Abstract
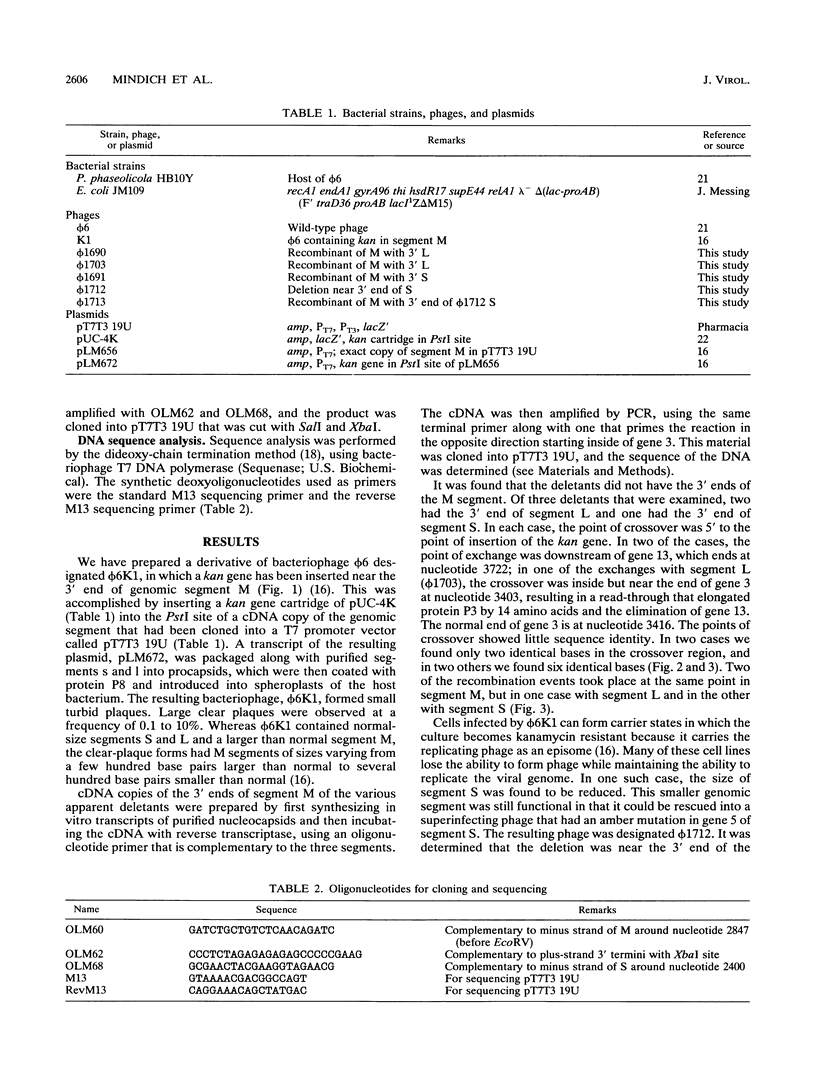
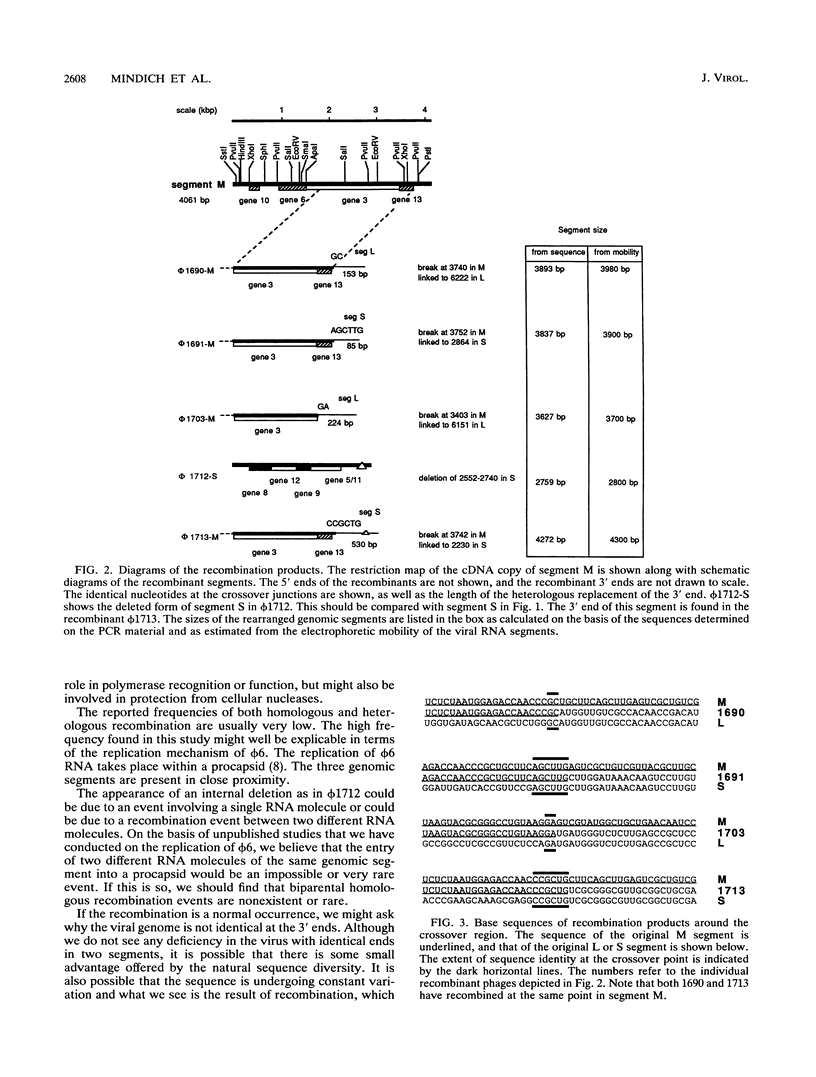
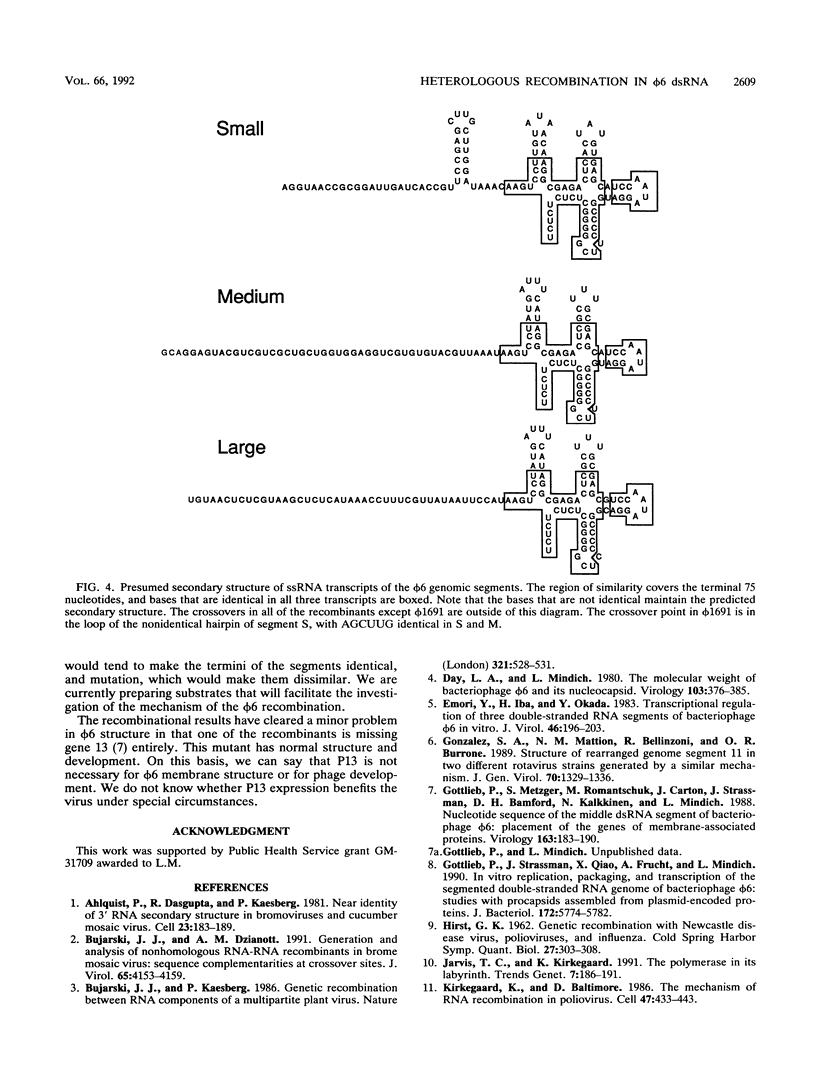
Bacteriophage phi 6 contains three double-stranded RNA genomic segments. We have constructed a virus with an insertion of a kanamycin resistance gene in genomic RNA segment M. The virus forms small, turbid plaques, and its genome is unstable. Virus from a single plaque contained from about 0.1 to 10% large clear-plaque forms of the virus; these were usually missing the kanamycin resistance gene, and in many cases, the resulting segment M was larger or smaller than its normal size. Sequence analysis of the genomic RNA of the apparent deletions showed that they were formed by recombination events between segment M and either segment S or L. These heterologous recombination events resulted in the loss of the kanamycin resistance gene from segment M and the replacement of the 3' end of segment M with the 3' end of segment S or L. Although the 3' ends of the single-stranded RNA transcripts of the genomic segments appear to have extensive secondary structure, the sequences at the 3' ends are not involved in the specificity of genomic packaging.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Dzianott A. M. Generation and analysis of nonhomologous RNA-RNA recombinants in brome mosaic virus: sequence complementarities at crossover sites. J Virol. 1991 Aug;65(8):4153–4159. doi: 10.1128/jvi.65.8.4153-4159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. 1986 May 29-Jun 4Nature. 321(6069):528–531. doi: 10.1038/321528a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day L. A., Mindich L. The molecular weight of bacteriophage phi 6 and its nucleocapsid. Virology. 1980 Jun;103(2):376–385. doi: 10.1016/0042-6822(80)90196-8. [DOI] [PubMed] [Google Scholar]
