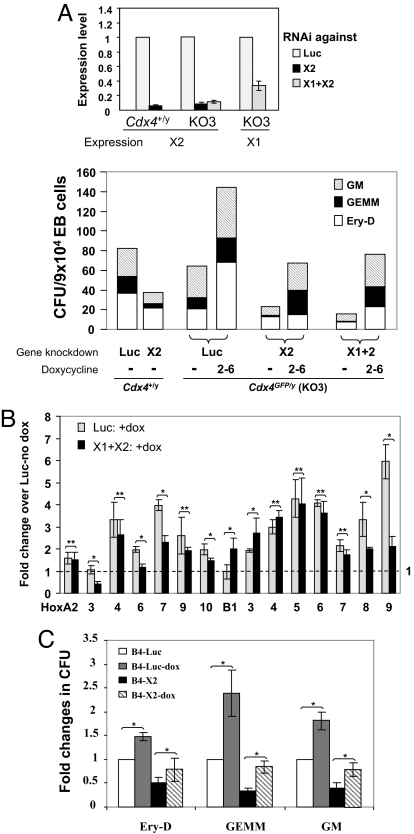
The combination of cdx4 and cdx1 deficiency in zebrafish leads to a completely bloodless embryo (6, 7). However, simultaneous reduction of Cdx1 and Cdx4 expression by Cdx1 knockdown in the Cdx4+/y or Cdx4GFP/y ESCs decreased only the formation of erythroid progenitors more significantly than single Cdx1 knockdown or Cdx4 knockout (Fig. S4), demonstrating functional differences of Cdx genes in mammals from zebrafish. We thus examined the effect of compound Cdx deficiency including Cdx2 reduction by knocking down Cdx2 alone or both Cdx1 and Cdx2 in the Cdx4+/y or Cdx4GFP/y ESCs (KO3). Consistent with our results on Cdx2−/− ESCs, Cdx2 knockdown resulted in significantly reduced blood progenitor formation (Fig. 5A, Fig. S4). In addition, the combination of Cdx4 knockout with Cdx1 and Cdx2 knockdown almost abolished blood formation from day 6 EBs (Fig. 5A, Fig. S4). Taken together, these results support an essential role of Cdx2 in murine hematopoiesis and suggest that the dosage of Cdx genes is an important permissive factor in hematopoietic specification.
Fig. 5.
Functional redundancy among Cdx family members. (A) Hematopoietic colony formation upon gene knockdown of Cdx2 alone or Cdx1 and Cdx2 in Cdx4+/y or Cdx4GFP/y (KO3) ESCs. (Upper) Expression of Cdx1 and Cdx2 was analyzed by real-time RT-PCR on day 4 EBs upon gene knockdown of Cdx1 (X1) and/or Cdx2 (X2) compared with control shRNA against Luc. (Lower) Hematopoietic colony formation from day 6 EBs upon gene knockdown of Cdx2 (X2), Cdx1 and Cdx2 (X1+X2), or Luc in Cdx4+/y or Cdx4GFP/y (KO3) ESCs. Cells were treated without (−) or with dox from day 2–6 of EB development (as indicated below x axis). Data are averaged CFU from triplicates in one representative experiment reproduced from three additional experiments. (B) Relative Hox gene expression level as measured with real-time RT-PCR analysis. Samples were Cdx4GFP/y day 6 EBs with either control shRNA against Luc or shRNA against Cdx1 and Cdx2 (X1+X2). Both samples were treated with dox from day 2–6 of EB development, and their Hox gene expression level was relative to Cdx4+/y day 6 EBs with control shRNA against Luc without dox treatment. Data represent the averaged fold changes of gene expression from triplicates in two independent experiments. (C) Ectopic HoxB4 expression rescues impaired hematopoietic progenitor formation caused by Cdx2 deficiency. Hematopoietic colony formation was examined from tetracycline-inducible HoxB4 ESCs with shRNA against either Luc or Cdx2 (X2) in the absence or presence of dox. Data represent as averaged fold change in cfu from triplicates in two independent experiments. (A–C) Error bars represent ±1 SD. *, P value <0.01; **, P value >0.01.
