Abstract
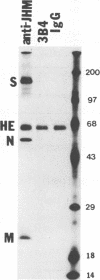
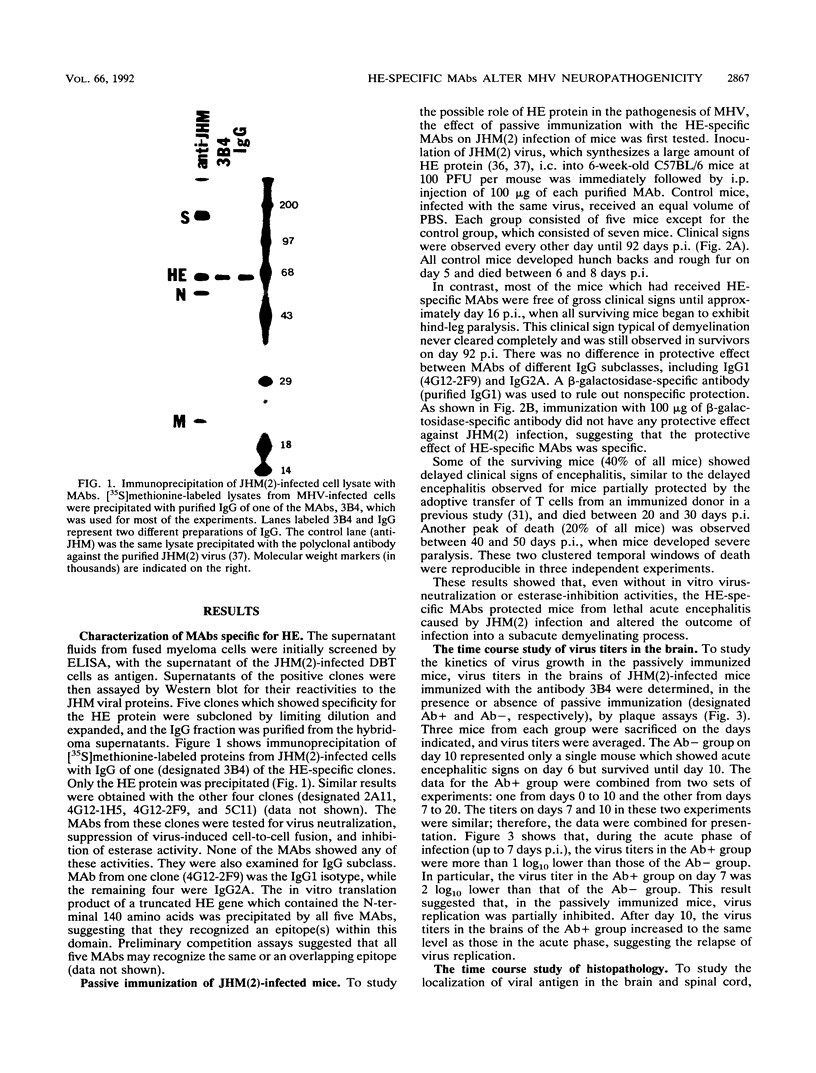
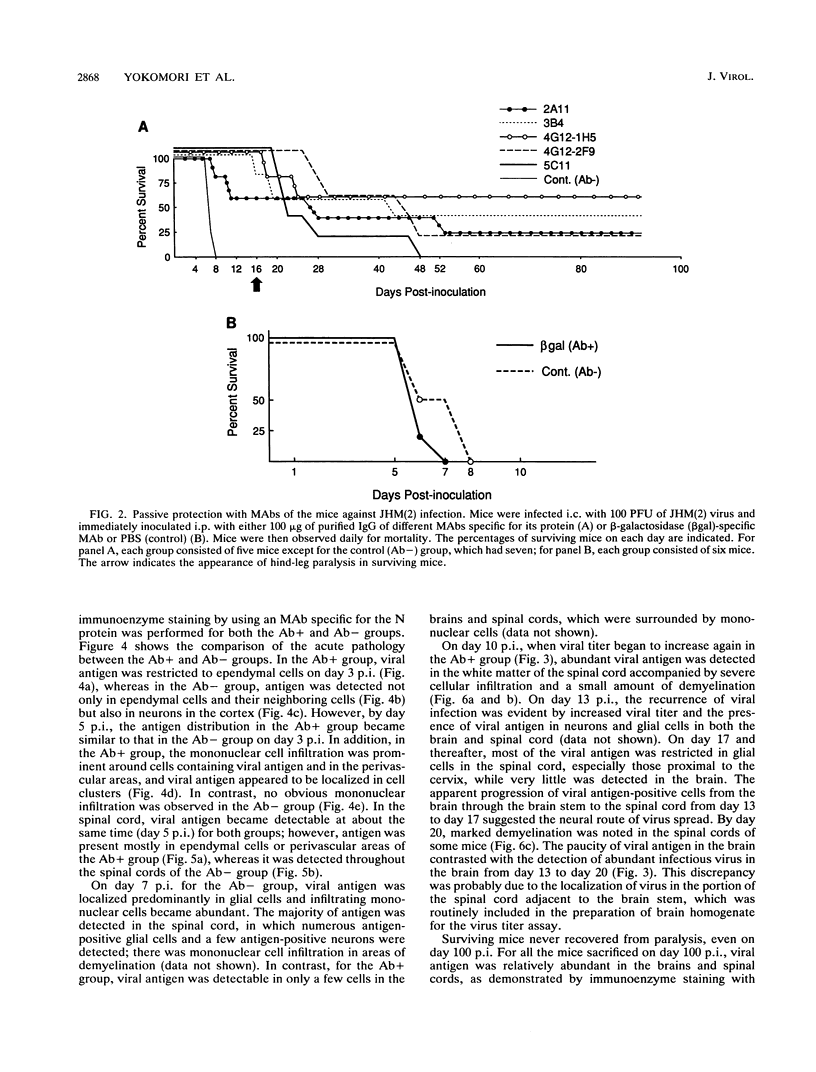
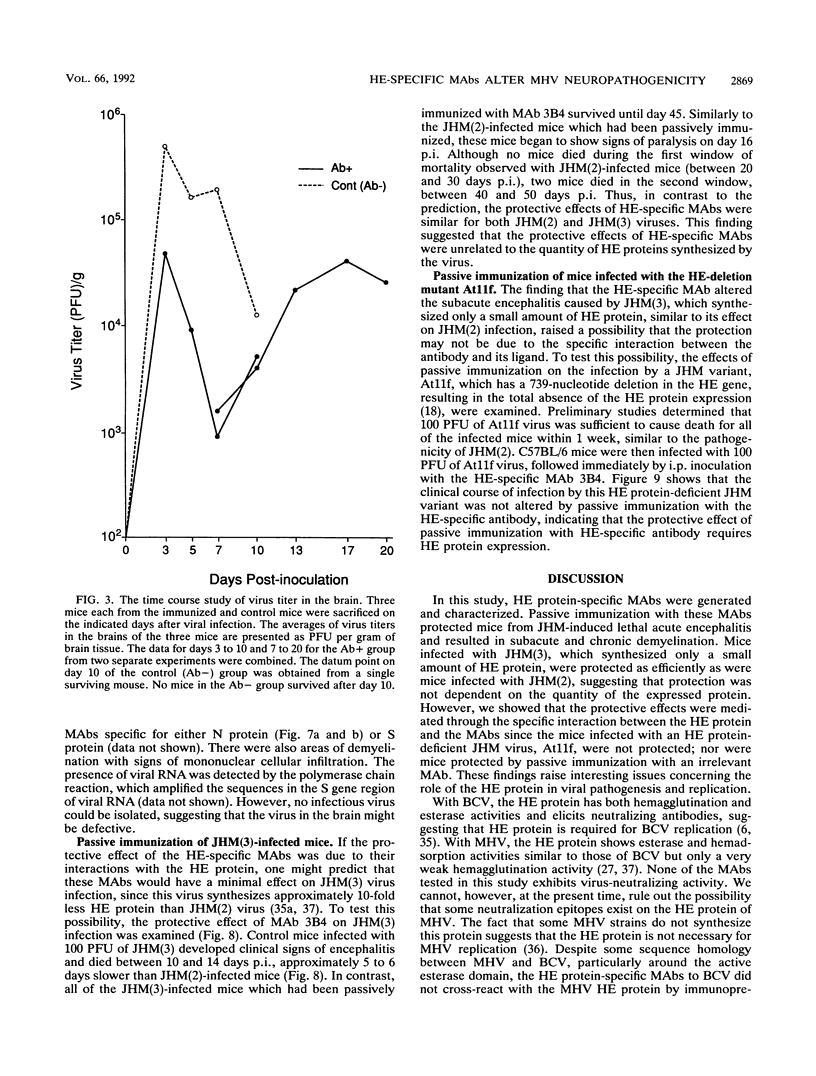
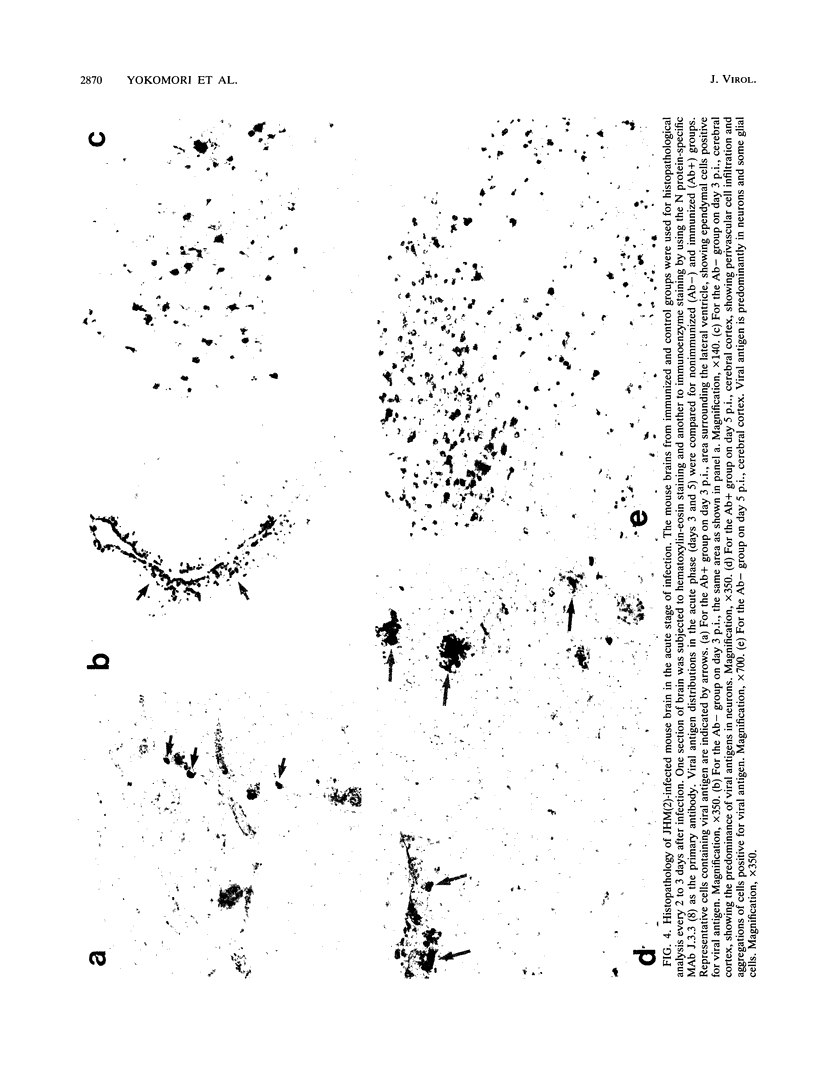
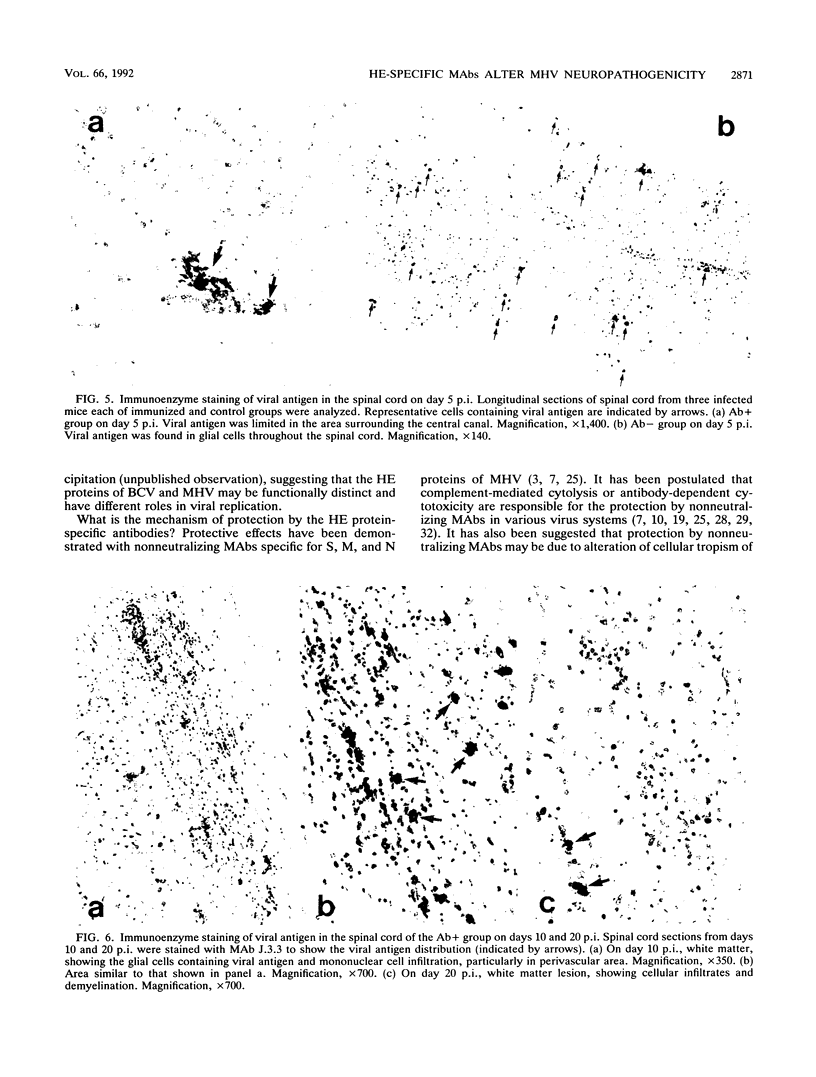
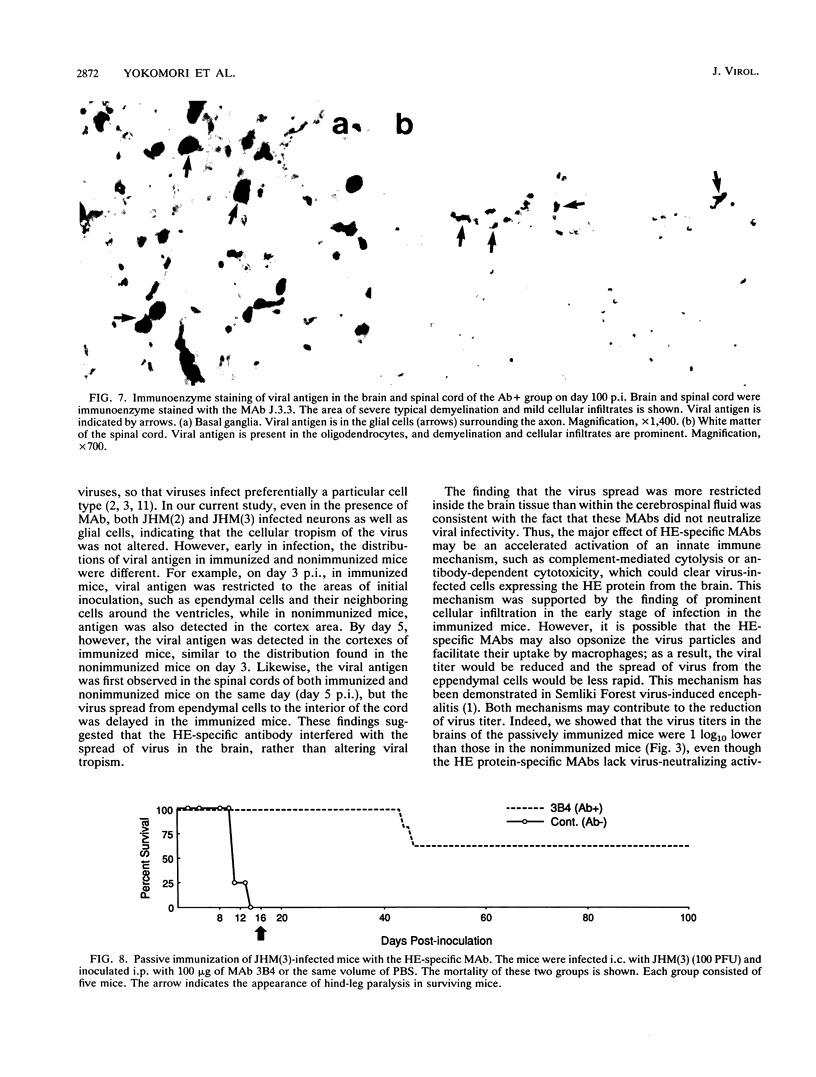
Some of mouse hepatitis virus strains contain an optional envelope glycoprotein, hemagglutinin-esterase (HE) protein. To understand the functional significance of this protein, monoclonal antibodies (MAbs) specific for this protein were generated and used for passive immunization of mice. None of these MAbs showed any virus-neutralizing activity in vitro; however, mice passively immunized with the purified MAbs were protected from lethal infection by the JHM strain of mouse hepatitis virus. Passive immunization altered the pathogenicity such that the virus caused subacute and chronic demyelination instead of acute lethal encephalitis. Virus titers in the brains of the immunized mice were significantly lower than those for the nonimmunized control mice, suggesting that the virus replication or spread was inhibited. In addition, histopathological analysis indicated that the spread of virus in the brain and spinal cord was significantly inhibited in the immunized mice. Furthermore, the mononuclear cell infiltration in the immunized mice appeared earlier than in the nonimmunized mice, suggesting that the exogenous antibody might have activated