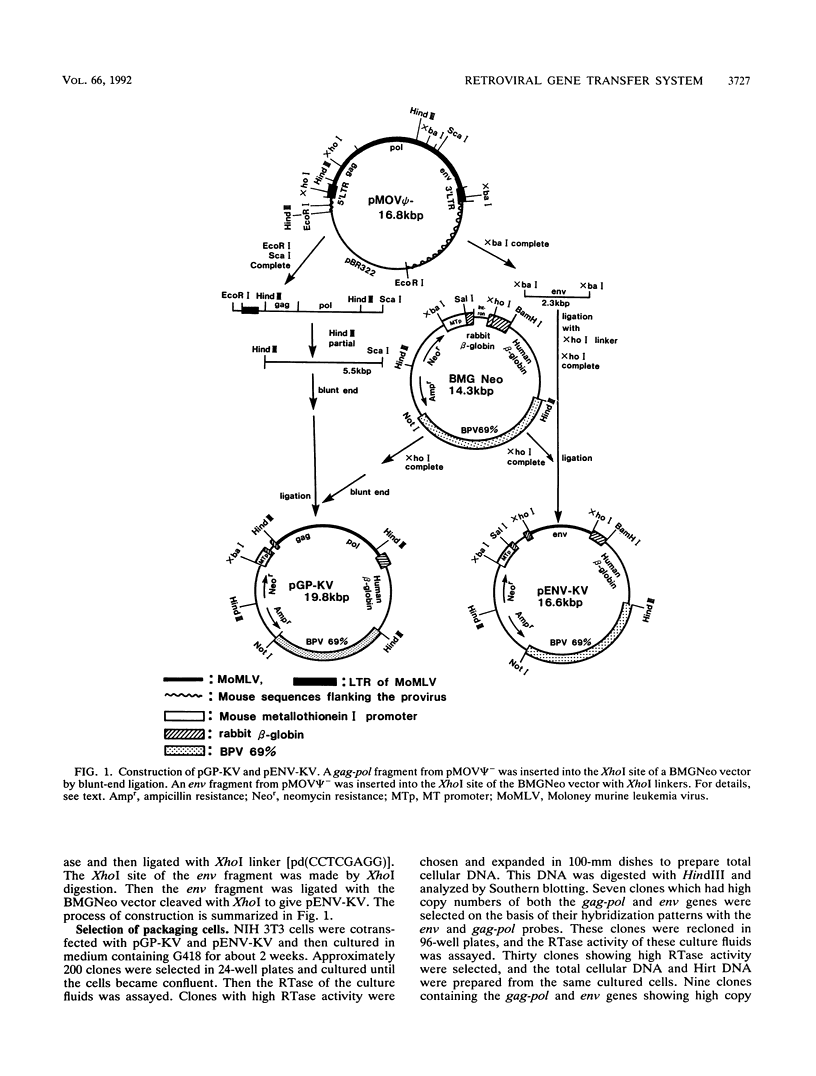
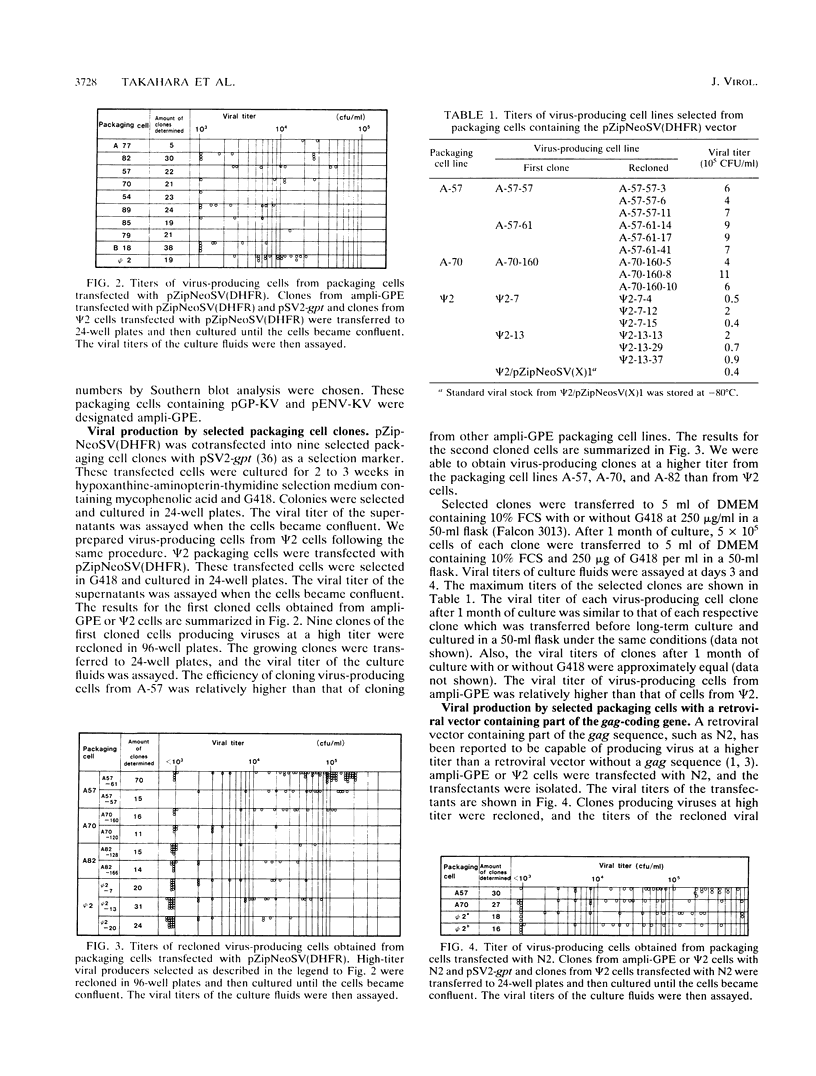
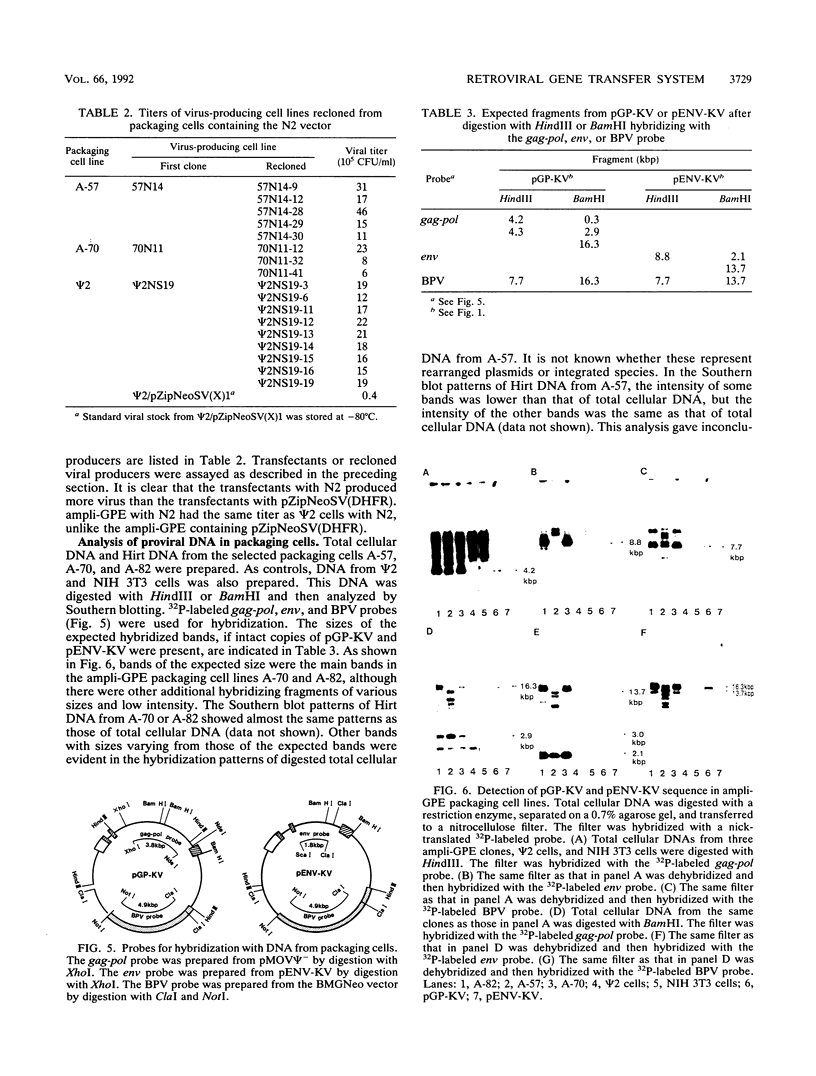
Abstract
The retroviral gene transfer system is a powerful tool for somatic gene therapy. A retroviral stock with a high viral titer and lacking replication-competent virus (RCV) is desirable for this type of gene transfer. To fulfill these requirements, we made a new packaging cell line, designated ampli-GPE. To reduce the homology between proviral DNA in the packaging cell and retroviral vector, the gag-pol and env genes of Moloney murine leukemia virus were separated onto two different plasmids, pGP-KV and pENV-KV, respectively, in which the 5' long terminal repeat and the 3' long terminal repeat had been replaced by the mouse metallothionein I promoter or the human beta-globin gene containing the polyadenylation site as control units for the gag-pol and env genes. In addition, these plasmids contained 69% of the bovine papillomavirus gene for gene amplification to obtain production of virus at a high titer. NIH 3T3 clones containing approximately 20 to 50 copies of the gag-pol and env genes were selected and designated ampli-GPE. When ampli-GPE was transfected with the N2 vector or pZipNeoSV(DHFR) derived from pZipNeoSV(X)1, we established clones producing titers of 5 x 10(6) and 1 x 10(6) CFU/ml, respectively. There was no sign of RCV generation in any virus-producing cells from ampli-GPE. However, virus-producing cells derived from psi 2 cells transfected with N2 did generate RCV. Thus, we showed that ampli-GPE, possessing the minimum complement of proviral genes, has potential for the development of a gene transfer system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Gelinas R. E., Miller A. D. A majority of mice show long-term expression of a human beta-globin gene after retrovirus transfer into hematopoietic stem cells. Mol Cell Biol. 1989 Apr;9(4):1426–1434. doi: 10.1128/mcb.9.