Abstract
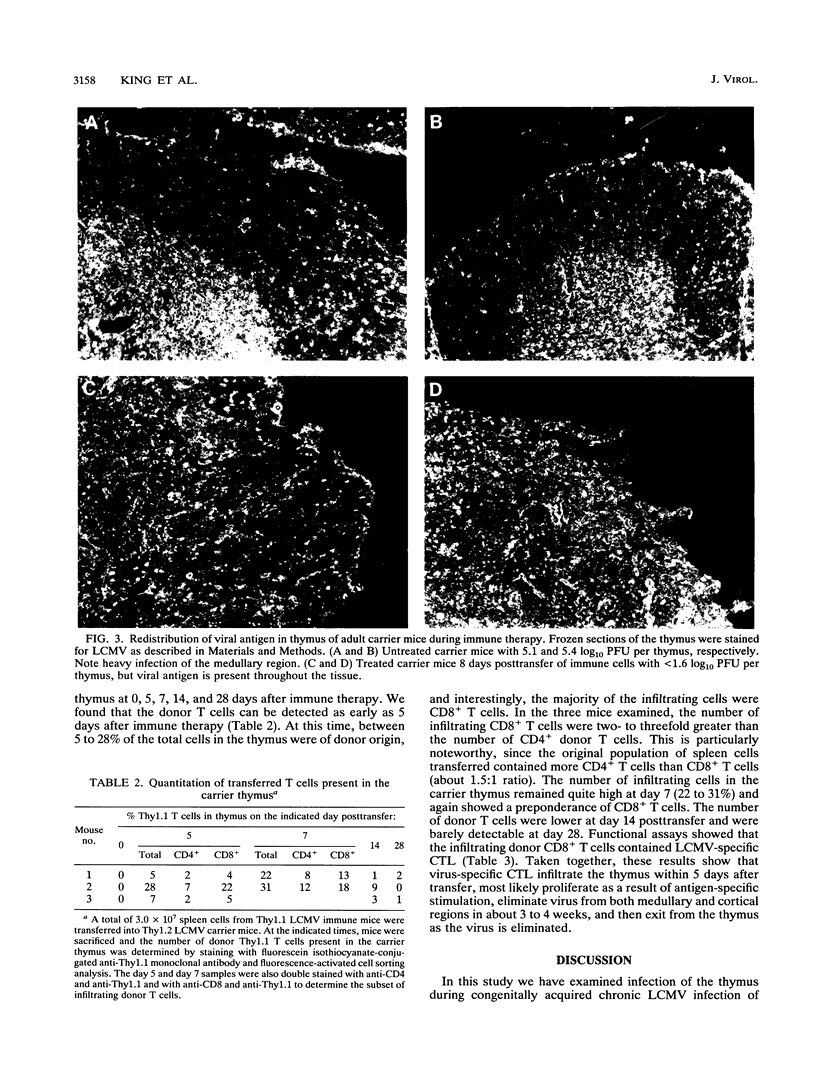
We have examined infection of the thymus during congenitally acquired chronic lymphocytic choriomeningitis virus (LCMV) infection of mice, a classic model of antigen-specific T-cell tolerance. Our results show that (i) infection starts at the fetal stage and is maintained throughout adulthood, and (ii) this chronic infection of the thymus can be eliminated by transfer of virus-specific cytotoxic T lymphocytes (CTL) that infiltrate the thymus and clear all viral products from both medullary and cortical regions. Elimination of virus from the thymus results in abrogation of tolerance. During the fetal stage, the predominant cell type infected is the earliest precursor of T cells with a surface phenotype of Thy1+ CD4- CD8- J11d+. In the adult thymus, infection is confined primarily to the cortisone-resistant thymocytes present in the medullary region. The infected cells are CD4+ and J11d+. The presence of J11d, a marker usually associated with immature thymocytes, on infected single positive CD4+ "mature" thymocytes is intriguing and suggests that infection by this noncytolytic virus may affect development of T cells. There is minimal infection of the CD8+ medullary thymocytes or of the double positive (CD4+ CD8+) cells present in the cortex. Infection within the cortex is confined to the stromal cells. Interestingly, there is infection of the double negative (CD4- CD8-) thymocytes in the adult thymus, showing that even during adulthood the newly developing T cells are susceptible to infection by LCMV. Virus can be eliminated from the thymuses of these carrier mice by adoptive transfer of medullary region first and then from the thymic cortex. This result clearly shows the need to reevaluate the widely held notion that mature T cells are unable to reenter the thymus. In fact, in our experiments the donor T cells made up to 20 to 30% of the total cells in the thymus at 5 to 7 days after the transfer. The number of donor T cells declined as virus was eliminated from the thymus, and at 1 month posttransfer, the donor T cells were hardly detectable. The results of this study examining the dynamics of viral infection and clearance from the thymus, the primary site of T-cell development, have implications for understanding tolerance induction in chronic viral infections.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus D. B., Surh C. D., Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991 May 1;173(5):1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Jamieson B. D., Porter D. D. Immune therapy of a persistent and disseminated viral infection. J Virol. 1987 Dec;61(12):3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., King C. C., Oldstone M. B. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987 May;61(5):1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Moennig V., Horzinek M. C. Recent advances in pestivirus research. J Gen Virol. 1989 Feb;70(Pt 2):253–266. doi: 10.1099/0022-1317-70-2-253. [DOI] [PubMed] [Google Scholar]
- Doyle M. V., Oldstone M. B. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J Immunol. 1978 Oct;121(4):1262–1269. [PubMed] [Google Scholar]
- Fowlkes B. J., Pardoll D. M. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- Gossmann J., Löhler J., Lehmann-Grube F. Entry of antivirally active T lymphocytes into the thymus of virus-infected mice. J Immunol. 1991 Jan 1;146(1):293–297. [PubMed] [Google Scholar]
- Hirokawa K., Makinodan T. Thymic involution: effect on T cell differentiation. J Immunol. 1975 Jun;114(6):1659–1664. [PubMed] [Google Scholar]
- Hirokawa K., Utsuyama M., Sado T. Immunohistological analysis of immigration of thymocyte-precursors into the thymus: evidence for immigration of peripheral T cells into the thymic medulla. Cell Immunol. 1989 Mar;119(1):160–170. doi: 10.1016/0008-8749(89)90232-3. [DOI] [PubMed] [Google Scholar]
