Abstract
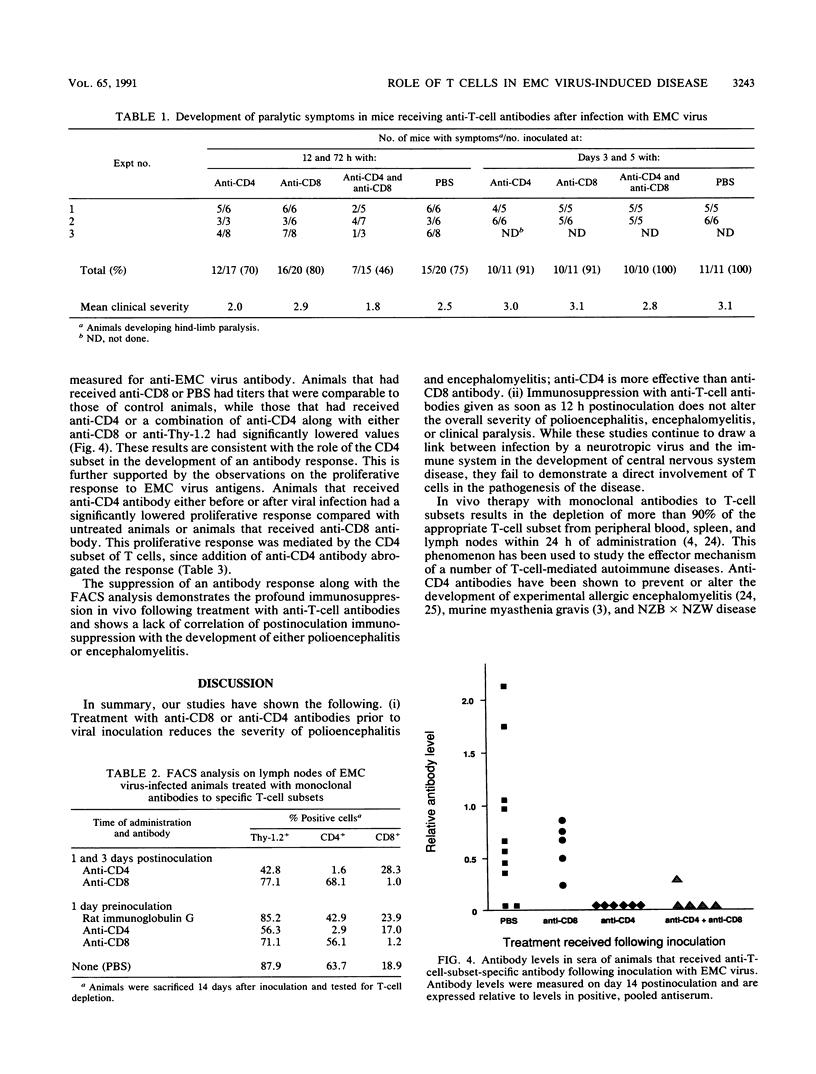
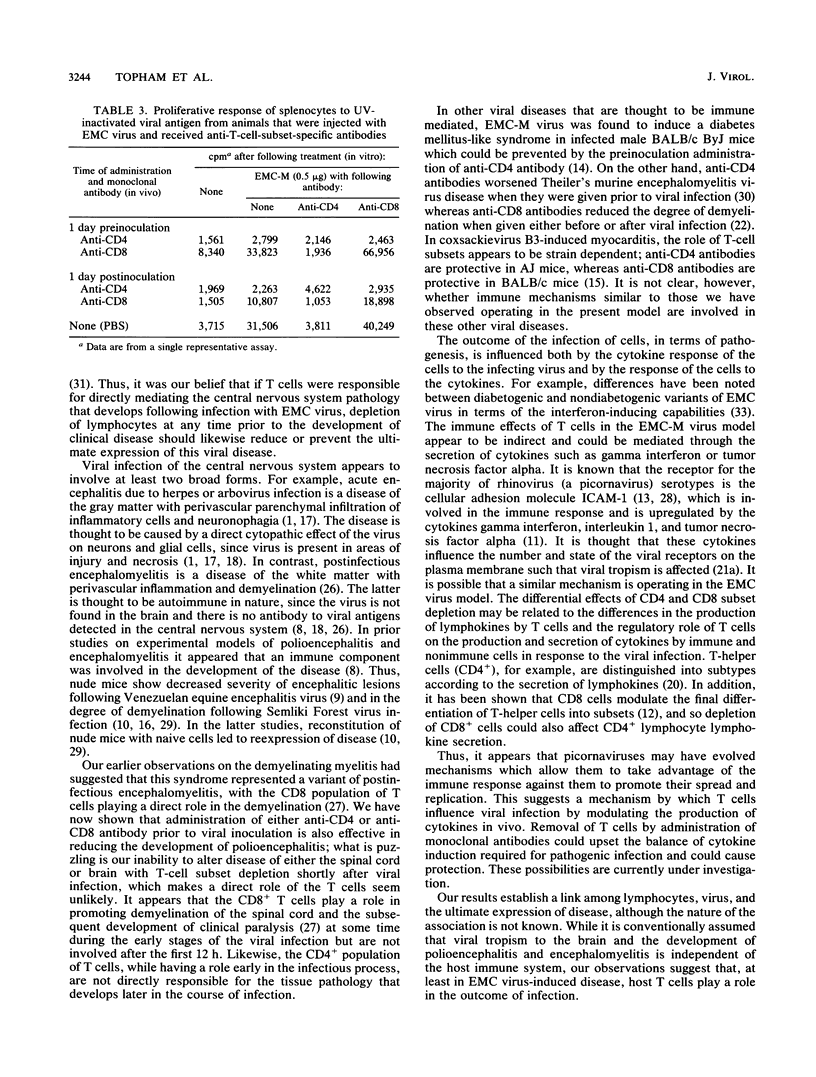
Infection of female BALB/c mice with encephalomyocarditis virus results in the development of a paralytic syndrome in 7 to 10 days postinoculation. Previous studies had suggested the involvement of an immune component in the development of central nervous system pathology. We have examined the effects of T-cell depletion on the development of polioencephalitis (neuronal necrosis and inflammation of the brain and brain stem) and the relative contribution of the CD4+ and CD8+ subsets following the establishment of viremia. We show that monoclonal antibody depletion of T cells is effective in the reduction of polioencephalitis when given prior to viral inoculation. However, administration of the antibodies 12 h or more after viral inoculation failed to alter the development of polioencephalitis or encephalomyelitis. We conclude that T cells are involved in the development of central nervous system disease during the initial stages of infection but are not responsible for the later progression of disease.
Full text
PDF

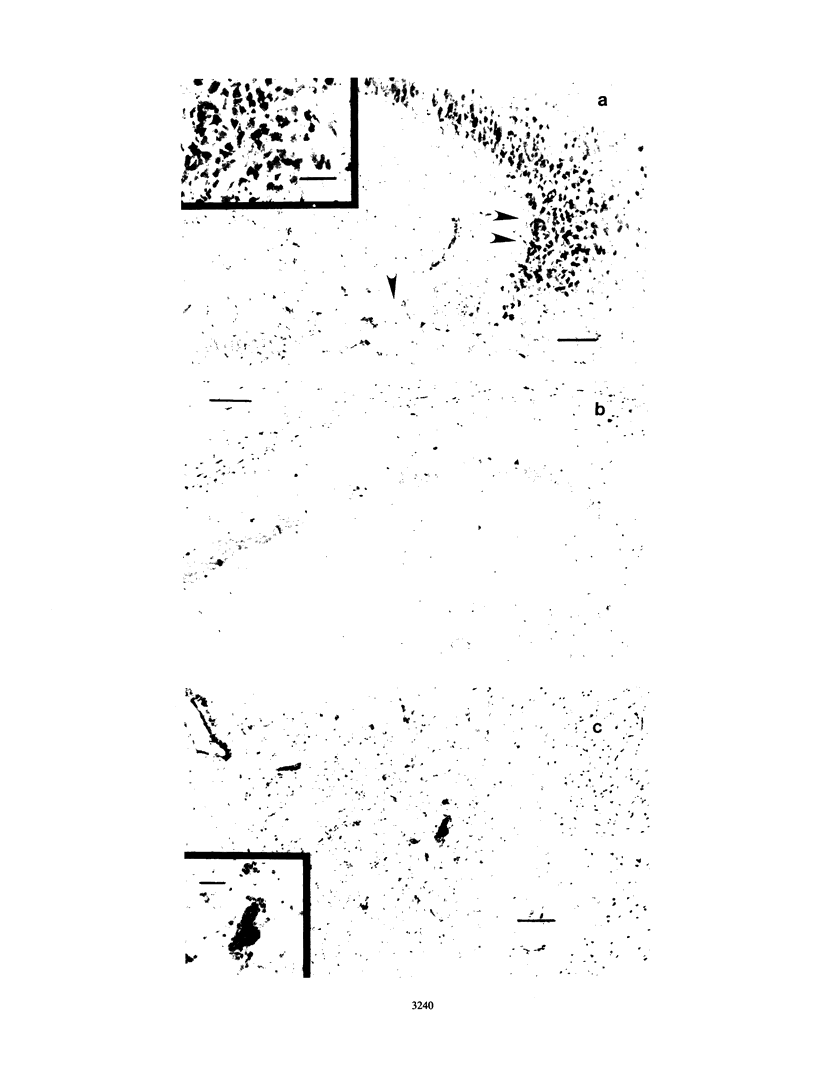
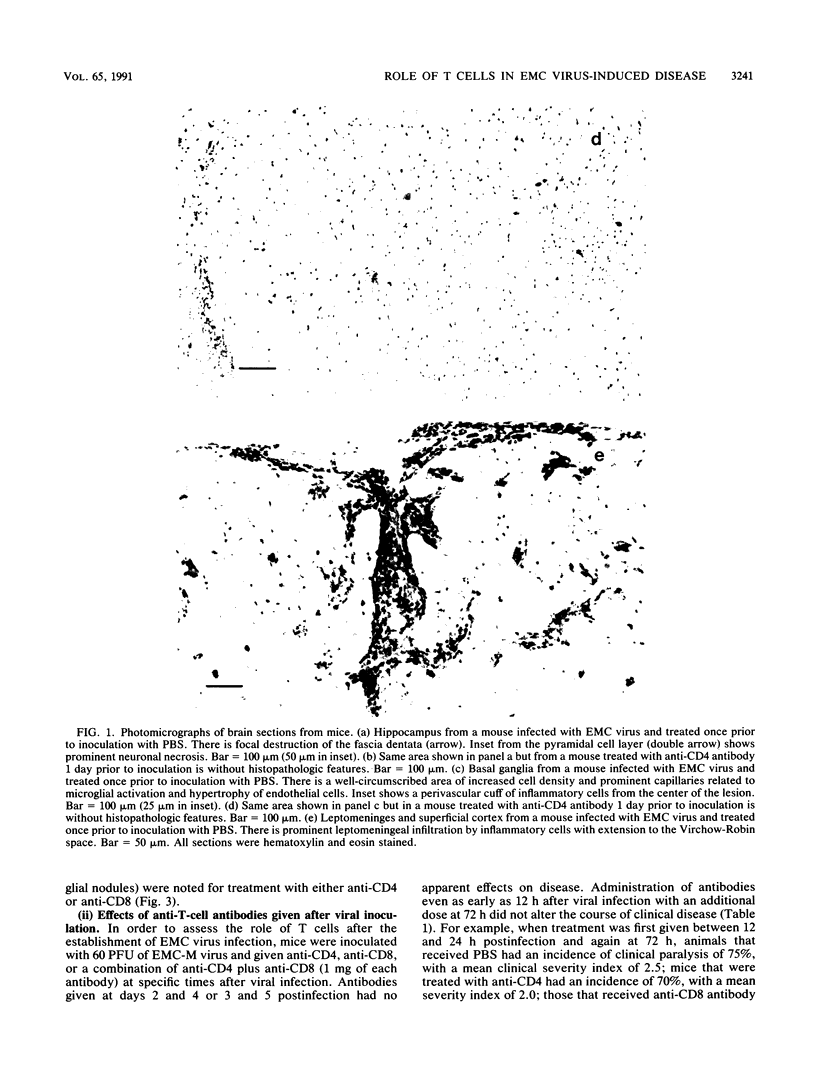
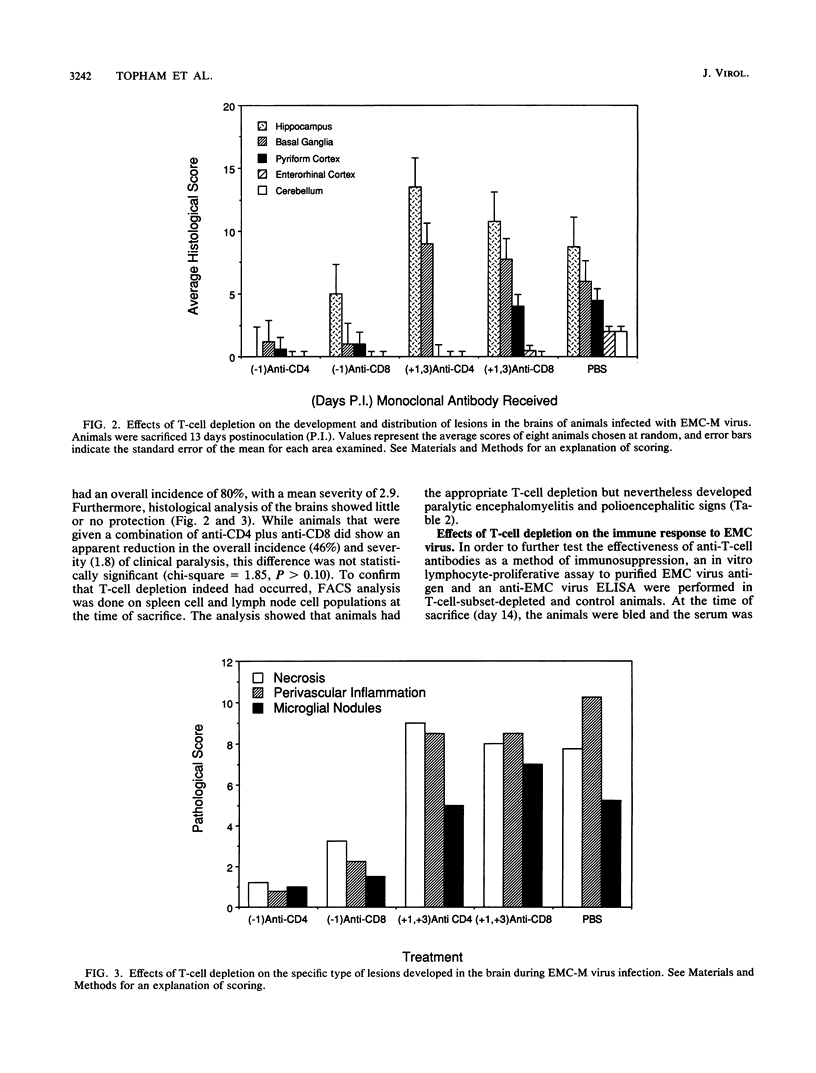

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht P. Pathogenesis of neurotropic arbovirus infections. Curr Top Microbiol Immunol. 1968;43:44–91. doi: 10.1007/978-3-642-46118-7_2. [DOI] [PubMed] [Google Scholar]
