Abstract
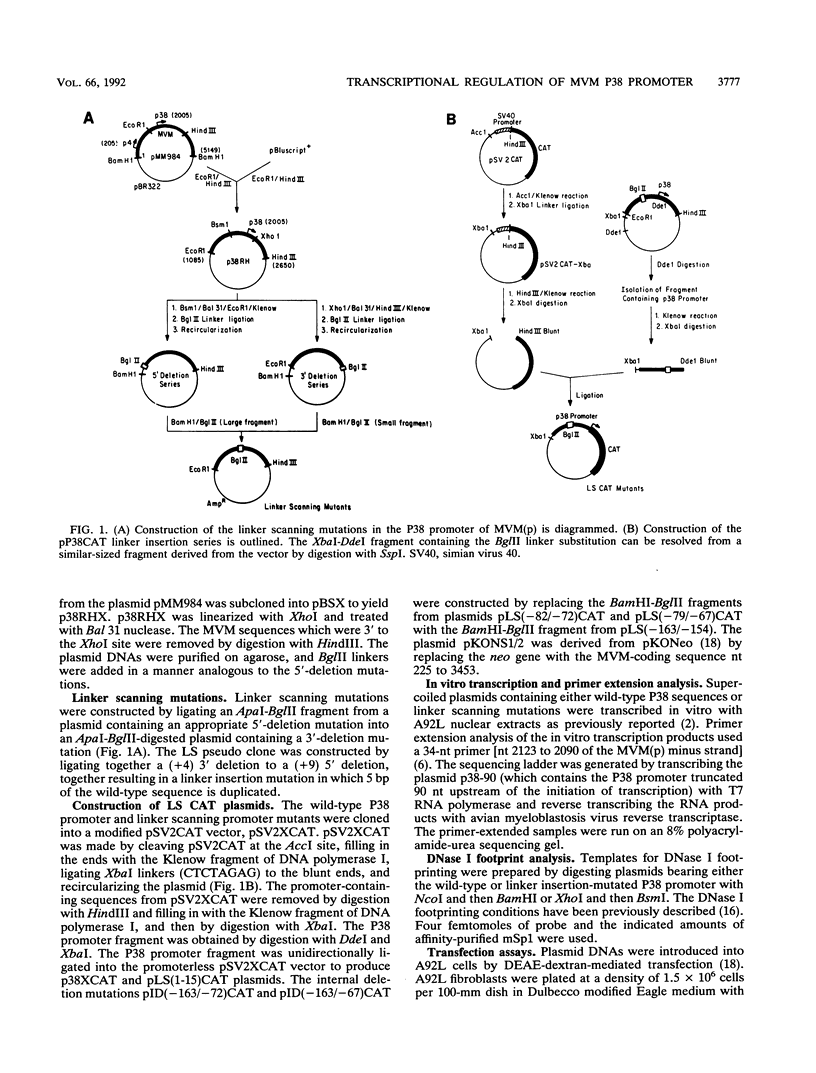
