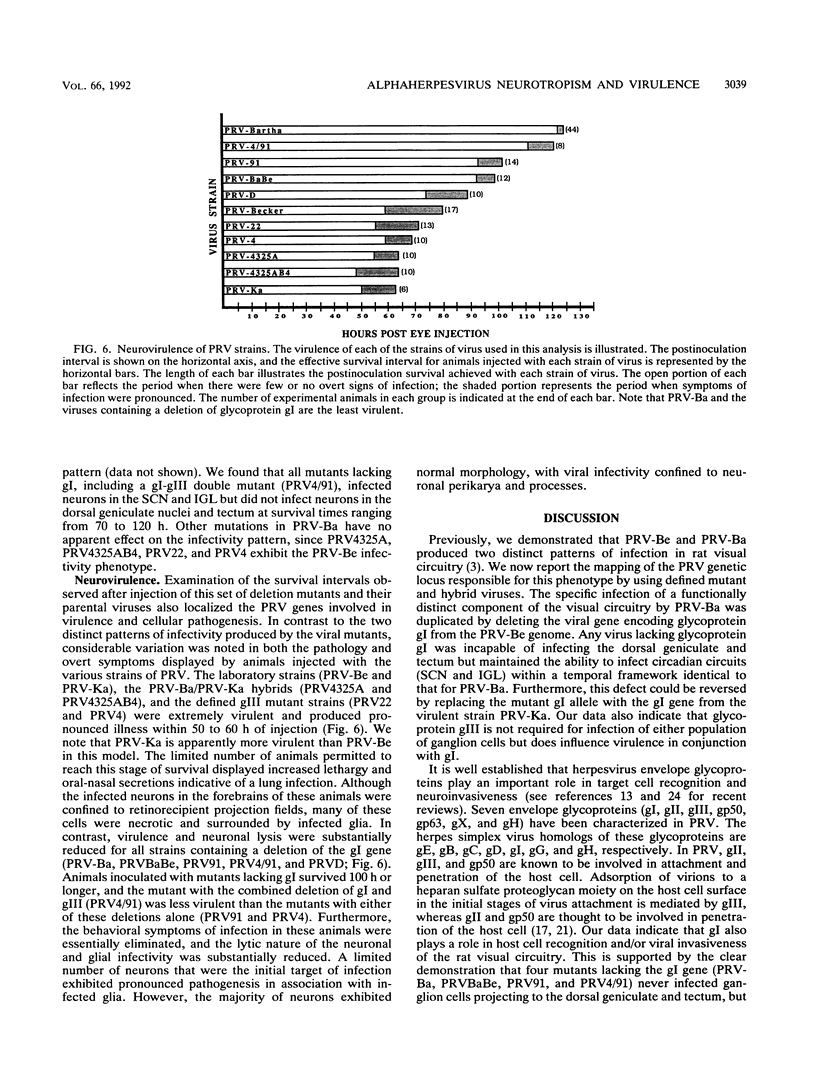
Abstract
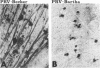
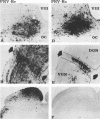
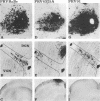
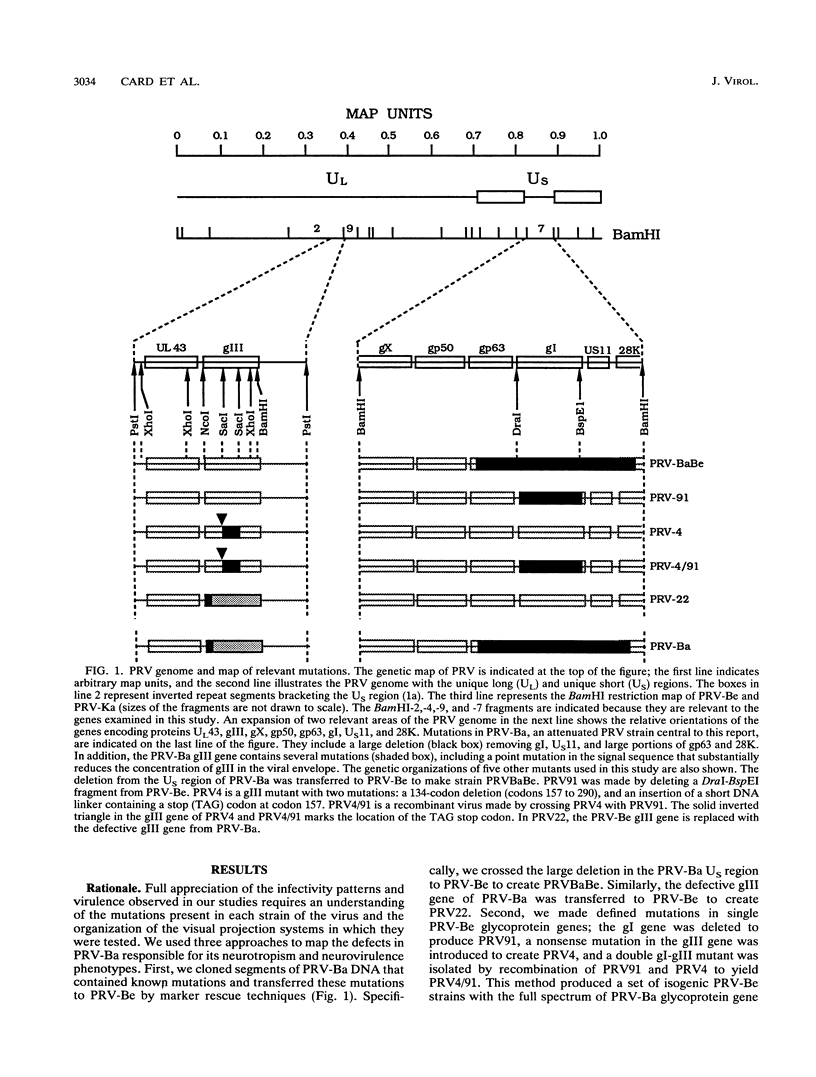
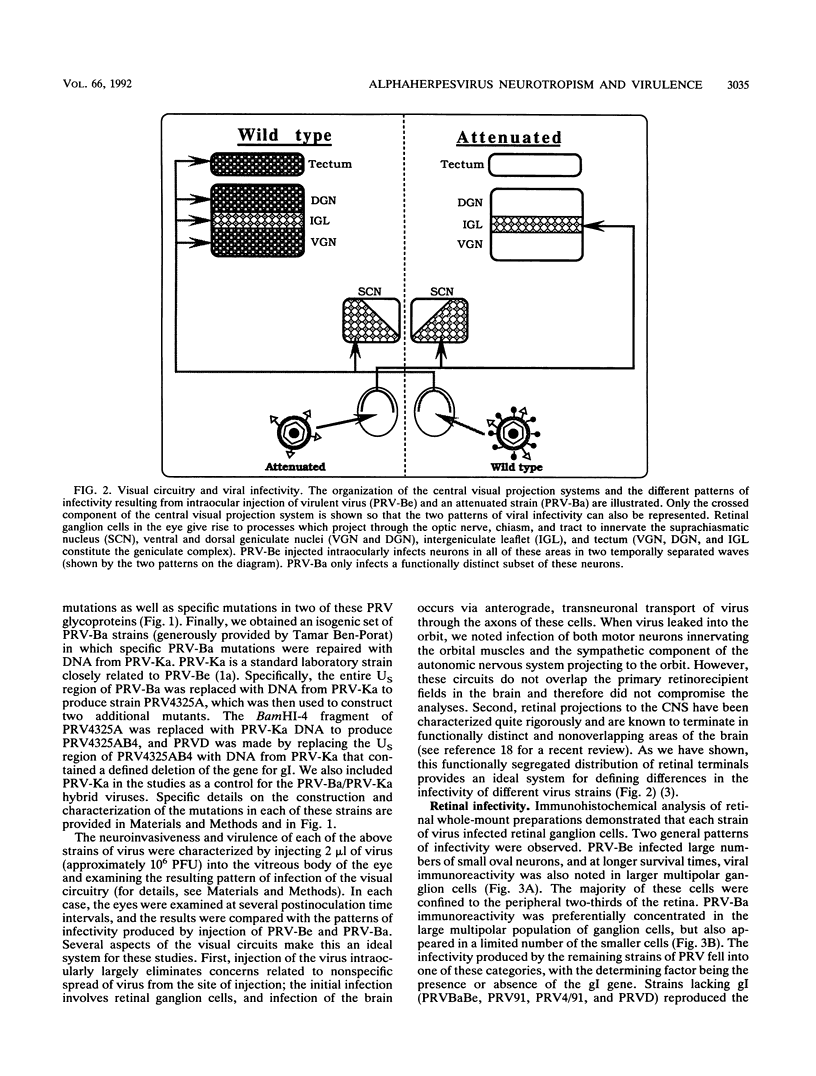
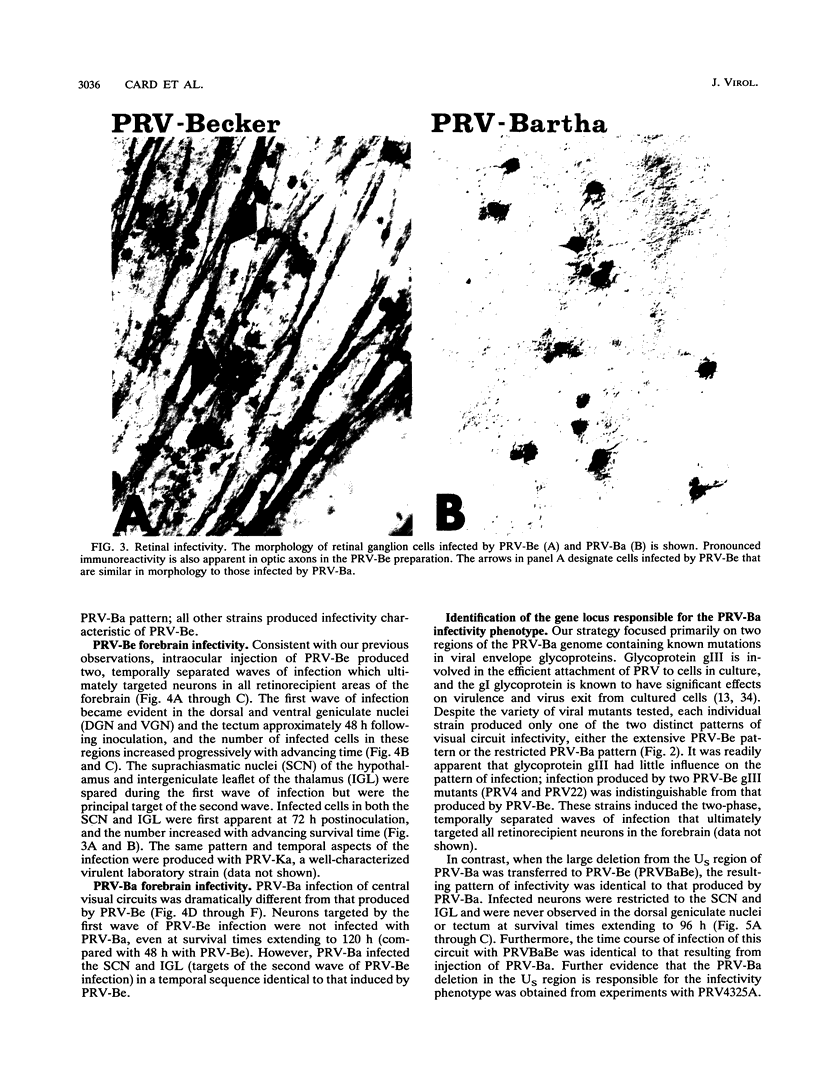
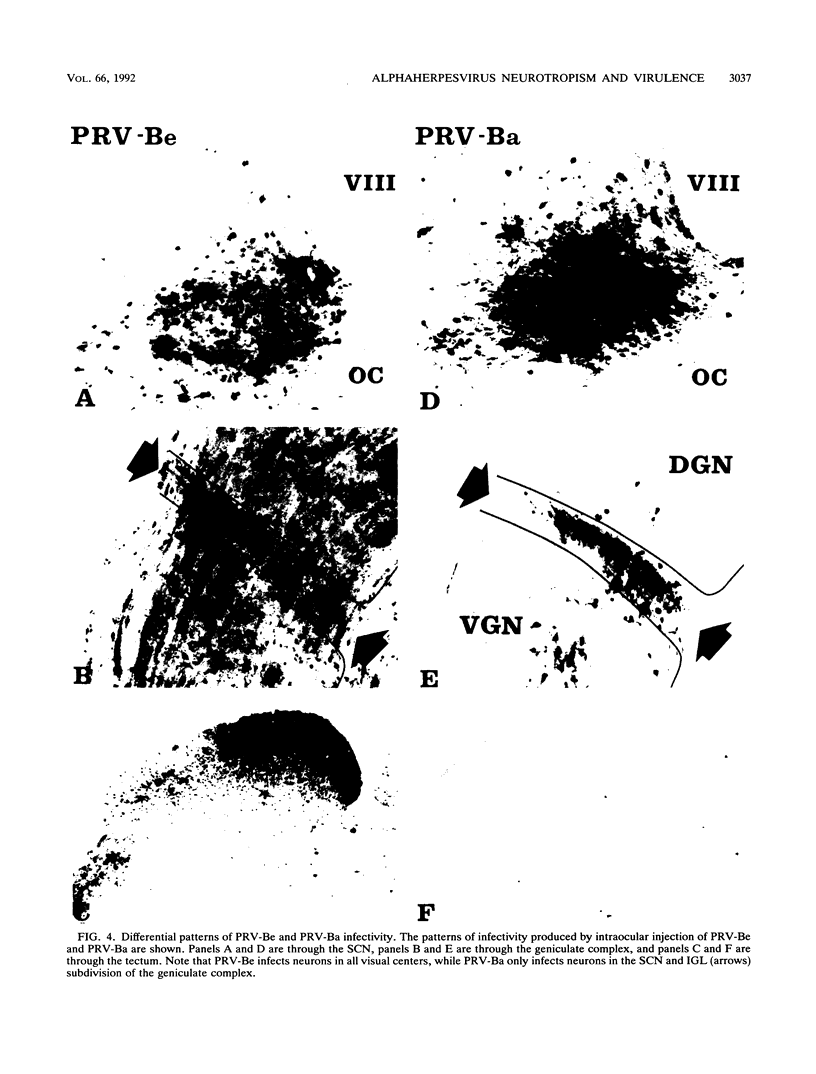
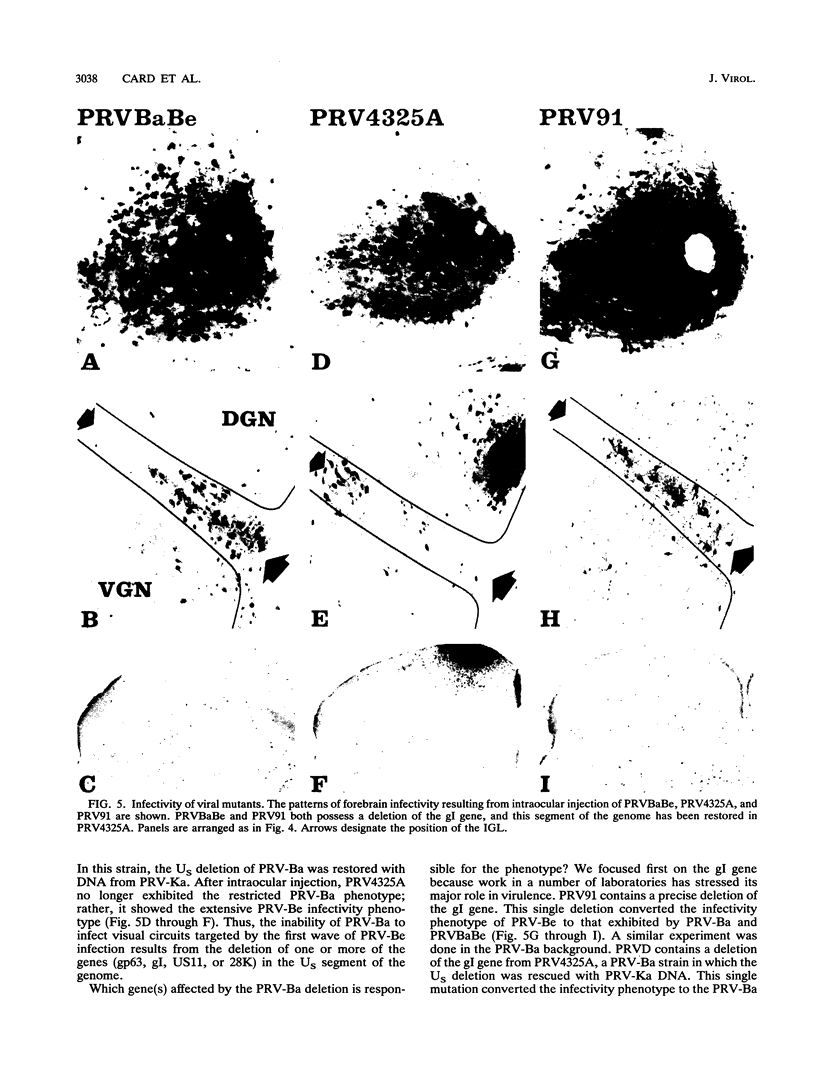
We previously demonstrated that intraocular injections of virulent and attenuated strains of pseudorabies virus (PRV) produce transneuronal infection of functionally distinct central visual circuits in the rat. The virulent Becker strain of PRV induces two temporally separated waves of infection that ultimately target all known retinorecipient neurons; the attenuated Bartha strain only infects a functionally distinct subset of these neurons. In this study, we demonstrate that deletion of a single viral gene encoding glycoprotein gI is sufficient to reproduce both the novel pattern of infectivity and the reduced neurovirulence of the Bartha strain of PRV. Glycoprotein gIII, a major viral membrane protein required for efficient adsorption of virus in cell culture, has no obvious role in determining the pattern of neuronal infectivity, but appears to function with gI to influence neurovirulence. These data suggest that