- Babu P. G., Huber S., Sriram S., Craighead J. E. Genetic control of multisystem autoimmune disease in encephalomyocarditis virus infected BALB/cCUM and BALB/cBYJ mice. Curr Top Microbiol Immunol. 1985;122:154–161. doi: 10.1007/978-3-642-70740-7_23. [DOI] [PubMed] [Google Scholar]
- Christadoss P., Dauphinee M. J. Immunotherapy for myasthenia gravis: a murine model. J Immunol. 1986 Apr 1;136(7):2437–2440. [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Pathogenicity of the M and E variants of the encephalomyocarditis (EMC) virus. II. Lesions of the pancreas, parotid and lacrimal glands. Am J Pathol. 1966 Mar;48(3):375–386. [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E. Viral diabetes mellitus in man and experimental animals. Am J Med. 1981 Jan;70(1):127–134. doi: 10.1016/0002-9343(81)90419-8. [DOI] [PubMed] [Google Scholar]
- Cronin M. E., Love L. A., Miller F. W., McClintock P. R., Plotz P. H. The natural history of encephalomyocarditis virus-induced myositis and myocarditis in mice. Viral persistence demonstrated by in situ hybridization. J Exp Med. 1988 Nov 1;168(5):1639–1648. doi: 10.1084/jem.168.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G. Experimental models of virus-induced demyelination of the central nervous system. Ann Neurol. 1982 Feb;11(2):109–127. doi: 10.1002/ana.410110202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. Virus-induced demyelination. Production by a viral temperature-sensitive mutant. J Neurol Sci. 1979 Jun;42(1):155–168. doi: 10.1016/0022-510x(79)90159-x. [DOI] [PubMed] [Google Scholar]
