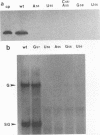
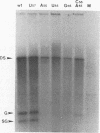
Abstract
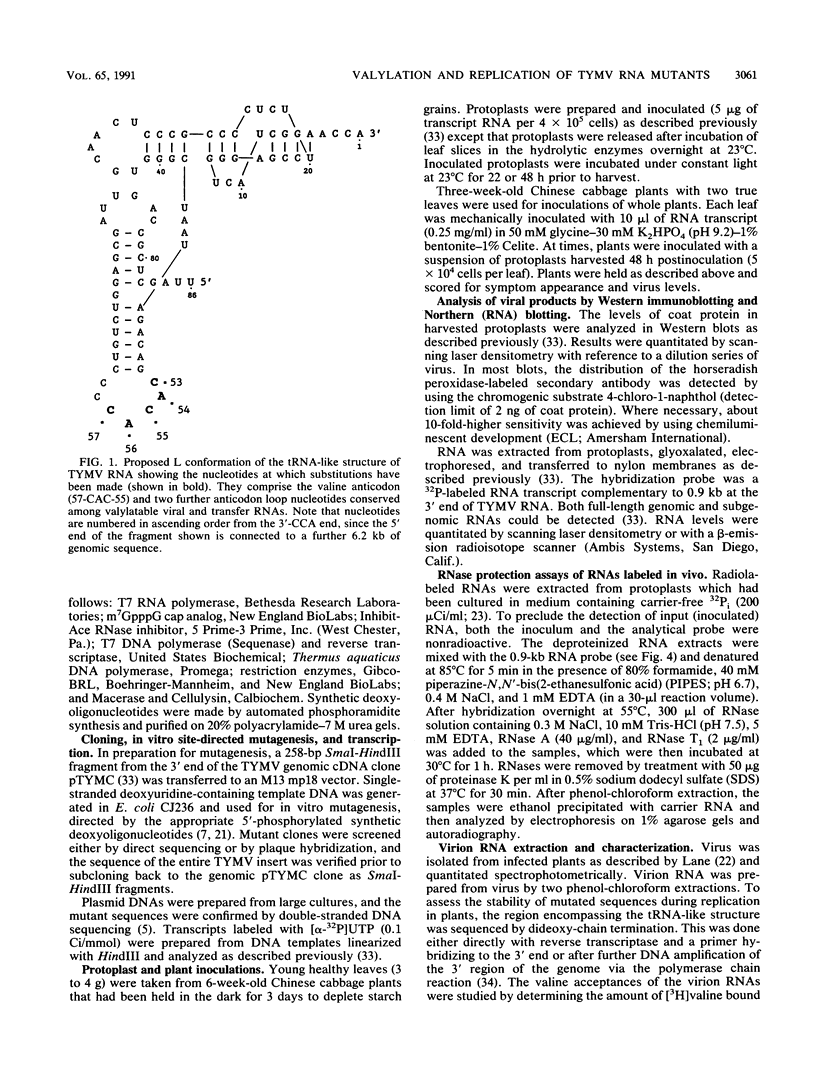
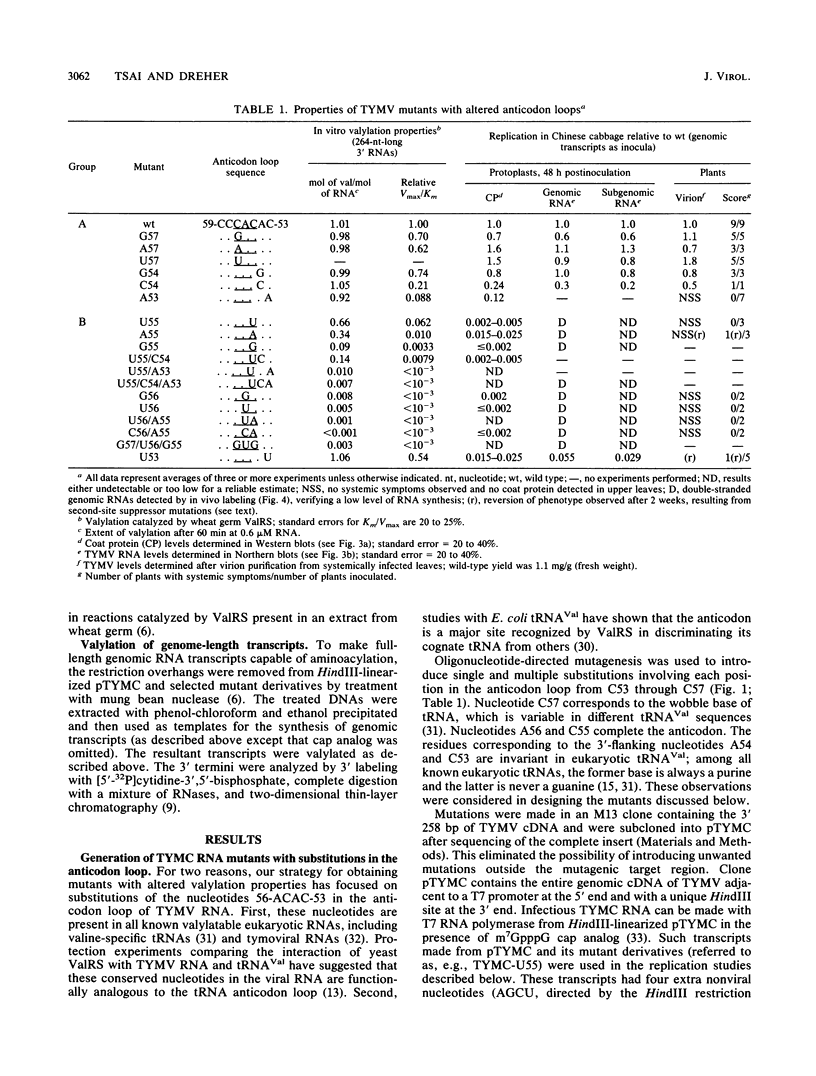
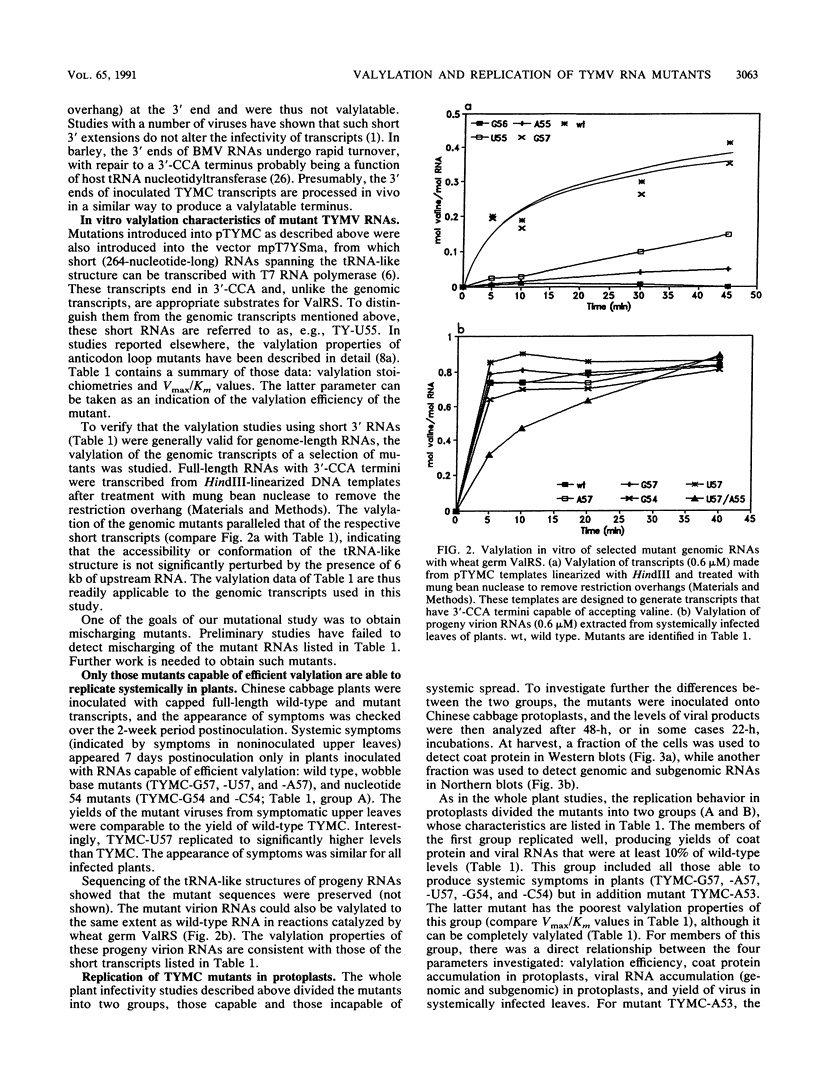
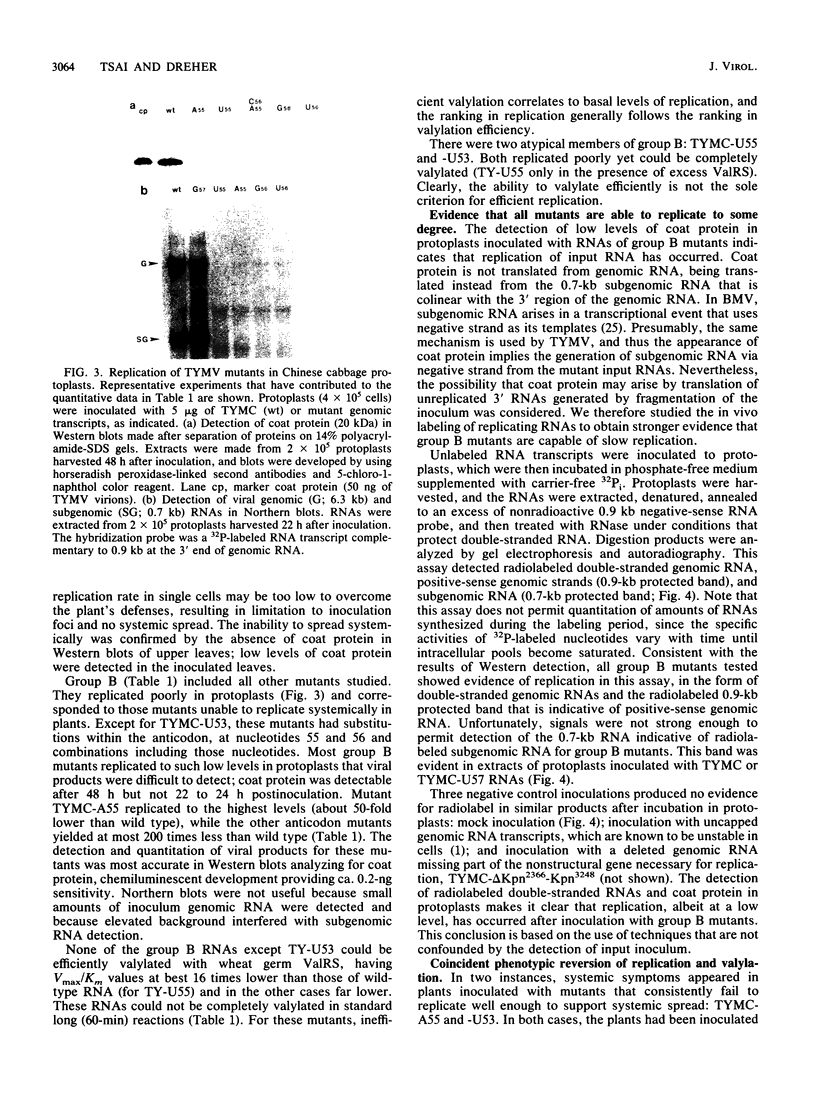
Single and multiple nucleotide substitutions have been introduced into the anticodon loop of the tRNA-like structure of turnip yellow mosaic virus (TYMV) genomic RNA. We studied the effects of these mutations on in vitro valylation and on replication in Chinese cabbage protoplasts and plants. Only those mutants capable of efficient and complete valylation showed efficient replication in protoplasts and gave rise to systemic symptoms in whole plants. Mutants that accepted valine inefficiently (in some cases Vmax/Km values were less than 10(-3) relative to wild-type values) replicated to levels 200- to 500-fold below wild-type levels in protoplasts (estimated on the basis of coat protein and genomic RNA levels). These mutants could not support systemic spread in plants. In one plant inoculated with TYMC-A55 RNA, which replicates poorly in protoplasts, systemic symptoms developed after a delay. The reversion in replication was accompanied by improved valine acceptance and the appearance of a U57 second-site mutation. Our results indicate a correlation between valine acceptance activity and viral yield. Possible roles for valylation are discussed, and the results are compared with those of similar studies with brome mosaic virus which suggested that tyrosylation is not crucial for brome mosaic virus replication (T. W. Dreher, A. L. N. Rao, and T. C. Hall, J. Mol. Biol. 206:425-438, 1989).