Abstract
The p53 protein is one of the major tumor suppressor proteins. In response to DNA damage, p53 is prevented from degradation and accumulates to high levels. Ionizing radiation leads to hypophosphorylation of the p53 ubiquitin ligase Mdm2 at sites where phosphorylation is critical for p53 degradation and to the phosphorylation and activation of Akt/PKB, a kinase that phosphorylates and inhibits GSK-3. GSK-3, which normally phosphorylates Mdm2, is inactivated in response to ionizing radiation. We show that p53 accumulates in lymphoblasts from patients with the hereditary disorder ataxia telangiectasia in response to ionizing radiation despite the absence of a functional ATM kinase. Also, knockdown of ATR did not prevent p53 accumulation in response to ionizing radiation. Instead, p53 stabilization in response to ionizing radiation depended on the inactivation of GSK-3 and the presence of Akt/PKB. Akt/PKB is a target of DNA-PK, a kinase that is activated after ionizing radiation. Correspondingly, down-regulation of DNA-PK prevented phosphorylation of Akt/PKB and GSK-3 after ionizing radiation and strongly reduced the accumulation of p53. We therefore propose a signaling cascade for the regulation of p53 in response to ionizing radiation that involves activation of DNA-PK and Akt/PKB and inactivation of GSK-3 and Mdm2.
Keywords: ionizing radiation, Mdm2
Ionizing irradiation (IR) is a major inducer of DNA double-strand breaks (DSBs), a severe cellular lesion that leads to cell death, chromosomal rearrangements, and cancer (1). To maintain genomic stability despite recurring insults, defense mechanisms have evolved including cell cycle arrest, DNA repair, and apoptosis (1). DSBs are typically recognized by large phosphatidylinositol 3-kinase-like protein kinases such as ataxia telangiectasia mutated (ATM) and DNA-dependent protein kinase (DNA-PK), which signal to downstream effectors to implement the DNA damage response. Particularly at later time points after IR, a third kinase of the family, ATM and Rad3-related (ATR), may also contribute to the response.
One of the most critical components in the DNA damage response is the tumor suppressor protein p53, a protein with cell cycle arrest-inducing and proapoptotic functions. p53 is frequently mutated in human tumors, and mice that are null for p53 develop lymphomas and sarcomas (2). In resting cells, p53 protein levels are low because of the activity of several E3 ubiquitin ligases such as Mdm2, PirH2, and COP1 (3). In the presence of DNA lesions, p53 is released from this control and accumulates to high levels, which results in the transcription of p53 target genes (4). This increase in p53 abundance in response to DNA damage is thought to be brought about by several mechanisms (5). In response to IR, ATM appeared to be the major player. ATM phosphorylates Chk2 and p53 at serine-15. Activated Chk2 subsequently phosphorylates p53 at serine-20. In addition, ATM phosphorylates the p53 ubiquitin ligases Mdm2 and COP1. These various phosphorylations are thought to release p53 from its control (6–8).
In contrast to this model, replacement of serine-15, threonine-18, or all of the serines in the N terminus of p53 with an alanine did not alter p53 half-life or its accumulation in response to DNA damage (9–11). Moreover, p53 that accumulates after IR is still ubiquitylated (12). These discrepancies suggest the existence of alternative pathways.
To resolve this caveat we determined p53 accumulation in ATM-deficient cells and investigated the pathway that leads to p53 accumulation in response to IR. Here we show that Akt/PKB and DNA-PK are required for the stabilization of p53 in response to IR. GSK-3 and Akt/PKB are both phosphorylated in response to IR. However, whereas Akt/PKB is activated in response to IR, phosphorylation of GSK-3 corresponds to its inactivation. Down-regulation of Akt-2/PKBβ by siRNA prevented phosphorylation of GSK-3 and strongly reduced the accumulation of p53 after IR. IR predominantly activated Akt/PKB in the nuclear cell compartment in a DNA-PK-dependent manner. Consistently, down-regulation of DNA-PK by siRNA prevented phosphorylation and inactivation of GSK-3 and reduced p53 accumulation in response to IR.
Results and Discussion
p53 Accumulates After IR in Cells with Mutated ATM.
