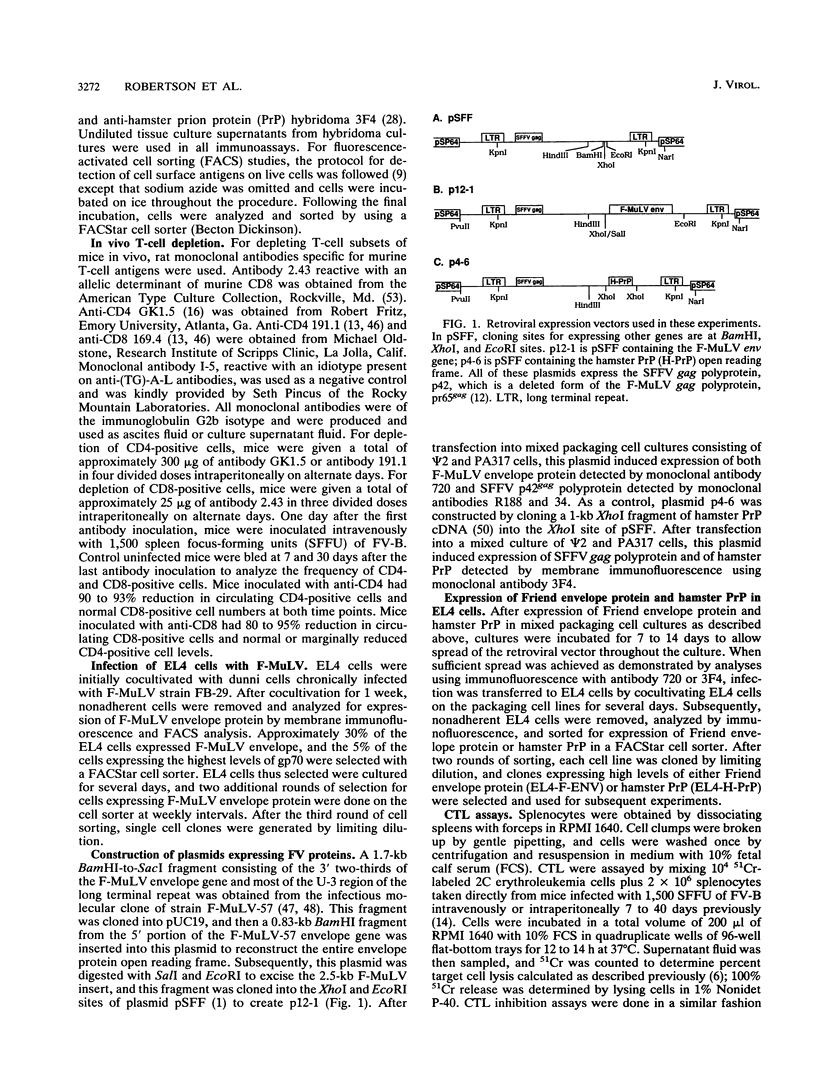
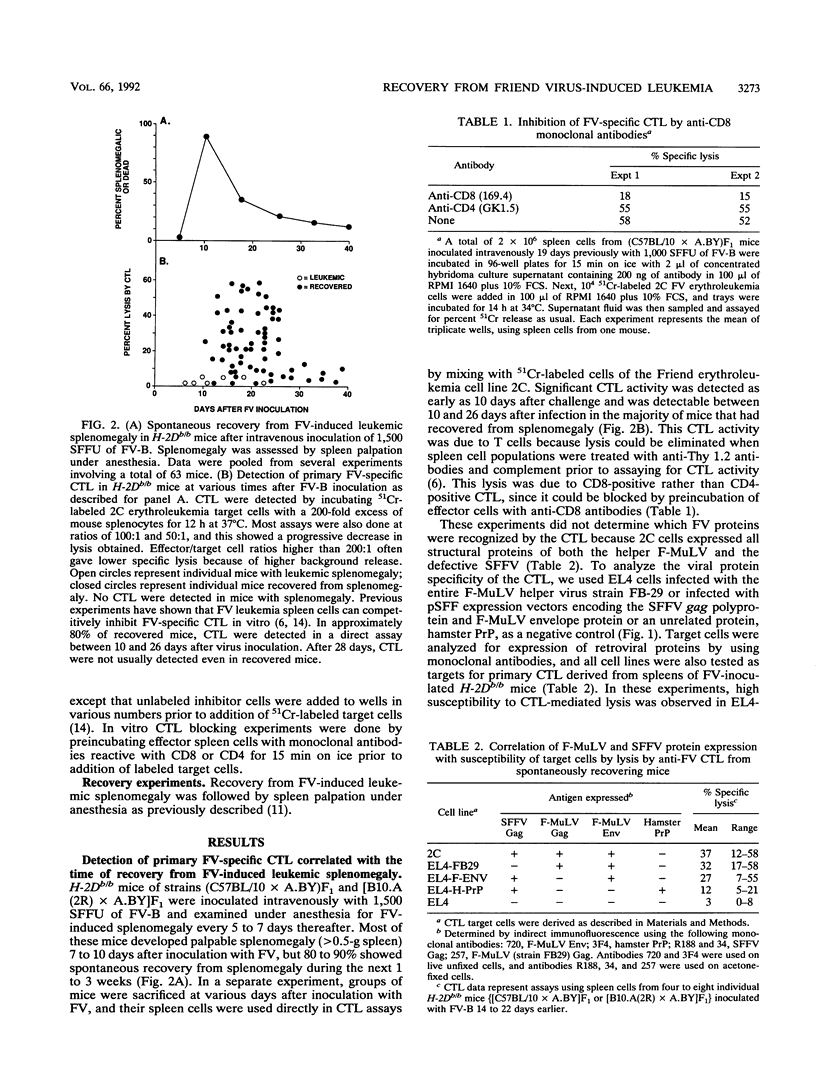
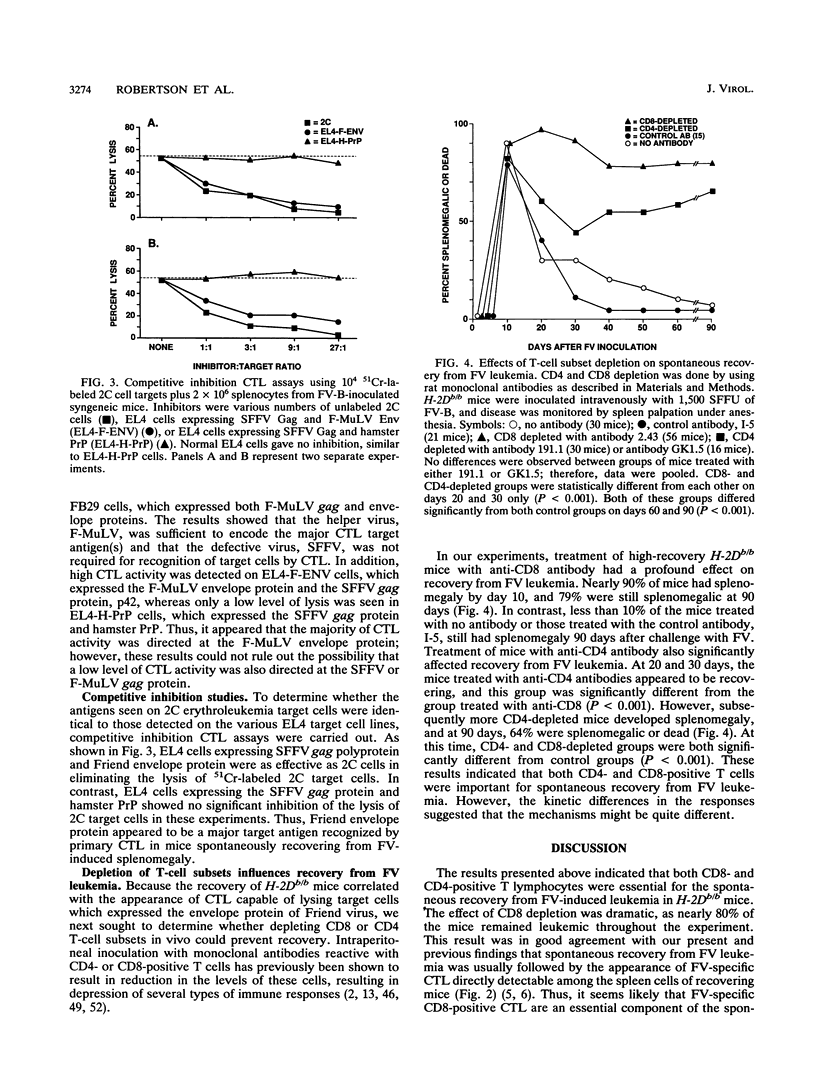
Abstract
Spontaneous recovery from Friend virus complex-induced leukemic splenomegaly in H-2Db/b mice correlated with the appearance of Friend virus complex-specific cytotoxic T lymphocytes (CTL) detectable directly in spleen cell populations. By testing CTL on target cells containing expression vectors encoding individual retroviral structural proteins, the main viral protein recognized was shown to be the Friend murine leukemia helper virus envelope glycoprotein. In vivo depletion of CD8-positive T cells drastically reduced the incidence of recovery, providing direct evidence for the role of CD8-positive CTL in the spontaneous recovery process. In vivo depletion of CD4-positive cells had little effect on the early stages of recovery but did cause a marked reduction in the final incidence of recovery at 60 to 90 days. Thus, CD8-positive cells were required for the initiation of the recovery process, whereas CD4-positive cells appeared to be required for maintenance of the recovered status.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bestwick R. K., Kozak S. L., Kabat D. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5404–5408. doi: 10.1073/pnas.85.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P., Tishon A., Oldstone M. B. Infection of lymphocytes by a virus that aborts cytotoxic T lymphocyte activity and establishes persistent infection. J Exp Med. 1991 Jul 1;174(1):203–212. doi: 10.1084/jem.174.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Chesebro B. H-2D control of recovery from Friend virus leukemia: H-2D region influences the kinetics of the T lymphocyte response to Friend virus. J Exp Med. 1983 Jun 1;157(6):1736–1745. doi: 10.1084/jem.157.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Britt W., Evans L., Wehrly K., Nishio J., Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983 May;127(1):134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Miyazawa M., Britt W. J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Chesebro K., Portis J. Characterization of Ia8 antigen, thy-1.2 antigen, complement receptors, and virus production in a group of murine virus-induced leukemia cell lines. J Immunol. 1976 Oct;117(4):1267–1274. [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Doig D., Nishio J. Antibody-induced modulation of Friend virus cell surface antigens decreases virus production by persistent erythroleukemia cells: influence of the Rfv-3 gene. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5784–5788. doi: 10.1073/pnas.76.