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., French R., Bujarski J. J. Molecular studies of brome mosaic virus using infectious transcripts from cloned cDNA. Adv Virus Res. 1987;32:215–242. doi: 10.1016/s0065-3527(08)60478-9. [DOI] [PubMed] [Google Scholar]
- Arraiano C. M., Yancey S. D., Kushner S. R. Stabilization of discrete mRNA breakdown products in ams pnp rnb multiple mutants of Escherichia coli K-12. J Bacteriol. 1988 Oct;170(10):4625–4633. doi: 10.1128/jb.170.10.4625-4633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Jonard G., Guilley H., Richards K., Hirth L. Nucleotide sequence (n=159) of the amino-acid-accepting 3'-OH extremity of turnip-yellow-mosaic-virus RNA and the last portion of its coat-protein cistron. Eur J Biochem. 1977 Feb;72(3):453–463. doi: 10.1111/j.1432-1033.1977.tb11269.x. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Florentz C., Giege R. Valylation of tRNA-like transcripts from cloned cDNA of turnip yellow mosaic virus RNA demonstrate that the L-shaped region at the 3' end of the viral RNA is not sufficient for optimal aminoacylation. Biochimie. 1988 Dec;70(12):1719–1727. doi: 10.1016/0300-9084(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Hall T. C. Mutational analysis of the sequence and structural requirements in brome mosaic virus RNA for minus strand promoter activity. J Mol Biol. 1988 May 5;201(1):31–40. doi: 10.1016/0022-2836(88)90436-6. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Rao A. L., Hall T. C. Replication in vivo of mutant brome mosaic virus RNAs defective in aminoacylation. J Mol Biol. 1989 Apr 5;206(3):425–438. doi: 10.1016/0022-2836(89)90491-9. [DOI] [PubMed] [Google Scholar]
- Dumas P., Moras D., Florentz C., Giegé R., Verlaan P., Van Belkum A., Pleij C. W. 3-D graphics modelling of the tRNA-like 3'-end of turnip yellow mosaic virus RNA: structural and functional implications. J Biomol Struct Dyn. 1987 Apr;4(5):707–728. doi: 10.1080/07391102.1987.10507674. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Zachau H. G. Charging of a yeast methionine tRNA with phenylalanine and its implication for the synthetase recognition problem. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):891–896. doi: 10.1515/bchm2.1977.358.2.891. [DOI] [PubMed] [Google Scholar]
- Florentz C., Giegé R. Contact areas of the turnip yellow mosaic virus tRNA-like structure interacting with yeast valyl-tRNA synthetase. J Mol Biol. 1986 Sep 5;191(1):117–130. doi: 10.1016/0022-2836(86)90427-4. [DOI] [PubMed] [Google Scholar]
- Giegé R., Briand J. P., Mengual R., Ebel J. P., Hirth L. Valylation of the two RNA components of turnip-yellow mosaic virus and specificity of the tRNA aminoacylation reaction. Eur J Biochem. 1978 Mar;84(1):251–256. doi: 10.1111/j.1432-1033.1978.tb12163.x. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Cedergren R. J., McKay W. Structure in tRNA data. Biochimie. 1982 Jun;64(6):387–397. doi: 10.1016/s0300-9084(82)80576-2. [DOI] [PubMed] [Google Scholar]