We studied the accumulation of p53 in response to IR in lymphoblastoid cell lines derived from three different patients with ataxia telangiectasia (AT) and compared it with the accumulation of p53 in wild-type cells. All three AT cell lines that we used expressed negligible amounts of the ATM kinase whereas ATM was clearly detectable in cells from a nonaffected donor or from a patient with Nijmegen breakage syndrome (Fig. 1A), a genetic disease that is caused by a mutation in the nibrin 1 gene (13). When we irradiated AT lymphoblasts, p53 accumulated in all AT cell lines [Fig. 1B and supporting information (SI) Fig. S1 A and B]. The accumulation of p53 was already detectable after irradiation with doses as low as 0.5 Gy in cells from AT patients and in cells from an unaffected donor and was further increased in a dose-dependent manner (Fig. 1C). In consistency with previous reports, cells from AT patients were almost completely devoid of radiation-induced phosphorylation of serine-15 and serine-20 of p53 (Fig. 1D and Fig. S1 A and C). This is particularly evident from those experiments, where we treated the cells with the proteasome inhibitor MG132, which enabled us to determine p53 phosphorylation after irradiation whereas p53 abundance was not changed. Even at 4 h after irradiation, phosphorylation of p53 at serine-15 was markedly reduced in AT cells compared with wild-type cells, and phosphorylation of serine-20 was hardly detectable even after long exposure times (Fig. S1 A and C). Likewise, treatment of human osteocarcinoma cells with an ATM inhibitor prevented phosphorylation of p53 at serine-15 but allowed p53 accumulation to a comparable extent as in mock-treated cells (Fig. 1E). Similarly, down-regulation of ATM by siRNA significantly reduced p53 phosphorylation but did not reduce p53 accumulation (Fig. 2). Down-regulation of ATR, a kinase of the same family of kinases, by siRNA did not affect phosphorylation of p53 or accumulation of p53 at all. Likewise, down-regulation of both ATM and ATR did not reduce p53 accumulation. Interestingly, down-regulation of both kinases did not reduce phosphorylation of p53 or p53 accumulation any further than down-regulation of ATM alone (Fig. 2 and Fig. S2). Down-regulation of ATM, ATR, or both also did not affect phosphorylation of GSK-3 or Akt/PKB (Fig. S2). These results demonstrate that ATM and phosphorylation of p53 at serine-15 and serine-20 are negligible for p53 accumulation. This result is, however, in contrast to a previous report by Kastan et al. (14). The discrepancy between the two observations is most likely due to the different cell types that were used and their varying response to ATM mutations. Whereas Kastan et al. (14) investigated primary fibroblasts, which grow poorly in the absence of ATM activity, we used lymphoid cells where mutations in the atm gene do not affect cell growth. p53 induction in response to IR, however, depends on cell proliferation (15). The fact that down-regulation of ATM and ATR did not reduce p53 phosphorylation further than down-regulation of ATM alone suggests that ATR is most likely not the kinase that mediates the residual phosphorylation of p53 on serine-15 in the absence of ATM (Fig. 1D, Fig. 2, and Fig. S1). In summary, our data demonstrate that an ATM-independent pathway exists for the control of p53 levels in response to IR. Nevertheless, because we have not investigated carefully induction of p53 in wild-type cells at time points earlier than 1 h after irradiation in the AT cell lines, we cannot exclude the possibility that ATM contributes to p53 induction at very early time points.
Fig. 1.
p53 accumulates in AT cells in response to IR. (A) Lymphoblasts from AT patients (719; 1526; and 3189), from a patient with Nijmegen breakage syndrome (NBS), and from an unaffected control were probed for ATM and for proliferating cell nuclear antigen (PCNA) for loading control. (B) Lymphoblasts from AT patients and an unaffected control were irradiated, harvested at the indicated time points, and probed for p53 and for PCNA for loading control. (C) Lymphoblasts from AT patients and an unaffected control were irradiated with the indicated doses, harvested 4 h after irradiation, and probed for p53 and PCNA. (D) Lymphoblasts from AT patients and an unaffected control were incubated in the presence of 10 μM MG132 for 4 h. Fifteen, 30, or 60 min before harvest, cells were irradiated, lysed, and probed for p53 phosphorylated at serine-15, serine-20, pan-p53, and PCNA. (E) Wild-type lymphoblasts were incubated with 10 μM MG132 for 5 h or left untreated for control. Thirty minutes before irradiation an ATM kinase inhibitor (10 μM) was added. Cells were lysed 1.5 h after irradiation and probed for p53 and PCNA. Lysates from cells that had been incubated with MG132 were also probed for p53 phosphorylated at serine-15.
Fig. 2.
Down-regulation of ATM and/or ATR does not prevent the accumulation of p53 in response to IR. U2OS cells were transfected with siRNA directed against ATM, ATR, ATM and ATR, or a nonspecific control siRNA. Ninety-six hours after transfection, cells were irradiated. Cells were harvested 4 h after irradiation. ATM, p53, and PCNA expression was determined by Western blotting. A second membrane was hybridized with antibodies directed against ATR, p53 phosphorylated at serine-15, and PCNA. Signals for p53 and PCNA were quantified. The graph shows mean values and standard deviations of three independent experiments. The relative amount of p53 in unirradiated cells was set to 1.