Because our Cdx4GFP/y ESC line KO3 was derived from a tetracycline-inducible Cdx4 ESC line, ectopic Cdx4 expression could be induced upon doxycycline (dox) treatment, even though its endogenous Cdx4 locus had been disrupted by GFP cDNA insertion (SI Text). Therefore, this cell line was used to investigate whether activation of a Cdx4 transgene could rescue the blood defects caused by Cdx gene deficiency. Interestingly, ectopic Cdx4 expression not only rescued the blood forming defects caused by loss of function of Cdx4 but also compensated for the reduced blood formation because of Cdx2 or compound Cdx gene deficiency (Fig. 5A, Fig. S4). Thus, these results support our hypothesis that Cdx family members function redundantly during embryonic hematopoiesis.
Because the Hox genes are good candidates for mediating the effects of the Cdx genes in hematopoiesis, we further examined whether Hox gene expression levels could be restored in Cdx-deficient cells by activation of a Cdx4 transgene. As shown in Fig. 5B, the expression level of most Hox genes was indeed restored to normal, with some posterior Hox genes being up-regulated significantly by ectopic Cdx4 expression (e.g., HoxA7, -A9, -B8, -B9). Compared to a luciferase (Luc) shRNA control, the expression levels of HoxB1 and HoxB3 appeared to be higher in the Cdx-deficient cells (Fig. 5B). Because Cdx genes typically do not influence the expression of the most anterior Hox genes, we suspect the effects are most likely due to inappropriate expression level or timing of the dox-driven Cdx4 transgene.
Finally, to determine whether Hox genes act downstream of Cdx family members in hematopoiesis, we examined whether ectopic HoxB4 expression could rescue the impaired hematopoietic commitment caused by Cdx2 deficiency. We chose HoxB4 in this analysis, because its expression was significantly reduced in Cdx2 null cells. In addition, it has been shown that Cdx4 activates HoxB4 in hematopoietic progenitors, and HoxB4 promotes hematopoietic specification from ESCs (8, 21). We thus introduced a shRNA construct against Cdx2 into inducible HoxB4 ESCs (21) and evaluated hematopoietic progenitor formation after dox induction of HoxB4 expression. We observed that overexpression of the HoxB4 transgene indeed rescued the impaired blood progenitor formation due to Cdx2 deficiency, suggesting HoxB4 is downstream of Cdx2 (Fig. 5C). Taken together, these data suggest that proper Hox gene expression patterns, as regulated by Cdx genes, contribute to embryonic hematopoietic specification in mammals.
Discussion
Although the functional requirement for cdx4 and cdx1 during hematopoiesis has been demonstrated in zebrafish, the roles of Cdx genes during hematopoietic commitment are poorly understood in mammals. In this study, using the ESC/EB in vitro differentiation system and mouse models, we demonstrate that deficiency of Cdx genes perturbs hematopoiesis to variable extents, with Cdx2 deficiency most substantially compromising primitive embryonic hematopoiesis. These data establish the functional conservation of the role of Cdx genes in hematopoietic development between fish and mammals, but that a combination of Cdx gene deficiencies is required to produce substantial deficits implies a more complex and redundant role in mammals.
Our study reveals a previously undescribed role for Cdx2 during embryonic hematopoiesis. Because Cdx2 deficiency results in embryonic lethality in mice, future investigation of the specific role of Cdx2 in hematopoietic development will require construction of a conditional knockout. Our data demonstrated only modest hematopoietic defects in Cdx1 or Cdx4 null cells but severe deficiency due to loss of function of Cdx2 in the murine system. A zebrafish orthologue of cdx2 has not been identified; thus, it is possible that the critical function of Cdx4 in zebrafish hematopoiesis is shared by Cdx2 in mammals.
All Cdx genes can be detected in the posterior primitive streak of mouse embryos ≈7 dpc (14, 22–25), where the highest frequency of the first hematopoietic cells, the hemangioblast, is found before blood island formation in YS (26). In EBs, peak Cdx expression occurs from day 2 to 5 of ESC differentiation (data not shown), which corresponds to the window of primitive hematopoietic fate specification (1). In addition, the expression of Cdx2 and Cdx4 is enriched in CD41-sorted hematopoietic cells. These findings suggest an intrinsic requirement for Cdx genes in embryonic hematopoiesis. Consistent with this hypothesis, our chimera studies showed that the reduced hematopoietic colony formation of Cdx2 null ESCs could not be rescued by mixing with normal ESCs or injecting into normal blastocysts. Although deficiencies of Cdx genes might alter a number of different tissue fates, our observations suggest that the Cdx genes act cell autonomously, at least in part, during blood development.