- Emori Y., Iba H., Okada Y. Transcriptional regulation of three double-stranded RNA segments of bacteriophage phi 6 in vitro. J Virol. 1983 Apr;46(1):196–203. doi: 10.1128/jvi.46.1.196-203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S. A., Mattion N. M., Bellinzoni R., Burrone O. R. Structure of rearranged genome segment 11 in two different rotavirus strains generated by a similar mechanism. J Gen Virol. 1989 Jun;70(Pt 6):1329–1336. doi: 10.1099/0022-1317-70-6-1329. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., Metzger S., Romantschuk M., Carton J., Strassman J., Bamford D. H., Kalkkinen N., Mindich L. Nucleotide sequence of the middle dsRNA segment of bacteriophage phi 6: placement of the genes of membrane-associated proteins. Virology. 1988 Mar;163(1):183–190. doi: 10.1016/0042-6822(88)90245-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Qiao X. Y., Frucht A., Mindich L. In vitro replication, packaging, and transcription of the segmented double-stranded RNA genome of bacteriophage phi 6: studies with procapsids assembled from plasmid-encoded proteins. J Bacteriol. 1990 Oct;172(10):5774–5782. doi: 10.1128/jb.172.10.5774-5782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G. K. Genetic recombination with Newcastle disease virus, polioviruses, and influenza. Cold Spring Harb Symp Quant Biol. 1962;27:303–309. doi: 10.1101/sqb.1962.027.001.028. [DOI] [PubMed] [Google Scholar]
- Jarvis T. C., Kirkegaard K. The polymerase in its labyrinth: mechanisms and implications of RNA recombination. Trends Genet. 