Stabilization of p53 in Response to IR Is Regulated by Glycogen Synthase Kinase 3.
To identify the signaling cascade that leads to p53 accumulation after IR, we followed the observation that Mdm2 is dephosphorylated after IR at several consecutive phosphorylation sites where phosphorylation is vital for p53 degradation (16). Some of these sites, respectively, serine-240 and serine-254, are phosphorylated by glycogen synthase kinase-3β (GSK-3β), a serine/threonine kinase that was initially isolated as a kinase for glycogen synthase (18, 19). Mammalian cells possess two GSK-3 isoforms, GSK-3α and GSK-3β, which are constitutively active but become inactivated by phosphorylation of serine-9 of GSK-3β and serine-21 of GSK-3α (19). For full activity, GSK-3 usually requires phosphorylation of a priming site 3 aa downstream (S/T-XXX-S/T-P; amino acids in bold are phosphorylated by GSK-3). In response to IR, GSK-3β is phosphorylated at serine-9, which leads to its inactivation (Fig. 3B) (17, 20). This phosphorylation of GSK-3β is independent of ATM because it occurs in wild-type and AT cells to the same extent (Fig. 3C). Inhibition of GSK-3β, e.g., with the specific GSK-3 inhibitor alsterpaullone, increased p53 abundance (Fig. 3A) (17), indicating that GSK-3 activity is required for p53 turnover. In consistency with this principle, overexpression of a constitutively active mutant of GSK-3β where serine-9 had been replaced with an alanine, and which is therefore refractory to radiation-mediated inhibition, significantly reduced the accumulation of p53 in response to IR (Fig. 3D) (17). Moreover, in the presence of an Mdm2 mutant, in which one or both of the GSK-3 phosphorylation sites has been replaced with an aspartic acid and thus resembles phosphorylated Mdm2, p53 accumulation after IR was significantly reduced (Fig. 3E). It should also be mentioned that the S254D mutant was incapable of targeting p53 for degradation and the S240D/S254D was less competent in reducing p53 levels than wild-type Mdm2, indicating that not only the charge but also the amino acid backbone is important for Mdm2 function. Likewise, when we replaced one of the GSK-3 phosphorylation sites together with another phosphorylated site of Mdm2 (16) or with one of the GSK-3 priming sites (Fig. S3), with an aspartic acid accumulation of p53 after IR was similarly reduced, indicating that hypophosphorylation of the central domain of Mdm2 is crucial for the accumulation of p53 after IR (16).
Fig. 3.
Inactivation of GSK-3β contributes to the accumulation of p53 in response to IR. (A) U2OS cells were incubated with 10 μM alsterpaullone for the indicated time. p53 and PCNA levels were determined by Western blotting. (B) Wild-type lymphoblasts were irradiated and harvested after the indicated time. Phosphorylated GSK-3β, total GSK-3β, and PCNA were determined by Western blotting. (C) Lymphoblasts from AT patients and unaffected controls were irradiated and harvested after the indicated times. Phosphorylated GSK-3β and PCNA were determined by Western blotting. (D) U2OS cells were transfected with Flag-tagged wild-type GSK-3β, with Flag-tagged GSK-3β-S9A, or with vector for control, selected for 6 days with neomycin, irradiated, and harvested 4 h after irradiation. p53, phosphorylated GSK-3, Flag-tagged GSK-3β, and PCNA expression was determined by Western blotting. (E) H1299 cells were transfected with 0.2 μg of a plasmid expressing p53 together with 1 μg of a plasmid expressing wild-type Mdm2 or mutant Mdm2 where serine-240, serine-254, or both were replaced with an aspartic acid or with a vector DNA for control. Forty-eight hours after transfection, cells were irradiated with 7.5 Gy. Cells were harvested 4 h after irradiation. Hdm/Mdm2, p53, and PCNA levels were determined by Western blotting. Signals for p53 and PCNA were quantified. The graph shows mean values and standard deviations of three independent experiments. The relative amount of p53 in unirradiated cells was set to 100%.
Accumulation of p53 in Response to IR Requires Akt2/PKBβ and DNA-PK.