Recently, van Nes et al. reported that Cdx1 and Cdx4 double mutant mice were viable, and no hematopoietic defects were noted (5). In our study, the blood formation defects caused by Cdx deficiency were transient, primarily at the stage of YS hematopoiesis before 9 dpc. No significant blood alteration was observed in YS after 9 dpc (when circulation is established in embryos), in fetal liver at 14.5 dpc, in adult bone marrow, or in peripheral blood from Cdx1 or Cdx4 null mice (Figs. S1 and S2 and Dataset S1). The hematopoietic contribution from Cdx2-deficient ESCs at the fetal liver stage appeared to be indistinguishable from the WT control (data not shown). Thus, Cdx genes primarily affect primitive hematopoiesis during embryonic development, which explains why published accounts of Cdx knockout mice have not reported hematopoietic defects. It is possible that definitive hematopoiesis is less sensitive to the dosage of Cdx gene expression and has a more efficient compensation mechanism than YS hematopoiesis.
Cdx family members appear to function redundantly during embryonic hematopoiesis, because a more severe hematopoietic defect was observed with compound Cdx deficiency than loss of function of any single Cdx gene. Similar alterations in expression levels of posterior Hox A cluster genes (A6–10) were seen in each of the different Cdx-deficient ESC lines, demonstrating that the Cdx family members shared common posterior Hox gene targets. In addition, activation of the Cdx4 transgene in Cdx-deficient EBs both rescued the hematopoietic defects and restored Hox gene expression. Therefore, it is reasonable to speculate that Cdx genes function redundantly during blood development by activating overlapping Hox gene targets. Although there is considerable evidence for functional redundancy, loss of function of specific Cdx genes showed some unique effects on hematopoiesis, perhaps mediated through the differential effects on the Hox gene targets.
Perturbation in the anterior expression boundaries of Hox genes alters cell fate. This finding has led to the “Hox code” hypothesis that a specific Hox gene expression pattern determines the tissue identity along the anterior–posterior axis (27). Ectopic expression and loss-of-function studies have implicated many Hox genes in normal hematopoiesis and leukemogenesis (28). Cdx genes have been shown to act directly as Hox gene regulators, and this is corroborated by the homeotic transformations in Cdx knockout studies (2, 4, 20, 29). Furthermore, blood defects due to Cdx4 deficiency in zebrafish or loss of function of Mll in mouse ESCs can be rescued by ectopic expression of Cdx4 or particular posterior Hox genes (6, 9). In this study, we observed that the Hox gene expression profile, possibly representing a “Hox code,” was altered in CD41+ hematopoietic cells upon Cdx deficiency, and that this alteration correlated with reduced blood formation. In addition, Hox gene expression levels were restored when blood formation was rescued by ectopic Cdx4 expression in Cdx single or compound deficient cells, and activation of a HoxB4 transgene partially compensated for the impaired blood progenitor formation caused by Cdx2 deficiency. Together with previous studies, our data provide compelling evidence that a proper Hox gene code, initiated by Cdx genes, is important for embryonic hematopoietic specification in mammals.
The ESC/EB system is a powerful, sensitive, and convenient in vitro model to explore the molecular pathways that specify the earliest stages of hematopoiesis during embryonic development, especially in cases where gene deficiency leads to embryonic lethality. In this study, by using the ESC/EB in vitro differentiation system and mouse models, we demonstrated an intrinsic requirement or the Cdx-Hox gene pathway during primitive embryonic hematopoiesis in mouse. Our findings advance an understanding of the role of Cdx genes in normal hematopoiesis and, given the links between Cdx genes and leukemogenesis, illuminate how reactivation of embryonic pathways might contribute to disease.
Materials and Methods
Cell Culture.
ESCs were maintained, differentiated, and harvested in vitro according to published protocols (8, 21). OP9 coculture was performed as described (8, 21), and the colony number was counted on day 6 after plating. Hematopoietic colony formation assay were performed according to published protocol (15).
Establishing and screening Cdx4 knockout/GFP knockin ESC lines and mouse are described in details in SI Text.
Obtaining Cdx1-Deficient ESC Lines and Cdx1 Genotyping.