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., McDonagh K. T., Brandt S. J., Ney P. A., Agricola B., Byrne E., Nienhuis A. W. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., Hsu R. Y., Bruszewski J., Hu S., Martin F., Nicolson M. Replication-defective chimeric helper proviruses and factors affecting generation of competent virus: expression of Moloney murine leukemia virus structural genes via the metallothionein promoter. Mol Cell Biol. 1987 May;7(5):1797–1806. doi: 10.1128/mcb.7.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey C. A., DeSilva A. D., Holland C. A., Williams D. A. Serial transplantation of methotrexate-resistant bone marrow: protection of murine recipients from drug toxicity by progeny of transduced stem cells. Blood. 1990 Jan 15;75(2):337–343. [PubMed] [Google Scholar]
- Correll P. H., Fink J. K., Brady R. O., Perry L. K., Karlsson S. Production of human glucocerebrosidase in mice after retroviral gene transfer into multipotential hematopoietic progenitor cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8912–8916. doi: 10.1073/pnas.86.22.8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P. W., Jolly J. D., Jones J. B., Anderson W. F., Lothrop C. D., Jr Gene transfer into hematopoietic progenitor cells from normal and cyclic hematopoietic dogs using retroviral vectors. Blood. 1988 Mar;71(3):717–722. [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P. W., Gillio A. P., McLachlin J. R., Bordignon C., Eglitis M. A., Kernan N. A., Moen R. C., Kohn D. B., Yu S. F., Karson E. Expression of human adenosine deaminase in nonhuman primates after retrovirus-mediated gene transfer. J Exp Med. 1987 Jul 1;166(1):219–234. doi: 10.1084/jem.166.