One of the kinases that directly phosphorylate GSK-3 is AKT/PKB. Native Akt/PKB is inactive, but the kinase can be rapidly activated by phosphatidylinositol 3-kinases, which leads to phosphorylation of Akt/PKB at threonine-308 and serine-473 (21). For full activity, phosphorylation of both sites (threonine-308 and serine-473) is required. As reported previously, both sites, threonine-308 and serine-473, are phosphorylated in response to IR both in the cytoplasm and in the nucleus (Fig. 4A) (22, 23). This phosphorylation of Akt/PKB is independent of ATM because Akt/PKB was also phosphorylated in cells from AT patients (Fig. 4B). Although the phosphorylation of Akt/PKB after IR and the fact that GSK-3 is a target pointed toward a contribution of Akt/PKB in the signaling chain leading to GSK-3 phosphorylation, we were puzzled by the fact that Akt/PKB is known as a primarily cytoplasmic kinase. DNA lesions and accumulation of p53 occur, though, in the nuclear compartment of the cell. To resolve this caveat, we prepared cytoplasmic and nuclear lysates and determined the amount and phosphorylation of Akt/PKB and GSK-3β in cytoplasmic and nuclear fractions. Notably, a significant part of Akt/PKB was localized in the nucleus, although cytoplasmic Akt/PKB protein levels were considerably higher (Fig. 4A). Most interestingly, nuclear Akt/PKB was phosphorylated at serine-473 at much earlier time points after IR than the cytoplasmic protein and preceded GSK-3β phosphorylation. Moreover, the overall increase in phosphorylation was significantly higher for nuclear Akt/PKB. Correspondingly, phosphorylation of GSK-3β was clearly stronger in the nuclear compartment than in the cytoplasm. Cytoplasmic Akt/PKB was also phosphorylated at threonine-308 in response to IR, although phosphorylation of threonine-308 was always weaker than phosphorylation of serine-473 and usually did not exceed a factor of 2. However, we failed to detect a significant increase in phosphorylation of threonine-308 in the nucleus (Fig. 4A). To monitor that nuclear Akt/PKB is activated after IR, we determined the capacity of nuclear and cytoplasmic Akt/PKB to phosphorylate bacterially expressed and purified GST-GSK-3β. Surprisingly, cytoplasmic Akt/PKB was hardly activated in response to IR whereas nuclear Akt/PKB phosphorylated GSK-3β very efficiently (Fig. 4C). The observation that nuclear Akt/PKB is activated upon IR almost exclusively suggests that all active Akt/PKB translocated into the nucleus or, even more likely, that activation of Akt/PKB after IR occurred within the nuclear compartment. In this context, phosphorylation of Mdm2 at serine-166 and serine-186 by Akt/PKB, which leads to nuclear translocation of Mdm2, can be disregarded (24). In consistency with the kinase assay, GSK-3β was predominantly phosphorylated in the nucleus, although phosphorylation of cytoplasmic GSK-3β was still detectable (Fig. 4C).
Fig. 4.
Akt/PKB phosphorylates GSK-3β in response to IR. (A) Wild-type lymphoblasts were irradiated, harvested at the indicated times, and separated into cytoplasmic and nuclear fractions. Phosphorylation of Akt/PKB at threonine-308 and serine-473, total Akt/PKB, phosphorylation of GSK-3β at serine-9, total GSK-3β, and PCNA were determined by Western blotting. (B) Lymphoblasts from AT patients and unaffected controls were irradiated and harvested after the indicated times. Phosphorylated Akt, total Akt, and PCNA were determined by Western blotting. (C) Wild-type lymphoblasts were irradiated and harvested after 4 h. Where indicated, cells were fractionated into nuclei and cytoplasm. (I) Akt/PKB was immunoprecipitated from 600 μg of lysates and incubated with 1.5 μg of GST-GSK-3β and [γ-32P]ATP. For control, total cell lysate of irradiated cells was incubated with IgG coupled to protein A Agarose (IgG) and processed as described. Samples were separated by electrophoresis and blotted. The membrane was exposed onto an x-ray film (32P-GSK-3β) before hybridization with antibodies directed against Akt/PKB and GST. (II) Lysates were separated by SDS/PAGE, blotted, and processed as described in the legend to A. Signals for phosphorylated Akt/PKB, total Akt/PKB, phosphorylated GSK-3β, and total GSK-3β were quantified, and the relative increase in phosphorylation after IR was calculated. Relative phosphorylation of Akt/PKB and GSK-3β of nonirradiated cells were set to 1. (D) U2OS cells were transfected with siRNA directed against Akt-1/PKBα, Akt-2/PKBβ, or a nonspecific control siRNA or left untransfected for control. Seventy-two hours after transfection, cells were irradiated. Cell were harvested 4 h after irradiation and probed for Akt/PKB (directed specifically against Akt-2/PKBβ in the second part of the figure), phosphorylated GSK-3β, total GSK-3β, and PCNA. A second membrane was probed for p53 and PCNA. Signals for phosphorylated GSK-3β and total GSK-3β were quantified. Relative values for GSK-3β phosphorylation of three independent experiments and standard deviations were plotted. Relative values for GSK-3β phosphorylation of unirradiated cells were set to 1.