Cdx1−/− and Cdx1+/+ ESCs were obtained from blastocysts of intercrossed Cdx1−/+ heterozygotes according to standard protocols (30). Cdx1 genotyping primers: sense-5′-GGCTCCTTGGCCCGGCGG-3′, antisense-5′-CCGAGCTGGCTGCTAACC-3′. PCR from the WT allele gave a 1.5-kb band, whereas the mutant allele gave a 400-bp band.
Western Blot Analysis.
Protein extract was prepared from dissociated Cdx1−/− and Cdx1+/+ day 4 EB, and Western blot analysis of Cdx1 and β-actin was performed according to published protocol (24).
Embryo Dissection and Analysis.
The age of embryo was defined as 0.5 dpc at 8:00 a.m. on the day of vaginal plug observation. In the chimera study, donor blastocysts were collected from C57BL/6 female mice intercrossed with ROSA26-lacZ male mice. Expression of lacZ in chimeric embryos was revealed according to published protocols (9). For lacZ expression in the colonies from YS, 400 μl of X-Gal staining solution (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/ml X-Gal) was added in drops into methylcult 9 days after plating of YS cells. Colonies were counted before and 12 h after X-Gal staining. All experimental procedures were conducted in accordance with the Animal Welfare Act and Public Health Service Policy.
Quantitative Real-Time PCR.
Cells were harvested in TRIzol. Total RNA and cDNAs were prepared according to the manufacturer's instructions (Invitrogen). All RNA samples were treated with DNaseI and then purified by RNeasy MinElute kit (Qiagen). Real-time PCR was performed on Stratagene MX3000P instrument and analyzed as described (8). Primer sequences were listed in Dataset S2.
FACS Analysis.
All antibodies were purchased from PharMingen, BD Biosciences. Propidium iodide was added to exclude dead cells. Samples were acquired on LSR II Flow Cytometer (BD Biosciences). CD41 sorting was done on Becton Dickinson FACSVantage SE Flow Cytometer with FITC-labeled CD41 antibody. The sorting purity was checked to ensure >90% purity for all sorted samples.
Microarray hybridization and data processing are described in detail in SI Text.
RNA Interference.
Lentivirus-based shRNA against Luc, Cdx1 or Cdx2 were generated as described (31). Detailed shRNA sequences and selection procedure are described in SI Text.
Statistical Analysis.
P values were calculated from Student's t test for all comparisons, except in Fig. 4A, Fisher's exact test was used to calculate the P values.
Supplementary Material
Acknowledgments.
We thank the following people who provided reagents: Cdx1 knockout mice and genotyping protocol from Dr. Jacqueline Deschamps (Netherlands Institute for Developmental Biology, Utrecht, The Netherlands), ROSA26-lacZ mice and PGK-dta vector from Dr. Philippe Soriano (Fred Hutchinson Cancer Research Center, Seattle), Cdx1 antibody from Dr. Peter Gruss (Max Planck Institute, Munich), parental Cdx4 targeting vector from Dr. Brian Huntly (Children's Hospital Boston, Boston), and V6.5 ESC line from Dr. Rudolf Jaenisch (Whitehead Institute, Massachusetts Institute of Technology, Boston). We thank Dr. Yuji Mishina for advice and Gregory J. Scott and Toni Ward for technical support in the chimera study. We also thank Il-Ho Jang for help in the cfu assays. We appreciate the critical comments during manuscript preparation from Drs. Alan J. Davidson, Thomas Eling, Paul Wade, and Yuji Mishina. This study is supported by the intramural research program of the National Institute on Environmental Health Sciences. G.Q.D. was supported by grants from the National Institutes of Health and by the National Institutes of Health Director's Pioneer Award of the National Institutes of Health Roadmap for Medical Research. G.Q.D. is also the recipient of Clinical Scientist Awards in Translational Research from the Burroughs Wellcome Fund and the Leukemia and Lymphoma Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708951105/DCSupplemental.