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly: mapping of a gene within the major histocompatability complex. J Exp Med. 1974 Dec 1;140(6):1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Studies on the role of the host immune response in recovery from Friend virus leukemia. II. Cell-mediated immunity. J Exp Med. 1976 Jan 1;143(1):85–99. doi: 10.1084/jem.143.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Complete nucleotide sequence of an infectious clone of Friend spleen focus-forming provirus: gp55 is an envelope fusion glycoprotein. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5037–5041. doi: 10.1073/pnas.80.16.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Collins J. K., Britt W. J., Chesebro B. Cytotoxic T lymphocyte recognition of gp70 on Friend virus-induced erythroleukemia cell clones. J Immunol. 1980 Sep;125(3):1318–1324. [PubMed] [Google Scholar]
- Collins J. K., Chesebro B. Spontaneous cessation of Friend murine leukemia virus production by leukemia cell line Y57: overgrowth by nonproducer cells. J Natl Cancer Inst. 1980 May;64(5):1153–1159. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Doig D., Chesebro B. Anti-Friend virus antibody is associated with recovery from viremia and loss of viral leukemia cell-surface antigens in leukemic mice. Identification of Rfv-3 as a gene locus influencing antibody production. J Exp Med. 1979 Jul 1;150(1):10–19. doi: 10.1084/jem.150.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn J. P., McCoy J. L., Fefer A. Cross-resistance to the transplantation of syngeneic Friend, Moloney, and Rauscher virus-induced tumors. Cancer Res. 1968 Mar;28(3):434–439. [PubMed] [Google Scholar]
- Herberman R. B., Aoki T., Nunn M., Lavrin D. H., Soares N., Gazdar A., Holden H., Chang K. S. Specificity of 51Cr-release cytotoxicity of lymphocytes immune to murine sarcoma virus. J Natl Cancer Inst. 1974 Oct;53(4):1103–1111. doi: 10.1093/jnci/53.4.1103. [DOI] [PubMed] [Google Scholar]
- Holt C. A., Osorio K., Lilly F. Friend virus-specific cytotoxic T lymphocytes recognize both gag and env gene-encoded specificities. J Exp Med. 1986 Jul 1;164(1):211–226. doi: 10.1084/jem.164.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsmann G., Schneider J., Schulz A. Immunoprevention of Friend virus-induced erythroleukemia by vaccination with viral envelope glycoprotein complexes. Virology. 1981 Sep;113(2):602–612. doi: 10.1016/0042-6822(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Ishihara C., Miyazawa M., Nishio J., Chesebro B. Induction of protective immunity to Friend murine leukemia virus in genetic nonresponders to virus envelope protein. J Immunol. 1991 Jun 1;146(11):3958–3963. [PubMed] [Google Scholar]