Akt/PKB represents a family of three serine/threonine kinases (Akt-1/PKBα, Akt-2/PKBβ, and Akt-3/PKBγ). Two of them, Akt-1/PKBα and Akt-2/PKBβ, are widely expressed in human tissues (25). To determine which of the Akt/PKB isoforms is responsible for GSK-3β phosphorylation after IR, we down-regulated both isoforms individually by siRNA. Transfection of U2OS cells with siRNA against Akt-1/PKBα or Akt-2/PKBβ efficiently down-regulated the respective Akt/PKB isoform (Fig. S4). However, whereas down-regulation of Akt-1/PKBα did not affect phosphorylation of GSK-3β, down-regulation of Akt-2/PKBβ almost completely blocked phosphorylation of GSK-3β in response to IR (Fig. 4D). Most importantly, down-regulation of Akt-2/PKBβ also strongly reduced the accumulation of p53 in response to IR whereas down-regulation of Akt-1/PKBα only slightly reduced p53 stabilization, which is probably because of some cross-reactivity of the Akt-1/PKBα siRNA with the Akt-2/PKBβ transcript (Fig. 4D and Fig. S4). Although phosphorylation of Akt/PKB at serine-473 correlated with phosphorylation of GSK-3 and stabilization of p53, these data do not exclude the possibility that Akt/PKB might be modified at other, as yet unknown sites after IR, which might be even more important for the stabilization of p53. Such a possibility would be consistent with the very strong activation of Akt/PKB in the nucleus after IR and the sometimes rather weak phosphorylation of serine-473. Nevertheless, these data strongly support the requirement of Akt-2/PKBβ for p53 stabilization in response to IR.
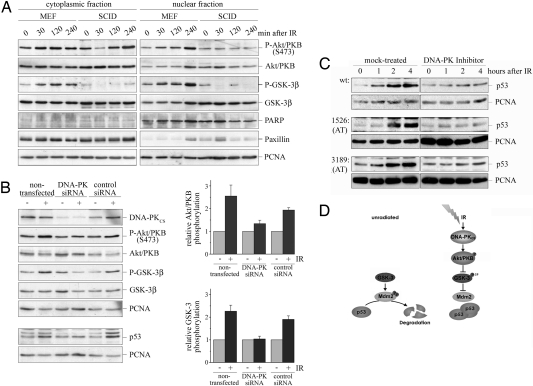
Because one of the kinases that phosphorylates Akt/PKB at serine-473 is DNA-PK, a serine/threonine kinase that is specifically activated by DNA DSBs (26), we determined Akt/PKB phosphorylation in embryonal fibroblasts from mice with severe combined immunodeficiency syndrome (SCID). SCID cells have been reported to lack DNA-PK function (27). In consistency with previous reports (28), fibroblasts from SCID mice showed no increase in Akt/PKB phosphorylation at serine-473 after IR. Correspondingly, phosphorylation of GSK-3β at serine-9 after IR was undetectable (Fig. 5A). Likewise, down-regulation of DNA-PK by siRNA prevented radiation-induced phosphorylation of Akt/PKB at serine-473 and phosphorylation of GSK-3β (Fig. 5B). Most importantly, accumulation of p53 in response to IR was significantly reduced when DNA-PK was down-regulated (Fig. 5B), while down-regulation of the p110 alpha subunit of PI3-kinase had no effect (Fig. S5). Likewise, when a DNA-PK inhibitor was applied to the cells before irradiation, p53 accumulation was significantly reduced. (Fig. 5C).
Fig. 5.