- Frohman E. M., Frohman T. C., Dustin M. L., Vayuvegula B., Choi B., Gupta A., van den Noort S., Gupta S. The induction of intercellular adhesion molecule 1 (ICAM-1) expression on human fetal astrocytes by interferon-gamma, tumor necrosis factor alpha, lymphotoxin, and interleukin-1: relevance to intracerebral antigen presentation. J Neuroimmunol. 1989 Jul;23(2):117–124. doi: 10.1016/0165-5728(89)90030-1. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Haynes M. K., Huber S. A., Craighead J. E. Helper-inducer T-lymphocytes mediate diabetes in EMC-infected BALB/c ByJ mice. Diabetes. 1987 Jul;36(7):877–881. doi: 10.2337/diab.36.7.877. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Weller A., Herzum M., Lodge P. A., Estrin M., Simpson K., Guthrie M. Immunopathogenic mechanisms in experimental picornavirus-induced autoimmunity. Pathol Immunopathol Res. 1988;7(4):279–291. doi: 10.1159/000157123. [DOI] [PubMed] [Google Scholar]
- Jenkins H. G., Tansey E. M., Macefield F., Ikeda H. Evidence for a T-cell related factor as the cause of demyelination in mice following Semliki Forest virus infection. Brain Res. 1988 Aug 30;459(1):145–147. doi: 10.1016/0006-8993(88)90294-6. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Burke D. S., Elwell M., Leake C. J., Nisalak A., Hoke C. H., Lorsomrudee W. Japanese encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann Neurol. 1985 Nov;18(5):567–573. doi: 10.1002/ana.410180510. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. The pathogenesis of acute viral encephalitis and postinfectious encephalomyelitis. J Infect Dis. 1987 Mar;155(3):359–364. doi: 10.1093/infdis/155.3.359. [DOI] [PubMed] [Google Scholar]
- MURNANE T. G., CRAIGHEAD J. E., MONDRAGON H., SHELOKOV A. Fatal disease of swine due to encephalomyocarditis virus. Science. 1960 Feb 19;131(3399):498–499. doi: 10.1126/science.131.3399.498. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Rodriguez M., Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988 May 1;140(9):2950–2955. [PubMed] [Google Scholar]
- Shanley J. D. Enzyme-linked immunosorbent assay for immunoglobulin G antibody to encephalomyocarditis virus. J Clin Microbiol. 1980 Nov;12(5):663–666. doi: 10.1128/jcm.12.5.663-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S., Carroll L., Fortin S., Cooper S., Ranges G. In vivo immunomodulation by monoclonal anti-CD4 antibody. II. Effect on T cell response to myelin basic protein and experimental allergic encephalomyelitis. J Immunol. 1988 Jul 15;141(2):464–468. [PubMed] [Google Scholar]