- Jennings S. R., Bonneau R. H., Smith P. M., Wolcott R. M., Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991 Mar;133(1):234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast W. M., Bronkhorst A. M., de Waal L. P., Melief C. J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med. 1986 Sep 1;164(3):723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarnet J. P., Kern D. E., Okuno K., Holt C., Lilly F., Greenberg P. D. FBL-reactive CD8+ cytotoxic and CD4+ helper T lymphocytes recognize distinct Friend murine leukemia virus-encoded antigens. J Exp Med. 1989 Feb 1;169(2):457–467. doi: 10.1084/jem.169.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiser C., Schneider J., Bayer H., Hunsmann G. Immunoprevention of Friend leukaemia virus-induced erythroleukaemia by vaccination with aggregated gp70. J Gen Virol. 1986 Sep;67(Pt 9):1901–1907. doi: 10.1099/0022-1317-67-9-1901. [DOI] [PubMed] [Google Scholar]
- Kolaitis G., Doymaz M., Rouse B. T. Demonstration of MHC class II-restricted cytotoxic T lymphocytes in mice against herpes simplex virus. Immunology. 1990 Sep;71(1):101–106. [PMC free article] [PubMed] [Google Scholar]
- Lander M. R., Chattopadhyay S. K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ectropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984 Nov;52(2):695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc J. C., Gomard E., Levy J. P. Cell-mediated reaction against tumors induced by oncornaviruses. I. Kinetics and specificity of the immune response in murine sarcoma virus (MSV)-induced tumors and transplanted lymphomas. Int J Cancer. 1972 Nov;10(3):589–601. doi: 10.1002/ijc.2910100318. [DOI] [PubMed] [Google Scholar]
- Lilly F., Steeves R. A. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV). Virology. 1973 Oct;55(2):363–370. doi: 10.1016/0042-6822(73)90176-1. [DOI] [PubMed] [Google Scholar]
- Lilly F. The effect of histocompatibility-2 type on response to friend leukemia virus in mice. J Exp Med. 1968 Mar 1;127(3):465–473. doi: 10.1084/jem.127.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A. E., Morrison L. A., Braciale V. L., Malissen B., Braciale T. J. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985 Jul 1;162(1):171–187. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Chesebro B. Genetic control of T cell responsiveness to the Friend murine leukemia virus envelope antigen. Identification of class II loci of the H-2 as immune response genes. J Exp Med. 1988 Nov 1;168(5):1587–1605. doi: 10.1084/jem.168.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Wehrly K., Chesebro B. Influence of MHC genes on spontaneous recovery from Friend retrovirus-induced leukemia. J Immunol. 1992 Jan 15;148(2):644–647. [PubMed] [Google Scholar]