neuroinvasiveness and virulence are the products of an interaction of viral envelope glycoproteins with as yet unidentified cellular receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Card J. P., Rinaman L., Schwaber J. S., Miselis R. R., Whealy M. E., Robbins A. K., Enquist L. W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990 Jun;10(6):1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card J. P., Whealy M. E., Robbins A. K., Moore R. Y., Enquist L. W. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991 Jun;6(6):957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- Dolivo M., Beretta E., Bonifas V., Foroglou C. Ultrastructure and function in sympathetic ganglia isolated from rats infected with pseudorabies virus. Brain Res. 1978 Jan 20;140(1):111–123. doi: 10.1016/0006-8993(78)90241-x. [DOI] [PubMed] [Google Scholar]
- Field H. J., Hill T. J. The pathogenesis of pseudorabies in mice following peripheral inoculation. J Gen Virol. 1974 May;23(2):145–157. doi: 10.1099/0022-1317-23-2-145. [DOI] [PubMed] [Google Scholar]
- Fraser G., Ramachandran S. P. Studies on the virus of Aujeszky's disease. I. Pathogenicity for rats and mice. J Comp Pathol. 1969 Oct;79(4):435–444. doi: 10.1016/0021-9975(69)90063-2. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Blankenship M. L., Ben-Porat T. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J Virol. 1984 Mar;49(3):970–979. doi: 10.1128/jvi.49.3.970-979.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Watanabe S., Ben-Porat T., Kaplan A. S. Genetic basis of the neurovirulence of pseudorabies virus. J Virol. 1984 Oct;52(1):198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Watanabe S., Ben-Porat T., Kaplan A. S. Genome location and identification of functions defective in the Bartha vaccine strain of pseudorabies virus. J Virol. 1987 Mar;61(3):796–801. doi: 10.1128/jvi.61.3.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin X., Dolivo M. Neuronal and transneuronal tracing in the trigeminal system of the rat using the herpes virus suis. Brain Res. 1983 Aug 29;273(2):253–276. doi: 10.1016/0006-8993(83)90850-8. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukàcs N., Rziha H. J. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985 Oct;56(1):307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C. Molecular biology of pseudorabies (Aujeszky's disease) virus. Comp Immunol Microbiol Infect Dis. 1991;14(2):151–163. doi: 10.1016/0147-9571(91)90128-z. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T., Kaplan A. S. Role of glycoprotein gIII of pseudorabies virus in virulence. J Virol. 1988 Aug;62(8):2712–2717. doi: 10.1128/jvi.62.8.2712-2717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Kaplan A. S., Ben-Porat T., Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987 Dec;61(12):4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Zuckermann F., Sugg N., Kern H., Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990 Jan;64(1):278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Gierman T. M., Post L. E. Deletions in vaccine strains of pseudorabies virus and their effect on synthesis of glycoprotein gp63. J Virol. 1986 Dec;60(3):1166–1169. doi: 10.1128/jvi.60.3.1166-1169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Gielkens A., Van Oirschot J., Berns A., Cuypers H. T. Construction and characterization of deletion mutants of pseudorabies virus: a new generation of 'live' vaccines. J Gen Virol. 1987 Feb;68(Pt 2):523–534. doi: 10.1099/0022-1317-68-2-523. [DOI] [PubMed] [Google Scholar]
- Rauh I., Mettenleiter T. C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991 Oct;65(10):5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Ryan J. P., Whealy M. E., Enquist L. W. The gene encoding the gIII envelope protein of pseudorabies virus vaccine strain Bartha contains a mutation affecting protein localization. J Virol. 