- Sriram S., Roberts C. A. Treatment of established chronic relapsing experimental allergic encephalomyelitis with anti-L3T4 antibodies. J Immunol. 1986 Jun 15;136(12):4464–4469. [PubMed] [Google Scholar]
- Sriram S., Steinman L. Postinfectious and postvaccinial encephalomyelitis. Neurol Clin. 1984 May;2(2):341–353. [PubMed] [Google Scholar]
- Sriram S., Topham D. J., Huang S. K., Rodriguez M. Treatment of encephalomyocarditis virus-induced central nervous system demyelination with monoclonal anti-T-cell antibodies. J Virol. 1989 Oct;63(10):4242–4248. doi: 10.1128/jvi.63.10.4242-4248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Suckling A. J., Pathak S., Jagelman S., Webb H. E. Virus-associated demyelination. A model using avirulent Semliki Forest virus infection of mice. J Neurol Sci. 1978 Nov;39(1):147–154. doi: 10.1016/0022-510x(78)90195-8. [DOI] [PubMed] [Google Scholar]
- Welsh C. J., Tonks P., Nash A. A., Blakemore W. F. The effect of L3T4 T cell depletion on the pathogenesis of Theiler's murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987 Jun;68(Pt 6):1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W., McClintock P. R., Onodera T., Notkins A. L. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J Exp Med. 1980 Oct 1;152(4):878–892. doi: 10.1084/jem.152.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. I. Polypeptide and ribonucleate components of the virus particle. Virology. 1974 Feb;57(2):531–542. doi: 10.1016/0042-6822(74)90192-5. [DOI] [PubMed] [Google Scholar]