host immune responses, and thus facilitated clearance of the virus or virus-infected cells. The same protective effects were observed for both JHM(2) and JHM(3) viruses, which expressed different amounts of the HE protein. In contrast, mice infected with At11f, a variant of JHM which does not express the HE protein, were not protected by these MAbs, suggesting that protection was mediated by the specific interaction between the MAb and the HE protein. Thus, the mechanism of protection by the exogenous HE-specific MAbs may represent the early activation of innate immune mechanisms in response to the interaction between the MAbs and the HE protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boere W. A., Benaissa-Trouw B. J., Harmsen M., Kraaijeveld C. A., Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983 Jun;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- Brandriss M. W., Schlesinger J. J., Walsh E. E., Briselli M. Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J Gen Virol. 1986 Feb;67(Pt 2):229–234. doi: 10.1099/0022-1317-67-2-229. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Talbot P. J., Knobler R. L. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology. 1984 Jan 30;132(2):261–270. doi: 10.1016/0042-6822(84)90033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. R., Knobler R. L., Powell H., Buchmeier M. J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell--cell fusion. Virology. 1982 Jun;119(2):358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea S., Tijssen P. Identification of the structural proteins of turkey enteric coronavirus. Arch Virol. 1988;99(3-4):173–186. doi: 10.1007/BF01311068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregt D., Babiuk L. A. Monoclonal antibodies to bovine coronavirus: characteristics and topographical mapping of neutralizing epitopes on the E2 and E3 glycoproteins. Virology. 1987 Dec;161(2):410–420. doi: 10.1016/0042-6822(87)90134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Shubin R. A., Sussman M. A., Casteel N., Stohlman S. A. Monoclonal antibodies to the matrix (E1) glycoprotein of mouse hepatitis virus protect mice from encephalitis. Virology. 1989 Jan;168(1):162–167. doi: 10.1016/0042-6822(89)90415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Stohlman S. A., Harmon R. C., Lai M. M., Frelinger J. A., Weiner L. P. Antigenic relationships of murine coronaviruses: analysis using monoclonal antibodies to JHM (MHV-4) virus. Virology. 1983 Dec;131(2):296–307. doi: 10.1016/0042-6822(83)90498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwes D. J., Reynolds D. J. The polypeptide structure of canine coronavirus and its relationship to porcine transmissible gastroenteritis virus. J Gen Virol. 1981 Jan;52(Pt 1):153–157. doi: 10.1099/0022-1317-52-1-153. [DOI] [PubMed] [Google Scholar]