Activation of Akt/PKB in response to IR requires DNA-PK. (A) Embryonal mouse fibroblasts (MEF) from healthy donors and from mice with SCID were irradiated, harvested at the indicated time points, fractionated into nuclei and cytoplasm, and probed for phosphorylated Akt/PKB (serine-473), total Akt/PKB, phosphorylated GSK-3β, total GSK-3β, for PARP and paxillin to monitor the quality of fractionation, and for PCNA. (B) U2OS cells were transfected with siRNA directed against the catalytic subunit of DNA-PK, a nonspecific control siRNA, or left untransfected for control. Ninety-six hours after transfection, cells were irradiated. Cells were harvested 4 h after irradiation. DNA-PK, phosphorylated Akt/PKB (serine-473), total Akt/PKB, phosphorylated GSK-3β, total GSK-3β, and PCNA were determined by Western blotting. Signals were quantified, and the relative amount of phosphorylated Akt/PKB and phosphorylated GSK-3β was calculated. Mean values and standard deviation of three independent experiments were plotted. The relative amount of phosphorylated Akt/PKB and GSK-3β was set to 1. A second membrane was hybridized with the anti-p53 antibody Ab-2 and an antibody directed against PCNA. (C) Lymphoblasts from AT patients and unaffected controls were incubated with 2 μM of an inhibitor of DNA-PK, irradiated, and harvested after the indicated time. p53 and PCNA levels were determined by Western blotting. (D) Model for the pathway for p53 control in response to IR. Activation of DNA-PK leads to the phosphorylation and activation of Akt/PKB and subsequently to the inactivation of GSK-3β. In consequence, Mdm2 is hypophosphorylated and incapable of mediating p53 degradation.
Because p53 levels were only reduced after IR in the presence of the DNA-PK inhibitor or after down-regulation of DNA-PK, but not completely prevented, we suspect that the inhibition of DNA-PK was incomplete or that another pathway exists that contributes to the control of p53 stability in response to IR. The latter possibility would be in line with previous observations that several mechanisms control p53 stability in response to DNA damage (5). Our result that DNA-PK participated in the stabilization of p53, though, disagrees with several previous investigations including our own (10, 29, 30). This discrepancy may be attributable to the fact that investigations of the p53 response of SCID cells are restricted to mouse cells, which may differ from human cells in their response. Alternatively, SCID cells may have recruited an alternative pathway that took over some of the functions of DNA-PK, which is absent upon transient down-regulation of DNA-PK by siRNA or inhibition of the kinase by a synthetic compound. It is also possible that the SCID mutation may not completely shut off the kinase activity in vivo (31, 32).
With this work we demonstrate the existence of an ATM-independent pathway for the accumulation of p53 in response to IR that is initiated by DNA-PK and which involves activation of Akt-2/PKBβ and inactivation of GSK-3β (Fig. 5D). Whether this pathway also affects degradation of p53 by other E3 ligases such as COP-1 or Pirh2 remains to be determined.
Materials and Methods
Cell Lines and Their Treatments.
GM02184 (wild type), GM0719 (AT), GM1526 (AT), GM3189 (AT), and GM15808 (NBS) lymphoblasts were purchased from Coriell Cell Repositories and cultured in RPMI medium 1640 with 15% heat-inactivated FCS and 100 units/ml penicillin/streptomycin. U2OS and H1299 cells were provided by G. Taucher-Scholz (Gesellschaft für Schwerionenforschung, Darmstadt, Germany). Mouse embryonal fibroblasts and SCID cells (33) were provided by B. Kaina (Mainz University, Mainz, Germany) and cultured in DMEM supplemented with 10% FCS and 100 units/ml penicillin/streptomycin. All cell lines were kept at 37°C and 5% CO2 in a humidified atmosphere. For some experiments, cells were incubated in DMEM supplemented with 0.5% FCS and 100 units/ml penicillin/streptomycin overnight before irradiation.
The ATM inhibitor 2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one (Calbiochem), alsterpaullone, and MG132 were used at a final concentration of 10 μM, and the DNA-PK inhibitor V (Calbiochem) was used at a final concentration of 2 μM. Neomycin was added at a concentration of 300 μg/ml. Cells were irradiated with 10 Gy (if not otherwise indicated) in culture medium using a cobalt-60 γ-source at a dose rate of 1.5 Gy/min.
Plasmids.
The plasmid pGEX-4T-2-WT-GSK-3β (amino acids 1–100) was created by PCR amplification of the N-terminal fragment of GSK-3β and ligated into pGEX-4T-2. Plasmids for p53 and Mdm2 were described earlier (16). The Mdm2 mutants S240D, S251D, S254D, S240D/S251D, S254D/S258D, and S240D/S254D were created by site-directed mutagenesis.
siRNA.
siRNAs were transfected by using LipofectamineTM2000 (Invitrogen) according to the manufacturer's recommendations. siRNAs against ATM and ATR were used at 60 nM, siRNA against Akt-2/PKBβ was used at a final concentration of 30 nM, and siRNAs against Akt-1/PKBα and DNA-PK were used at a final concentration of 75 nM. Cells were harvested at 3 days (ATM, ATR, Akt-1/PKBα, and Akt-2/PKBβ) and 4 days (DNA-PK) after transfection if not otherwise indicated. Sequences of siRNAs are available on request.