- Hall T. C. Transfer RNA-like structures in viral genomes. Int Rev Cytol. 1979;60:1–26. doi: 10.1016/s0074-7696(08)61257-7. [DOI] [PubMed] [Google Scholar]
- Joshi R. L., Faulhammer H., Chapeville F., Sprinzl M., Haenni A. L. Aminoacyl RNA domain of turnip yellow mosaic virus Val-RNA interacting with elongation factor Tu. Nucleic Acids Res. 1984 Oct 11;12(19):7467–7478. doi: 10.1093/nar/12.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R. L., Ravel J. M., Haenni A. L. Interaction of turnip yellow mosaic virus Val-RNA with eukaryotic elongation factor EF-1 [alpha]. Search for a function. EMBO J. 1986 Jun;5(6):1143–1148. doi: 10.1002/j.1460-2075.1986.tb04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Chapeville F., Haenni A. L. Length requirements for tRNA-specific enzymes and cleavage specificity at the 3' end of turnip yellow mosaic virus RNA. Nucleic Acids Res. 1982 Mar 25;10(6):1947–1962. doi: 10.1093/nar/10.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Chapeville F., Haenni A. L. Turnip yellow mosaic virus RNA is aminoacylated in vivo in Chinese cabbage leaves. EMBO J. 1982;1(8):935–938. doi: 10.1002/j.1460-2075.1982.tb01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Bujarski J. J., Dreher T. W., Hall T. C. Minus-strand initiation by brome mosaic virus replicase within the 3' tRNA-like structure of native and modified RNA templates. J Mol Biol. 1986 Feb 20;187(4):537–546. doi: 10.1016/0022-2836(86)90332-3. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Dreher T. W., Hall T. C. Synthesis of brome mosaic virus subgenomic RNA in vitro by internal initiation on (-)-sense genomic RNA. Nature. 1985 Jan 3;313(5997):68–70. doi: 10.1038/313068a0. [DOI] [PubMed] [Google Scholar]