- Harty J. T., Plagemann P. G. Monoclonal antibody protection from age-dependent poliomyelitis: implications regarding the pathogenesis of lactate dehydrogenase-elevating virus. J Virol. 1990 Dec;64(12):6257–6262. doi: 10.1128/jvi.64.12.6257-6262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N., Fujiwara K., Hino S., Matumoto M. Replication and plaque formation of mouse hepatitis virus (MHV-2) in mouse cell line DBT culture. Arch Gesamte Virusforsch. 1974;44(3):298–302. doi: 10.1007/BF01240618. [DOI] [PubMed] [Google Scholar]
- Hogue B. G., Brian D. A. Structural proteins of human respiratory coronavirus OC43. Virus Res. 1986 Aug;5(2-3):131–144. doi: 10.1016/0168-1702(86)90013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue B. G., King B., Brian D. A. Antigenic relationships among proteins of bovine coronavirus, human respiratory coronavirus OC43, and mouse hepatitis coronavirus A59. J Virol. 1984 Aug;51(2):384–388. doi: 10.1128/jvi.51.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Potts B. J., Brian D. A. Bovine coronavirus hemagglutinin protein. Virus Res. 1985 Feb;2(1):53–59. doi: 10.1016/0168-1702(85)90059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Lampert P. W., Oldstone M. B. Virus persistence and recurring demyelination produced by a temperature-sensitive mutant of MHV-4. Nature. 1982 Jul 15;298(5871):279–280. doi: 10.1038/298279a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- La Monica N., Banner L. R., Morris V. L., Lai M. M. Localization of extensive deletions in the structural genes of two neurotropic variants of murine coronavirus JHM. Virology. 