1991 Jun;7(6):186–191. doi: 10.1016/0168-9525(91)90434-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K., Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986 Nov 7;47(3):433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Baric R. S., Makino S., Keck J. G., Egbert J., Leibowitz J. L., Stohlman S. A. Recombination between nonsegmented RNA genomes of murine coronaviruses. J Virol. 1985 Nov;56(2):449–456. doi: 10.1128/jvi.56.2.449-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre M., Rosenbaum V., Rappold W., Desselberger M., Wood D., Desselberger U. Biophysical characterization of rotavirus particles containing rearranged genomes. J Gen Virol. 1987 Nov;68(Pt 11):2961–2966. doi: 10.1099/0022-1317-68-11-2961. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mindich L., MacKenzie G., Strassman J., McGraw T., Metzger S., Romantschuk M., Bamford D. cDNA cloning of portions of the bacteriophage phi 6 genome. J Bacteriol. 1985 Jun;162(3):992–999. doi: 10.1128/jb.162.3.992-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Nemhauser I., Gottlieb P., Romantschuk M., Carton J., Frucht S., Strassman J., Bamford D. H., Kalkkinen N. Nucleotide sequence of the large double-stranded RNA segment of bacteriophage phi 6: genes specifying the viral replicase and transcriptase. J Virol. 1988 Apr;62(4):1180–1185. doi: 10.1128/jvi.62.4.1180-1185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera S., Olkkonen V. M., Gottlieb P., Strassman J., Qiao X. Y., Bamford D. H., Mindich L. Construction of a transducing virus from double-stranded RNA bacteriophage phi6: establishment of carrier states in host cells. J Virol. 1992 Jan;66(1):190–196. doi: 10.1128/jvi.66.1.190-196.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Cohen J., Mindich L. The isolation of suppressible nonsence mutants of bacteriophage phi6. Virology. 1976 Nov;75(1):198–208. [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