- Rao A. L., Dreher T. W., Marsh L. E., Hall T. C. Telomeric function of the tRNA-like structure of brome mosaic virus RNA. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5335–5339. doi: 10.1073/pnas.86.14.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Hall T. C. Interference in trans with brome mosaic virus replication by RNA-2 bearing aminoacylation-deficient mutants. Virology. 1991 Jan;180(1):16–22. doi: 10.1016/0042-6822(91)90004-u. [DOI] [PubMed] [Google Scholar]
- Rietveld K., Pleij C. W., Bosch L. Three-dimensional models of the tRNA-like 3' termini of some plant viral RNAs. EMBO J. 1983;2(7):1079–1085. doi: 10.1002/j.1460-2075.1983.tb01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Olsson M. Transfer RNA pseudouridine synthases in Saccharomyces cerevisiae. J Biol Chem. 1990 May 25;265(15):8782–8787. [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon switching changes the identity of methionine and valine transfer RNAs. Science. 1988 Nov 4;242(4879):765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J. J., Dreher T. W. Infectious TYMV RNA from cloned cDNA: effects in vitro and in vivo of point substitutions in the initiation codons of two extensively overlapping ORFs. Nucleic Acids Res. 1989 Jun 26;17(12):4675–4687. doi: 10.1093/nar/17.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]