- Jamieson B. D., Ahmed R. T-cell tolerance: exposure to virus in utero does not cause a permanent deletion of specific T cells. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2265–2268. doi: 10.1073/pnas.85.7.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson B. D., Butler L. D., Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J Virol. 1987 Dec;61(12):3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson B. D., Somasundaram T., Ahmed R. Abrogation of tolerance to a chronic viral infection. J Immunol. 1991 Nov 15;147(10):3521–3529. [PubMed] [Google Scholar]
- King C. C., de Fries R., Kolhekar S. R., Ahmed R. In vivo selection of lymphocyte-tropic and macrophage-tropic variants of lymphocytic choriomeningitis virus during persistent infection. J Virol. 1990 Nov;64(11):5611–5616. doi: 10.1128/jvi.64.11.5611-5616.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostoff J. M., Nakada M. T., Faas S. J., Blank K. J., Gaulton G. N. Neonatal exposure to thymotropic gross murine leukemia virus induces virus-specific immunologic nonresponsiveness. J Exp Med. 1990 Dec 1;172(6):1765–1775. doi: 10.1084/jem.172.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M. HIV-1: the infective process in vivo. Cell. 1991 Jan 25;64(2):351–363. doi: 10.1016/0092-8674(91)90644-e. [DOI] [PubMed] [Google Scholar]
- Michie S. A., Kirkpatrick E. A., Rouse R. V. Rare peripheral T cells migrate to and persist in normal mouse thymus. J Exp Med. 1988 Nov 1;168(5):1929–1934. doi: 10.1084/jem.168.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Jones J. E., Hughes J. L., Price J., Raney A. K., McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A. 1990 Sep;87(17):6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Osoba D. Current concepts of the immunological function of the thymus. Physiol Rev. 1967 Jul;47(3):437–520. doi: 10.1152/physrev.1967.47.3.437. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Ben-Nun A., Holoshitz J., Reshef T., Frenkel A., Rosenberg M., Cohen I. R. T lymphocyte lines producing or vaccinating against autoimmune encephalomyelitis (EAE). Functional activation induces peanut agglutinin receptors and accumulation in the brain and thymus of line cells. Eur J Immunol. 1983 May;13(5):418–423. doi: 10.1002/eji.1830130513. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Holoshitz J., Eisenstein S., Reshef T., Rappaport S., Chemke J., Ben-Nun A., Cohen I. R. Effector T lymphocyte line cells migrate to the thymus and persist there. Nature. 1982 Nov 18;300(5889):262–264. doi: 10.1038/300262a0. [DOI] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., Zinkernagel R. M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989 Nov 30;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Popescu M., Löhler J., Lehmann-Grube F. Infectious lymphocytes in lymphocytic choriomeningitis virus carrier mice. J Gen Virol. 1979 Mar;42(3):481–492. doi: 10.1099/0022-1317-42-3-481. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. V., McGuire K. L., Boyer P. D. Molecular indices of functional competence in developing T cells. Immunol Rev. 1988 Aug;104:29–53. doi: 10.1111/j.1600-065x.1988.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Tishon A., Southern P. J., Oldstone M. B. Virus-lymphocyte interactions. II. Expression of viral sequences during the course of persistent lymphocytic choriomeningitis virus infection and their localization to the L3T4 lymphocyte subset. J Immunol. 1988 Feb 15;140(4):1280–1284. [PubMed] [Google Scholar]
- Watry D., Hedrick J. A., Siervo S., Rhodes G., Lamberti J. J., Lambris J. D., Tsoukas C. D. Infection of human thymocytes by Epstein-Barr virus. J Exp Med. 1991 Apr 1;173(4):971–980. doi: 10.1084/jem.173.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]