References
- 1.Lensch MW, Daley GQ. Origins of mammalian hematopoiesis: in vivo paradigms and in vitro models. Curr Top Dev Biol. 2004;60:127–196. doi: 10.1016/S0070-2153(04)60005-6. [DOI] [PubMed] [Google Scholar]
- 2.Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 3.Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci USA. 2004;101:7641–7645. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian V, Meyer BI, Gruss P. Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell. 1995;83:641–653. doi: 10.1016/0092-8674(95)90104-3. [DOI] [PubMed] [Google Scholar]
- 5.van Nes J, de Graaff W, Lebrin F, Gerhard M, Beck F, Deschamps J. The Cdx4 mutation affects axial development and reveals an essential role of Cdx genes in the ontogenesis of the placental labyrinth in mice. Development. 2006;133:419–428. doi: 10.1242/dev.02216. [DOI] [PubMed] [Google Scholar]
- 6.Davidson AJ, et al. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- 7.Davidson AJ, Zon LI. The caudal-related homeobox genes cdx1a and cdx4 act redundantly to regulate hox gene expression and the formation of putative hematopoietic stem cells during zebrafish embryogenesis. Dev Biol. 2006;292:506–518. doi: 10.1016/j.ydbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst P, Mabon M, Davidson AJ, Zon LI, Korsmeyer SJ. An Mll-dependent Hox program drives hematopoietic progenitor expansion. Curr Biol. 2004;14:2063–2069. doi: 10.1016/j.cub.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Bansal D, et al. Cdx4 dysregulates Hox gene expression and generates acute myeloid leukemia alone and in cooperation with Meis1a in a murine model. Proc Natl Acad Sci USA. 2006;103:16924–16929. doi: 10.1073/pnas.0604579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawat VP, et al. Ectopic expression of the homeobox gene Cdx2 is the transforming event in a mouse model of t(12;13)(p13;q12) acute myeloid leukemia. Proc Natl Acad Sci USA. 2004;101:817–822. doi: 10.1073/pnas.0305555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 13.Mitjavila-Garcia MT, et al. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development. 2002;129:2003–2013. doi: 10.1242/dev.129.8.2003. [DOI] [PubMed] [Google Scholar]
- 14.Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy M, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 16.Godin I, Cumano A. The hare and the tortoise: an embryonic haematopoietic race. Nat Rev Immunol. 2002;2:593–604. doi: 10.1038/nri857. [DOI] [PubMed] [Google Scholar]
- 17.Neubauer H, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 18.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 19.Ross J, Li L. Recent advances in understanding extrinsic control of hematopoietic stem cell fate. Curr Opin Hematol. 2006;13:237–242. doi: 10.1097/01.moh.0000231420.92782.8f. [DOI] [PubMed] [Google Scholar]
- 20.van den Akker E, et al. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development. 2002;129:2181–2193. doi: 10.1242/dev.129.9.2181. [DOI] [PubMed] [Google Scholar]
- 21.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 22.Gamer LW, Wright CV. Murine Cdx-4 bears striking similarities to the Drosophila caudal gene in its homeodomain sequence and early expression pattern. Mech Dev. 1993;43:71–81. doi: 10.1016/0925-4773(93)90024-r. [DOI] [PubMed] [Google Scholar]
- 23.Gaunt SJ, Drage D, Trubshaw RC. cdx4/lacZ and cdx2/lacZ protein gradients formed by decay during gastrulation in the mouse. Int J Dev Biol. 2005;49:901–908. doi: 10.1387/ijdb.052021sg. [DOI] [PubMed] [Google Scholar]
- 24.Meyer BI, Gruss P. Mouse Cdx-1 expression during gastrulation. Development. 1993;117:191–203. doi: 10.1242/dev.117.1.191. [DOI] [PubMed] [Google Scholar]
- 25.Lohnes D. The Cdx1 homeodomain protein: an integrator of posterior signaling in the mouse. BioEssays. 2003;25:971–980. doi: 10.1002/bies.10340. [DOI] [PubMed] [Google Scholar]
- 26.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 27.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 28.Abramovich C, Humphries RK. Hox regulation of normal and leukemic hematopoietic stem cells. Curr Opin Hematol. 2005;12:210–216. doi: 10.1097/01.moh.0000160737.52349.aa. [DOI] [PubMed] [Google Scholar]
- 29.Charite J, et al. Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development. 1998;125:4349–4358. doi: 10.1242/dev.125.22.4349. [DOI] [PubMed] [Google Scholar]
- 30.Abbondanzo SJ, Gadi I, Stewart CL. Derivation of embryonic stem cell lines. Methods Enzymol. 1993;225:803–823. doi: 10.1016/0076-6879(93)25052-4. [DOI] [PubMed] [Google Scholar]
- 31.Zaehres H, Daley GQ. Transgene expression and RNA interference in embryonic stem cells. Methods Enzymol. 2006;420:49–64. doi: 10.1016/S0076-6879(06)20004-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.