1991 Jun;182(2):883–888. doi: 10.1016/0042-6822(91)90635-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984 Jul;51(1):208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Bredenbeek P. J., Noten A. F., Horzinek M. C., Spaan W. J. Sequence of mouse hepatitis virus A59 mRNA 2: indications for RNA recombination between coronaviruses and influenza C virus. Virology. 1988 Oct;166(2):415–422. doi: 10.1016/0042-6822(88)90512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987 Sep;105(3):1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Lai M. M. Evolution of the 5'-end of genomic RNA of murine coronaviruses during passages in vitro. Virology. 1989 Mar;169(1):227–232. doi: 10.1016/0042-6822(89)90060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Taguchi F., Fujiwara K. Defective interfering particles of mouse hepatitis virus. Virology. 1984 Feb;133(1):9–17. doi: 10.1016/0042-6822(84)90420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V. L., Tieszer C., Mackinnon J., Percy D. Characterization of coronavirus JHM variants isolated from Wistar Furth rats with a viral-induced demyelinating disease. Virology. 1989 Mar;169(1):127–136. doi: 10.1016/0042-6822(89)90048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanaga K., Yamanouchi K., Fujiwara K. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J Virol. 1986 Jul;59(1):168–171. doi: 10.1128/jvi.59.1.168-171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. D., Cox G. J., Deregt D., Fitzpatrick D. R., Babiuk L. A. Cloning and in vitro expression of the gene for the E3 haemagglutinin glycoprotein of bovine coronavirus. J Gen Virol. 1989 Jan;70(Pt 1):155–164. doi: 10.1099/0022-1317-70-1-155. [DOI] [PubMed] [Google Scholar]
- Pfleiderer M., Routledge E., Herrler G., Siddell S. G. High level transient expression of the murine coronavirus haemagglutinin-esterase. J Gen Virol. 1991 Jun;72(Pt 6):1309–1315. doi: 10.1099/0022-1317-72-6-1309. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Walsh E. E. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J Immunol. 1985 Oct;135(4):2805–2809. [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Shieh C. K., Lee H. J., Yokomori K., La Monica N., Makino S., Lai M. M. Identification of a new transcriptional initiation site and the corresponding functional gene 2b in the murine coronavirus RNA genome. J Virol. 1989 Sep;63(9):3729–3736. doi: 10.1128/jvi.63.9.3729-3736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin R. A., Sussman M. A., Fleming J. O., Stohlman S. A. Relapsing encephalomyelitis following transfer of partial immunity to JHM virus. Microb Pathog. 1990 May;8(5):305–314. doi: 10.1016/0882-4010(90)90089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Cooper S. J., Griffin D. E. Monoclonal antibody cure and prophylaxis of lethal Sindbis virus encephalitis in mice. J Virol. 