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Bodine D. M., Perry L., Papayannopoulou T., Nienhuis A. W. Expression of the human beta-globin gene following retroviral-mediated transfer into multipotential hematopoietic progenitors of mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6062–6066. doi: 10.1073/pnas.85.16.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Laneuville P., Chang W., Kamel-Reid S., Fauser A. A., Dick J. E. High-efficiency gene transfer and expression in normal human hematopoietic cells with retrovirus vectors. Blood. 1988 Mar;71(3):811–814. [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lim B., Apperley J. F., Orkin S. H., Williams D. A. Long-term expression of human adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8892–8896. doi: 10.1073/pnas.86.22.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B., Williams D. A., Orkin S. H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol Cell Biol. 1987 Oct;7(10):3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. M., Miller A. D. Production of high-titer helper virus-free retroviral vectors by cocultivation of packaging cells with different host ranges. J Virol. 1991 Jul;65(7):3887–3890. doi: 10.1128/jvi.65.7.3887-3890.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead R. B., Kwok W. W., Storb R., Miller A. D. Canine model for gene therapy: inefficient gene expression in dogs reconstituted with autologous marrow infected with retroviral vectors. Blood. 1988 Mar;71(3):742–747. [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- Yang Z., Korman A. J., Cooper J., Pious D., Accolla R. S., Mulligan R. C., Strominger J. L. Expression of HLA-DR antigen in human class II mutant B-cell lines by double infection with retrovirus vectors. Mol Cell Biol. 1987 Nov;7(11):3923–3928. doi: 10.1128/mcb.7.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]