- Mizuochi T., Hügin A. W., Morse H. C., 3rd, Singer A., Buller R. M. Role of lymphokine-secreting CD8+ T cells in cytotoxic T lymphocyte responses against vaccinia virus. J Immunol. 1989 Jan 1;142(1):270–273. [PubMed] [Google Scholar]
- Morrison R. P., Earl P. L., Nishio J., Lodmell D. L., Moss B., Chesebro B. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature. 1987 Oct 22;329(6141):729–732. doi: 10.1038/329729a0. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Nishio J., Chesebro B. Influence of the murine MHC (H-2) on Friend leukemia virus-induced immunosuppression. J Exp Med. 1986 Feb 1;163(2):301–314. doi: 10.1084/jem.163.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Linemeyer D., Ruscetti S., Lowe R., Lowy D. R., Scolnick E. Subgenomic fragment of molecular cloned Friend murine leukemia virus DNA contains the gene(s) responsible for Friend murine leukemia virus-induced disease. J Virol. 1980 Sep;35(3):924–936. doi: 10.1128/jvi.35.3.924-936.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. L., Lodmell D. L. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J Virol. 1991 Jul;65(7):3429–3434. doi: 10.1128/jvi.65.7.3429-3434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis N. K., Sawh P. R., Wolfe G. C., Rubenstein R., Carp R. I., Innis M. A. Isolation of a cDNA clone encoding the leader peptide of prion protein and expression of the homologous gene in various tissues. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6377–6381. doi: 10.1073/pnas.83.17.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. N., Miyazawa M., Mori S., Caughey B., Evans L. H., Hayes S. F., Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J Virol Methods. 1991 Oct;34(3):255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988 May 1;140(9):2950–2955. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986 Dec 26;47(6):851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- Tite J. P. Evidence of a role for TNF-alpha in cytolysis by CD4+, class II MHC-restricted cytotoxic T cells. Immunology. 1990 Oct;71(2):208–212. [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Ruscetti S. K., Scolnick E. M. The molecular biology of Friend virus. Biochim Biophys Acta. 1980 Sep 22;605(3):305–324. doi: 10.1016/0304-419x(80)90014-1. [DOI] [PubMed] [Google Scholar]


