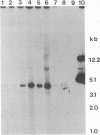
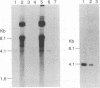
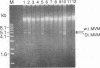
Abstract
The prototype strain of minute virus of mice [MVM(p)] is an autonomous parvovirus with a tropism for cells expressing a neoplastically transformed phenotype. To generate gene transfer vectors for tumor-specific gene expression, human interleukin-2 (IL-2) and murine interleukin-4 (IL-4) genes were cloned under the control of the p38 late promoter of MVM(p). Upon transfection into permissive cells, the recombinant MVMIL2 or MVMIL4 DNA was excised, amplified, and, in the presence of a helper plasmid, packaged into recombinant viral particles. The recombinant viruses were able to transfer fully functional IL-2 and IL-4 genes to permissive target cells and retained the oncotropic host range properties of the parental virus. Following infection with MVMIL2, nontransformed fibroblasts of rodent (FR3T3) or human (MRC-5) origin produced minimal IL-2 compared with the high levels of IL-2 production observed in their transformed derivatives (FREJ4 and MRC-5V1).
Full text
PDF
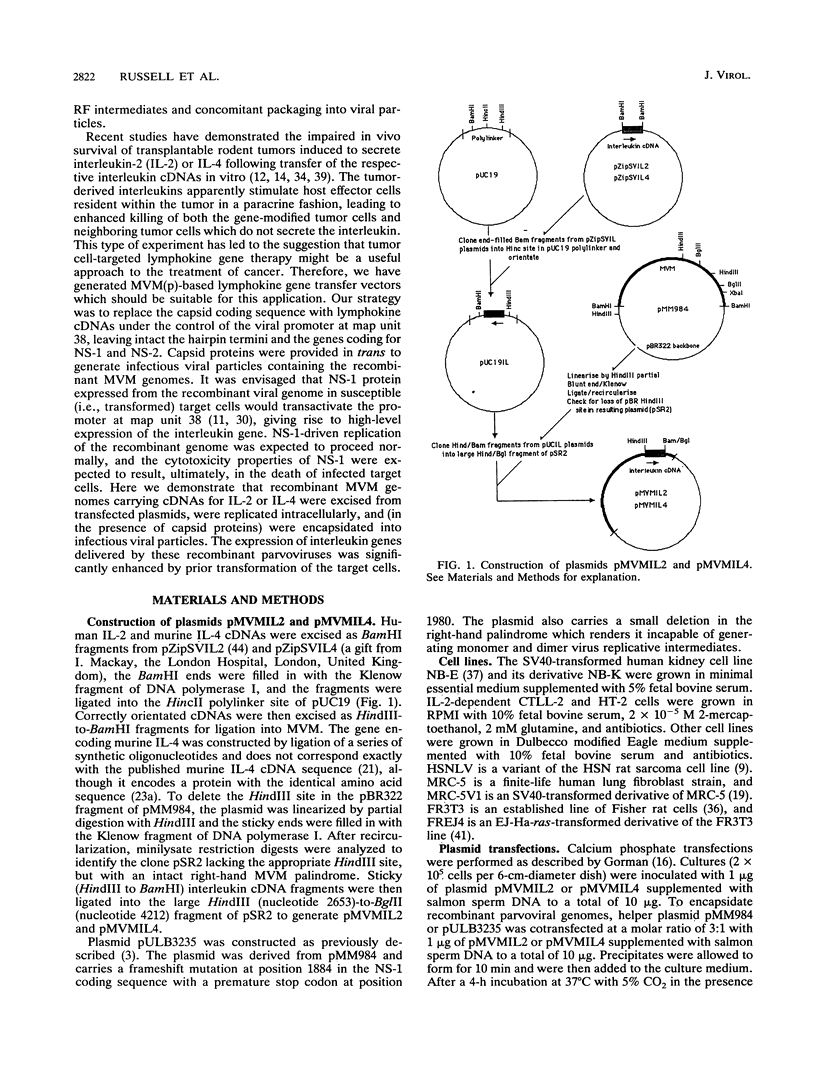

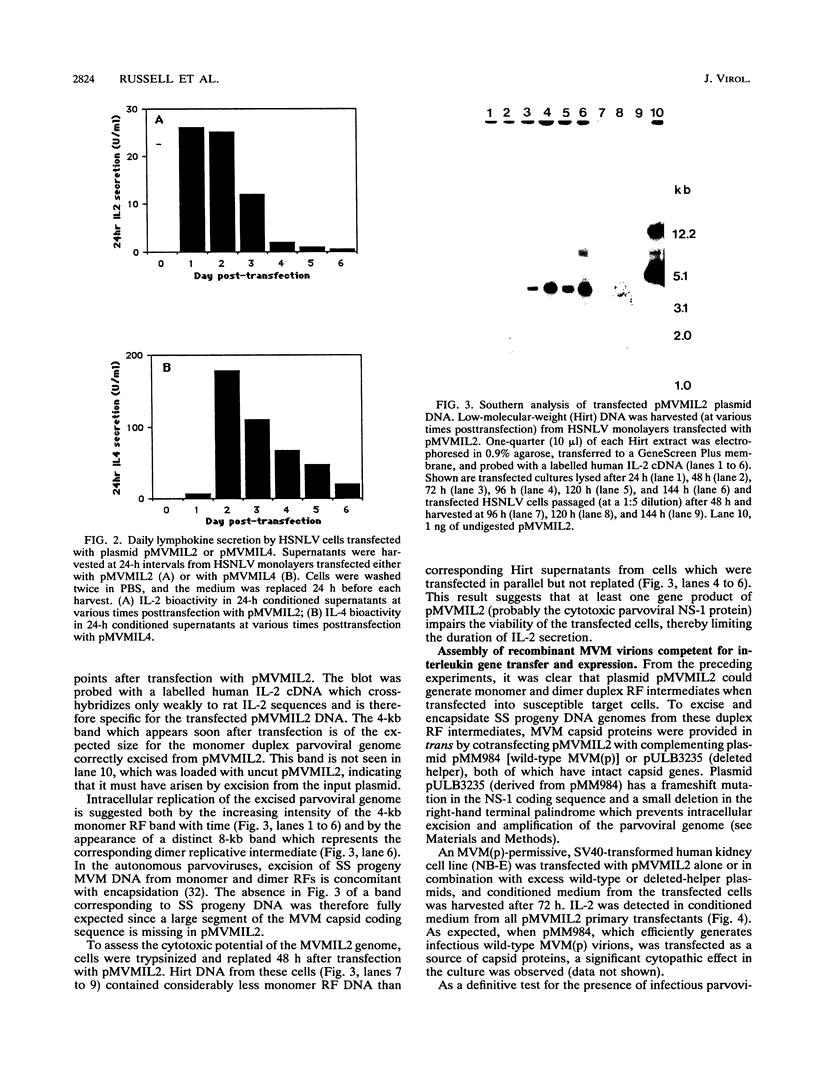
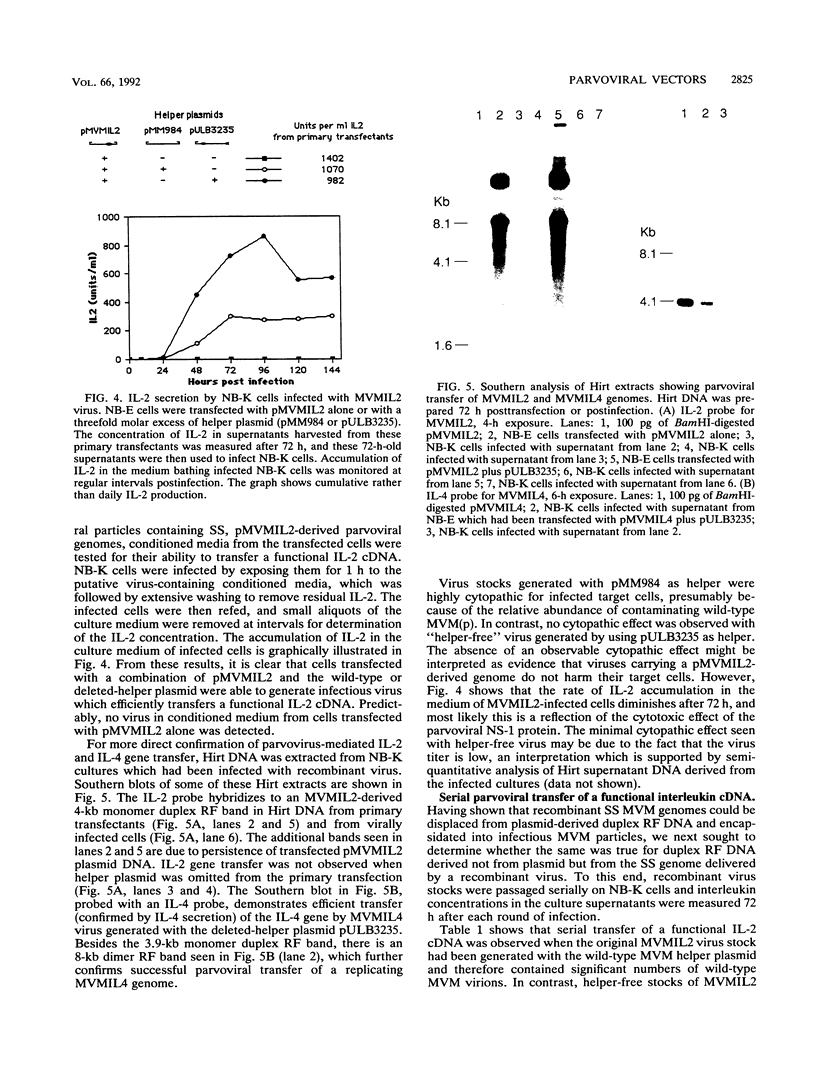
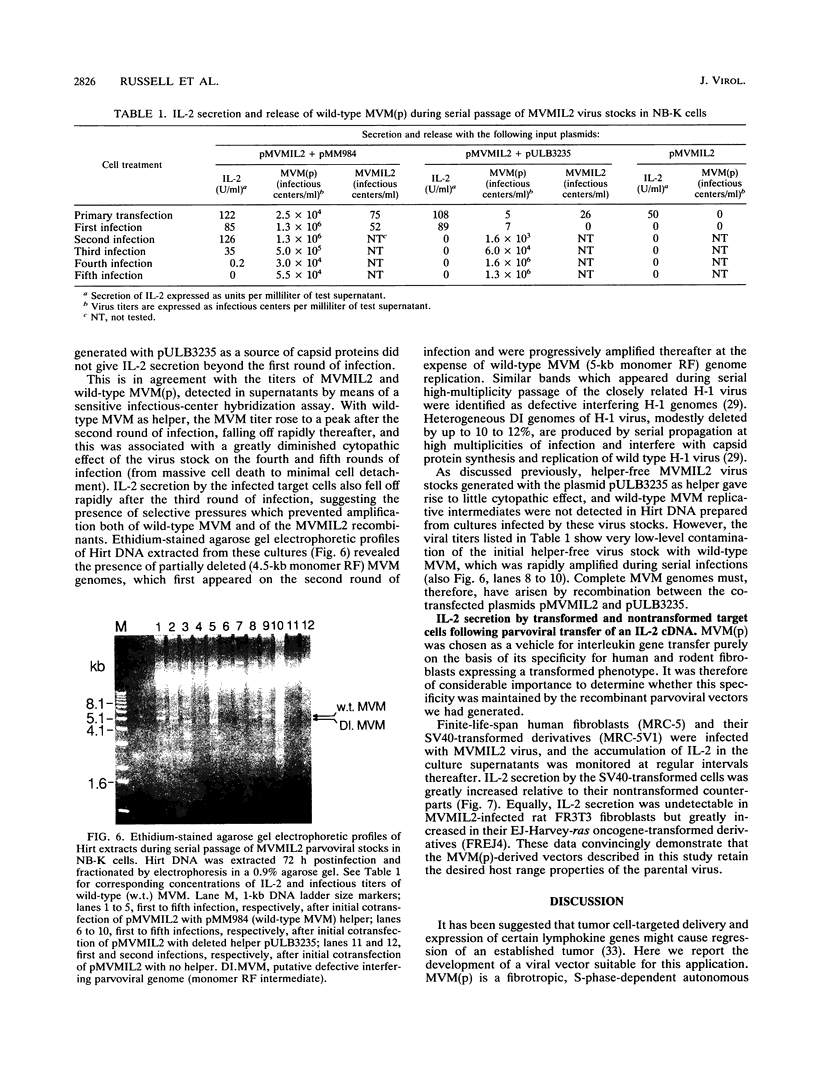
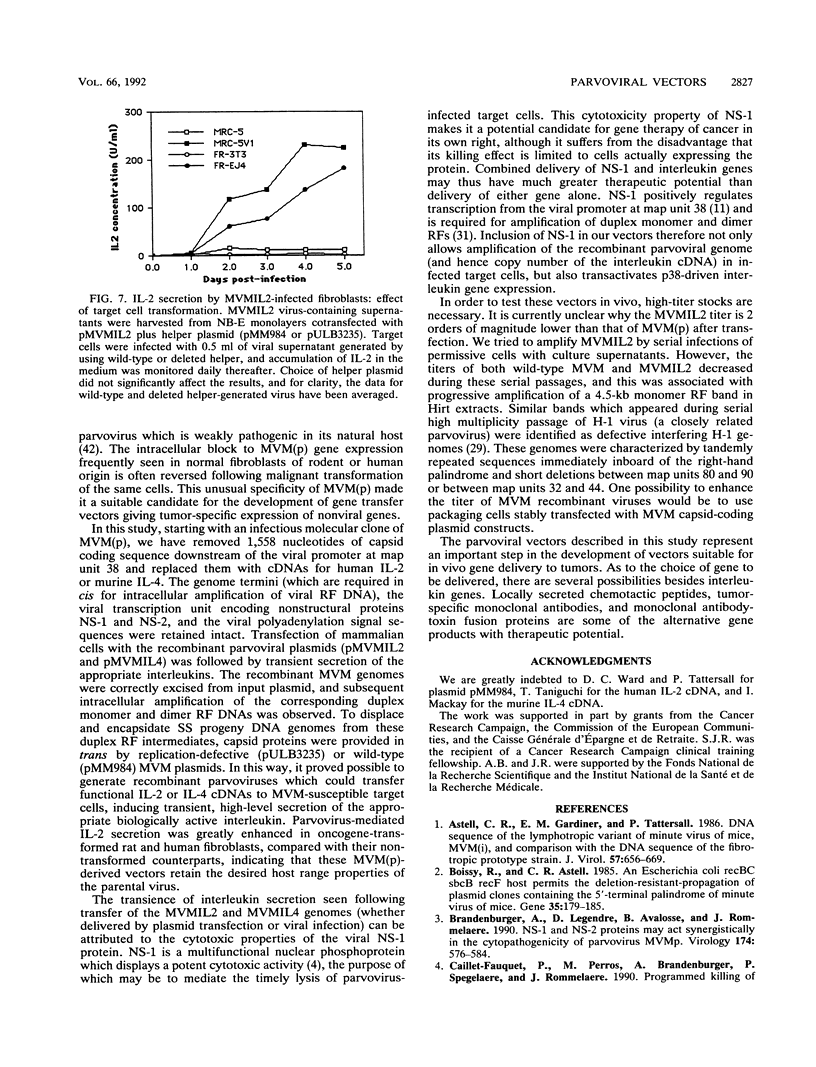

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Gardiner E. M., Tattersall P. DNA sequence of the lymphotropic variant of minute virus of mice, MVM(i), and comparison with the DNA sequence of the fibrotropic prototype strain. J Virol. 1986 Feb;57(2):656–669. doi: 10.1128/jvi.57.2.656-669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy R., Astell C. R. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5'-terminal palindrome of minute virus of mice. Gene. 1985;35(1-2):179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- Brandenburger A., Legendre D., Avalosse B., Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology. 1990 Feb;174(2):576–584. doi: 10.1016/0042-6822(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Cornelis J. J., Becquart P., Duponchel N., Salomé N., Avalosse B. L., Namba M., Rommelaere J. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J Virol. 1988 May;62(5):1679–1686. doi: 10.1128/jvi.62.5.1679-1686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis J. J., Chen Y. Q., Spruyt N., Duponchel N., Cotmore S. F., Tattersall P., Rommelaere J. Susceptibility of human cells to killing by the parvoviruses H-1 and minute virus of mice correlates with viral transcription. J Virol. 1990 Jun;64(6):2537–2544. doi: 10.1128/jvi.64.6.2537-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. A genome-linked copy of the NS-1 polypeptide is located on the outside of infectious parvovirus particles. J Virol. 1989 Sep;63(9):3902–3911. doi: 10.1128/jvi.63.9.3902-3911.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Gage J. O. Influence of tumour growth on the evolution of cytotoxic lymphoid cells in rats bearing a spontaneously metastasizing syngeneic fibrosarcoma. Br J Cancer. 1973 Aug;28(2):136–146. doi: 10.1038/bjc.1973.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Antonietti J. P., Beard P. Nonstructural protein of parvoviruses B19 and minute virus of mice controls transcription. J Virol. 