Cell Lysis, SDS/PAGE, and Western Blotting.
SDS/PAGE and Western blotting were performed as described (16). For some experiments, the lysis buffer was supplemented with the phosphatase inhibitor PhosSTOP (Roche) according to the manufacturer's recommendation. The following antibodies were used: Ab-2 (anti-p53; Oncogene Science), 15G8 (anti-phospho-serine-15/p53; Cell Signaling Technology), anti-phospho-serine-20/p53 (Cell Signaling Technology), clone 7 (anti-GSK-3β; BD Transduction Laboratories), 244F9 and 193H12 (anti-phospho-threonine-308/Akt and anti-phospho-serine-473/Akt; Cell Signaling Techmology), anti Akt/PKB and anti-Akt-2/PKBβ (Cell Signaling Technology), M2 (anti-Flag; Sigma), 4B2 (anti-Mdm2; Calbiochem), PC10 (anti-PCNA; Santa Cruz Biotechnology), anti-GST antibody (Rockland), anti-PARP (Roche), G-4 (anti-DNA-PK; Santa Cruz Biotechnology), anti-ATR (N-19; Santa Cruz Biotechnology), anti-phosphatidylinositol 3-kinase p110α (Cell Signaling Technology), and anti-paxillin (BD Biosciences). The ATM antibody MAT3 was kindly provided by Yossi Shiloh (Tel Aviv University, Tel Aviv).
For cell fractionation, cells were resuspended in lysis buffer (25 mM HEPES, pH 7.9/1.5 mM EDTA, pH 8.0/50 mM NaCl/0.5% Nonidet P-40) and incubated for 3 min on ice. Pre-B lymphoblasts were incubated for 2 min on ice. After centrifugation at 4,500 × g at 4°C for 5 min, the cytoplasmic fraction was transferred to a fresh tube. The nuclei were washed with 25 mM HEPES (pH 7.9)/1.5 mM EDTA (pH 8.0)/50 mM NaCl, resuspended in ice-cold lysis buffer, and lysed by mild sonification. Insoluble material was separated from the nucleic fraction by centrifugation at 16,000 × g for 10 min at 4°C.
Membranes were stripped at 50°C for 40 min in 62.5 mM Tris·HCl (pH 6.8), 2% SDS, and 50 mM DTT by constant shaking and washed four times in PBS/0.2% Tween.
Immunoprecipitation.
Cells were lysed in Nonidet P-40 buffer for 20 min. The protein extract was cleared by centrifugation at 16,000 × g at 4°C for 15 min, and the protein concentration was determined by the method of Bradford. A total of 0.5 μl of an antibody against Akt/PKB, precoupled to protein A-Agarose (Pierce), were added to 600 μg of the lysate and incubated for 1.5 h. The protein/antibody/Agarose complexes were washed three times with Nonidet P-40 lysis buffer and used as a kinase source for kinase assays.
Kinase Assay.
A total of 1.5 μg of a bacterially expressed GST-GSK-3β fusion protein was incubated with Akt/PKB fixed on Sepharose beads in 7 mM Mops, pH 7.3/20 mM MgCl2/0.2 mM EDTA/1 mM DTT/10 μM ATP/250 μCi/ml [γ-32P]ATP for 30 min at 30°C.
Supplementary Material
Acknowledgments.
We thank S. Jackson, Y. Shiloh, and M. Oren for critical reading of the manuscript and Y. Shiloh for providing us with antibodies. This work was supported by Grant 02S8223 from the Bundesministerium für Bildung und Forschung.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703423105/DCSupplemental.