1989 Jan;63(1):250–258. doi: 10.1128/jvi.63.1.250-258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Whealy M. E., Watson R. J., Enquist L. W. Pseudorabies virus gene encoding glycoprotein gIII is not essential for growth in tissue culture. J Virol. 1986 Sep;59(3):635–645. doi: 10.1128/jvi.59.3.635-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller E. M., Capt M., Dolivo M., De Ribaupierre F. Neuronal organization of the stapedius reflex pathways in the rat: a retrograde HRP and viral transneuronal tracing study. Brain Res. 1989 Jan 2;476(1):21–28. doi: 10.1016/0006-8993(89)91532-1. [DOI] [PubMed] [Google Scholar]
- Rouiller E. M., Capt M., Dolivo M., De Ribaupierre F. Tensor tympani reflex pathways studied with retrograde horseradish peroxidase and transneuronal viral tracing techniques. Neurosci Lett. 1986 Dec 23;72(3):247–252. doi: 10.1016/0304-3940(86)90521-5. [DOI] [PubMed] [Google Scholar]
- Ryan J. P., Whealy M. E., Robbins A. K., Enquist L. W. Analysis of pseudorabies virus glycoprotein gIII localization and modification by using novel infectious viral mutants carrying unique EcoRI sites. J Virol. 1987 Oct;61(10):2962–2972. doi: 10.1128/jvi.61.10.2962-2972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs C., Mettenleiter T. C., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988 Jul;62(7):2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack A. M., Loewy A. D. Pseudorabies virus: a highly specific transneuronal cell body marker in the sympathetic nervous system. J Neurosci. 1990 Jul;10(7):2139–2147. doi: 10.1523/JNEUROSCI.10-07-02139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack A. M., Sawyer W. B., Hughes J. H., Platt K. B., Loewy A. D. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989 Jul 3;491(1):156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Strack A. M., Sawyer W. B., Platt K. B., Loewy A. D. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989 Jul 10;491(2):274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- Zuckermann F. A., Mettenleiter T. C., Schreurs C., Sugg N., Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988 Dec;62(12):4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F., Zsak L., Reilly L., Sugg N., Ben-Porat T. Early interactions of pseudorabies virus with host cells: functions of glycoprotein gIII. J Virol. 1989 Aug;63(8):3323–3329. doi: 10.1128/jvi.63.8.3323-3329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oirschot J. T., Gielkens A. L. Intranasal vaccination of pigs against pseudorabies: absence of vaccinal virus latency and failure to prevent latency of virulent virus. Am J Vet Res. 1984 Oct;45(10):2099–2103. [PubMed] [Google Scholar]