1990 Jan;64(1):387–396. doi: 10.1128/jvi.64.1.387-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerig C., Hirt B., Beard P., Antonietti J. P. Minute virus of mice non-structural protein NS-1 is necessary and sufficient for trans-activation of the viral P39 promoter. J Gen Virol. 1988 Oct;69(Pt 10):2563–2573. doi: 10.1099/0022-1317-69-10-2563. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Guetta E., Graziani Y., Tal J. Suppression of Ehrlich ascites tumors in mice by minute virus of mice. J Natl Cancer Inst. 1986 Jun;76(6):1177–1180. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huschtscha L. I., Holliday R. Limited and unlimited growth of SV40-transformed cells from human diploid MRC-5 fibroblasts. J Cell Sci. 1983 Sep;63:77–99. doi: 10.1242/jcs.63.1.77. [DOI] [PubMed] [Google Scholar]
- Kimsey P. B., Engers H. D., Hirt B., Jongeneel C. V. Pathogenicity of fibroblast- and lymphocyte-specific variants of minute virus of mice. J Virol. 1986 Jul;59(1):8–13. doi: 10.1128/jvi.59.1.8-13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman A. H., Kurt-Jones E. A., Abbas A. K. B-cell stimulatory factor 1 and not interleukin 2 is the autocrine growth factor for some helper T lymphocytes. Proc Natl Acad Sci U S A. 1987 Feb;84(3):824–827. doi: 10.1073/pnas.84.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P., Bruning H., Armentrout R. W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977 Oct;24(1):211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer P. P., Humphries R. K., Moore J. G., Purcell R. H., Young N. S. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. 