References
- 1.Gasser S, Raulet D. The DNA damage response, immunity and cancer. Semin Cancer Biol. 2006;5:344–347. doi: 10.1016/j.semcancer.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Donehower LA, et al. Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev. 1995;9:882–895. doi: 10.1101/gad.9.7.882. [DOI] [PubMed] [Google Scholar]
- 3.Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–315. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine AJ, Hu W, Feng Z. The p53 pathway: What questions remain to be explored? Cell Death Differ. 2006;13:1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 5.Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006;6:941–950. doi: 10.1038/sj.cdd.4401925. [DOI] [PubMed] [Google Scholar]
- 6.Dornan D, et al. ATM engages autodegradation of the E3 ubiquitin ligase COP1 after DNA damage. Science. 2006;313:1122–1126. doi: 10.1126/science.1127335. [DOI] [PubMed] [Google Scholar]
- 7.Niida H, Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis. 2006;21:3–9. doi: 10.1093/mutage/gei063. [DOI] [PubMed] [Google Scholar]
- 8.Bode AM, Dong Z. Post-translational modifications of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 9.Hengstermann A, Whitaker NJ, Zimmer D, Zentgraf H, Scheffner M. Characterization of sequence elements involved in p53 stability regulation reveals cell type dependence for p53 degradation. Oncogene. 1998;17:2933–2941. doi: 10.1038/sj.onc.1202282. [DOI] [PubMed] [Google Scholar]
- 10.Blattner C, Tobiasch E, Litfin M, Rahmsdorf HP, Herrlich P. DNA damage induced p53 stabilization: No indication for an involvement of p53 phosphorylation. Oncogene. 1999;18:1723–1732. doi: 10.1038/sj.onc.1202480. [DOI] [PubMed] [Google Scholar]
- 11.Ashcroft M, Taya Y, Vousden K. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol. 2000;20:3224–3233. doi: 10.1128/mcb.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maki CG, Howley PM. Ubiquitination of p53 and p21 is differentially affected by ionizing and UV radiation. Mol Cell Biol. 1997;17:355–363. doi: 10.1128/mcb.17.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhou J, Lim CU. The role of NBS1 in DNA double strand break repair, telomere stability, and cell cycle checkpoint control. Cell Res. 2006;1:45–54. doi: 10.1038/sj.cr.7310007. [DOI] [PubMed] [Google Scholar]
- 14.Kastan MB, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 15.Midgley CA, et al. Coupling between gamma irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. J Cell Sci. 1995;108:1843–1848. doi: 10.1242/jcs.108.5.1843. [DOI] [PubMed] [Google Scholar]
- 16.Blattner C, Hay T, Meek DW, Lane DP. Hypophosphorylation of Mdm2 augments p53 stability. Mol Cell Biol. 2002;22:6170–6182. doi: 10.1128/MCB.22.17.6170-6182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulikov R, Boehme KA, Blattner C. Glycogen synthase kinase 3-dependent phosphorylation of Mdm2 regulates p53 abundance. Mol Cell Biol. 2005;25:7170–7180. doi: 10.1128/MCB.25.16.7170-7180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 19.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turenne GA, Price BD. Glycogen synthase kinase3 beta phosphorylates serine 33 of p53 and activates p53's transcriptional activity. BMC Cell Biol. 2001;2:12. doi: 10.1186/1471-2121-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 22.Contessa JN, et al. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cells. Oncogene. 2002;21:4032–4041. doi: 10.1038/sj.onc.1205500. [DOI] [PubMed] [Google Scholar]
- 23.Edwards E, et al. Phosphatidylinositol 3-kinase/Akt signaling in the response of vascular endothelium to ionizing radiation. Cancer Res. 2002;62:4671–4677. [PubMed] [Google Scholar]
- 24.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang ZZ, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 26.Lees-Miller SP, Chen YR, Anderson CW. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol. 1990;10:6472–6481. doi: 10.1128/mcb.10.12.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beamish HJ, et al. The C-terminal conserved domain of DNA-PKcs, missing in the SCID mouse, is required for kinase activity. Nucleic Acids Res. 2000;28:1506–1513. doi: 10.1093/nar/28.7.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 29.Fried LM, et al. The DNA damage response in DNA-dependent protein kinase-deficient SCID mouse cells: Replication protein A hyperphosphorylation and p53 induction. Proc Natl Acad Sci USA. 1996;93:13825–13830. doi: 10.1073/pnas.93.24.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurley KE, Kemp CJ. p53 induction, cell cycle checkpoints, and apoptosis in DNAPK-deficient scid mice. Carcinogenesis. 1996;17:2537–2542. doi: 10.1093/carcin/17.12.2537. [DOI] [PubMed] [Google Scholar]
- 31.Burma S, et al. DNA-dependent protein kinase-independent activation of p53 in response to DNA damage. J Biol Chem. 1999;274:17139–17143. doi: 10.1074/jbc.274.24.17139. [DOI] [PubMed] [Google Scholar]
- 32.Rathmell WK, Kaufmann WK, Hurt JC, Byrd LL, Chu G. DNA-dependent protein kinase is not required for accumulation of p53 or cell cycle arrest after DNA damage. Cancer Res. 1997;57:68–74. [PubMed] [Google Scholar]
- 33.Biedermann KA, Sun JR, Giacca AJ, Tosto LM, Brown JM. Acid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci USA. 1991;88:1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.