1986 Apr;58(1):107–115. doi: 10.1128/jvi.58.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Sefton B. M. Coronavirus proteins: structure and function of the oligosaccharides of the avian infectious bronchitis virus glycoproteins. J Virol. 1982 Dec;44(3):804–812. doi: 10.1128/jvi.44.3.804-812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Baric R. S., Nelson G. N., Soe L. H., Welter L. M., Deans R. J. Specific interaction between coronavirus leader RNA and nucleocapsid protein. J Virol. 1988 Nov;62(11):4288–4295. doi: 10.1128/jvi.62.11.4288-4295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Leider J., Spaan W., Palese P. The E3 protein of bovine coronavirus is a receptor-destroying enzyme with acetylesterase activity. J Virol. 1988 Dec;62(12):4686–4690. doi: 10.1128/jvi.62.12.4686-4690.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., Banner L. R., Lai M. M. Heterogeneity of gene expression of the hemagglutinin-esterase (HE) protein of murine coronaviruses. Virology. 1991 Aug;183(2):647–657. doi: 10.1016/0042-6822(91)90994-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., La Monica N., Makino S., Shieh C. K., Lai M. M. Biosynthesis, structure, and biological activities of envelope protein gp65 of murine coronavirus. Virology. 1989 Dec;173(2):683–691. doi: 10.1016/0042-6822(89)90581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]