1983 Mar 31-Apr 6Nature. 302(5907):426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- Mousset S., Cornelis J., Spruyt N., Rommelaere J. Transformation of established murine fibroblasts with an activated cellular Harvey-ras oncogene or the polyoma virus middle T gene increases cell permissiveness to parvovirus minute-virus-of-mice. Biochimie. 1986 Jul-Aug;68(7-8):951–955. doi: 10.1016/s0300-9084(86)81058-6. [DOI] [PubMed] [Google Scholar]
- Mousset S., Rommelaere J. Minute virus of mice inhibits cell transformation by simian virus 40. Nature. 1982 Dec 9;300(5892):537–539. doi: 10.1038/300537a0. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Both excision and replication of cloned autonomous parvovirus DNA require the NS1 (rep) protein. J Virol. 1989 Oct;63(10):4249–4256. doi: 10.1128/jvi.63.10.4249-4256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Defective interfering particles of parvovirus H-1. J Virol. 1978 Aug;27(2):347–356. doi: 10.1128/jvi.27.2.347-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R., Linser P., Armentrout R. W. Kinetics of assembly of a parvovirus, minute virus of mice, in synchronized rat brain cells. J Virol. 1977 Jun;22(3):778–793. doi: 10.1128/jvi.22.3.778-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. J., Eccles S. A., Flemming C. L., Johnson C. A., Collins M. K. Decreased tumorigenicity of a transplantable rat sarcoma following transfer and expression of an IL-2 cDNA. Int J Cancer. 1991 Jan 21;47(2):244–251. doi: 10.1002/ijc.2910470213. [DOI] [PubMed] [Google Scholar]
- Russell S. J. Lymphokine gene therapy for cancer. Immunol Today. 1990 Jun;11(6):196–200. doi: 10.1016/0167-5699(90)90081-j. [DOI] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc Soc Exp Biol Med. 1962 Mar;109:495–500. doi: 10.3181/00379727-109-27246. [DOI] [PubMed] [Google Scholar]
- Salomé N., van Hille B., Duponchel N., Meneguzzi G., Cuzin F., Rommelaere J., Cornelis J. J. Sensitization of transformed rat cells to parvovirus MVMp is restricted to specific oncogenes. Oncogene. 1990 Jan;5(1):123–130. [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Toolan H. W. The parvoviruses. Prog Exp Tumor Res. 1972;16:410–425. doi: 10.1159/000393383. [DOI] [PubMed] [Google Scholar]
- Van Hille B., Duponchel N., Salomé N., Spruyt N., Cotmore S. F., Tattersall P., Cornelis J. J., Rommelaere J. Limitations to the expression of parvoviral nonstructural proteins may determine the extent of sensitization of EJ-ras-transformed rat cells to minute virus of mice. Virology. 1989 Jul;171(1):89–97. doi: 10.1016/0042-6822(89)90514-x. [DOI] [PubMed] [Google Scholar]
- Yakobson B., Hrynko T. A., Peak M. J., Winocour E. Replication of adeno-associated virus in cells irradiated with UV light at 254 nm. J Virol. 1989 Mar;63(3):1023–1030. doi: 10.1128/jvi.63.3.1023-1030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada G., Kitamura Y., Sonoda H., Harada H., Taki S., Mulligan R. C., Osawa H., Diamantstein T., Yokoyama S., Taniguchi T. Retroviral expression of the human IL-2 gene in a murine T cell line results in cell growth autonomy and tumorigenicity. EMBO J. 1987 Sep;6(9):2705–2709. doi: 10.1002/j.1460-2075.1987.tb02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]