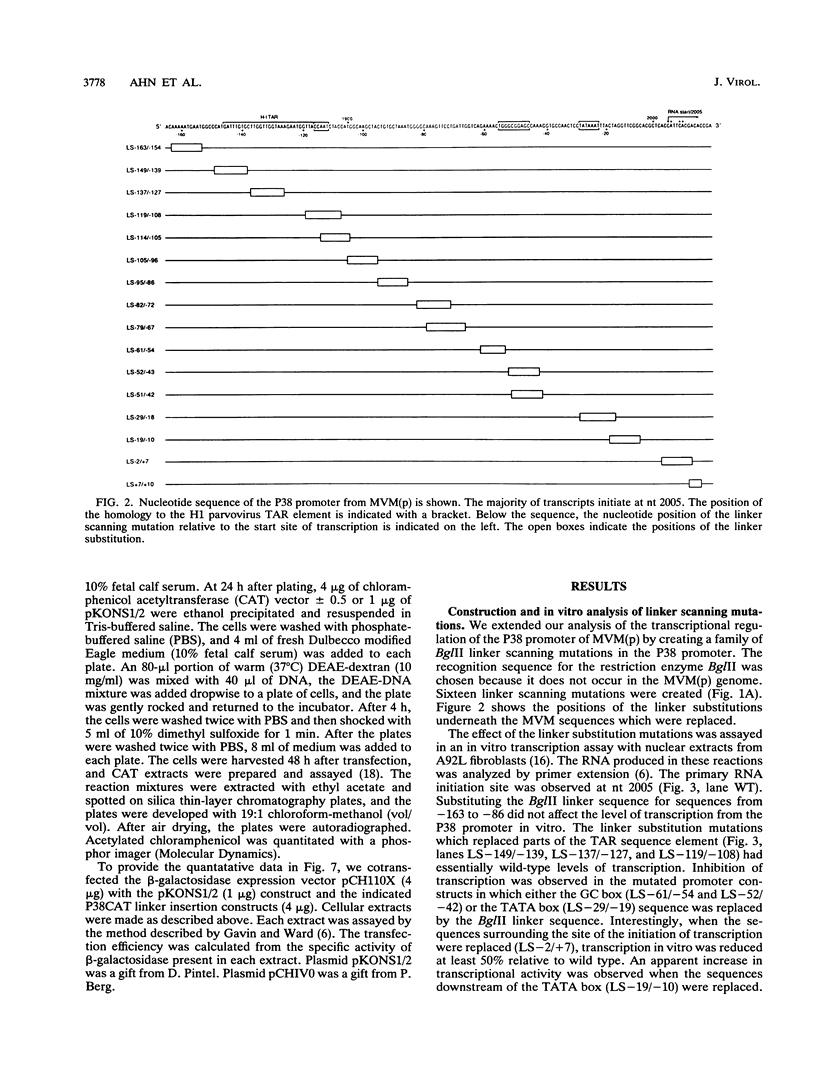
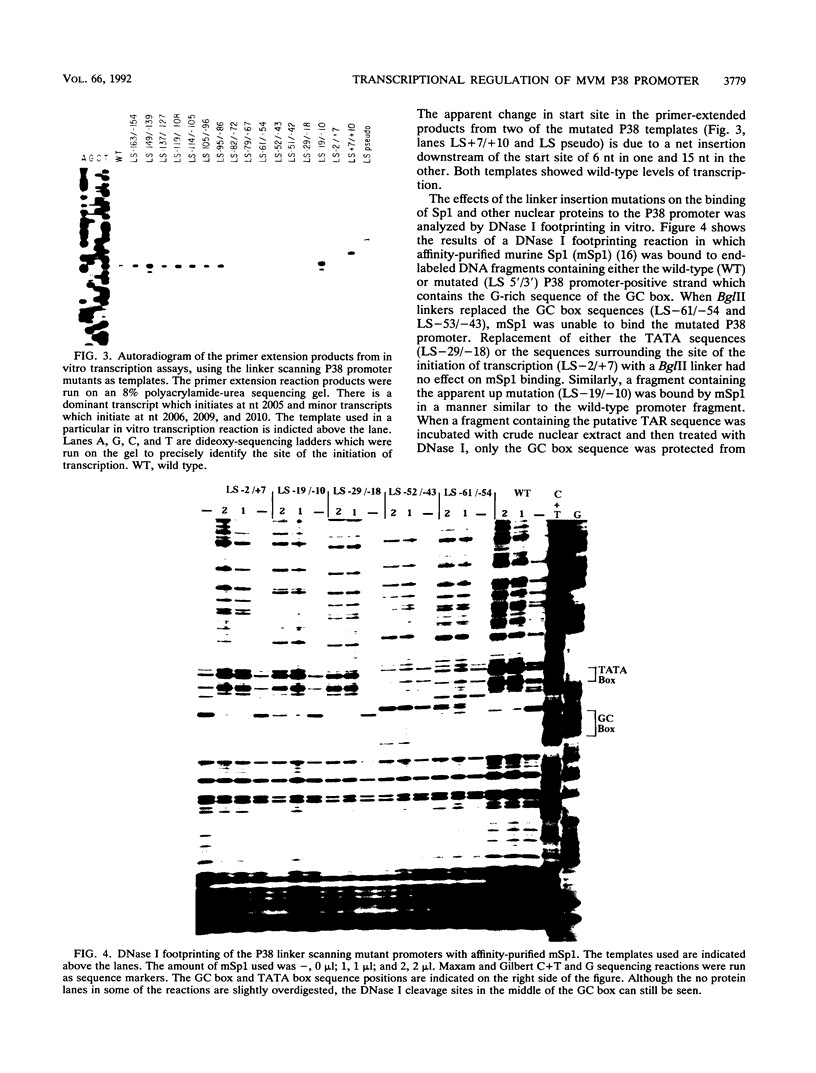
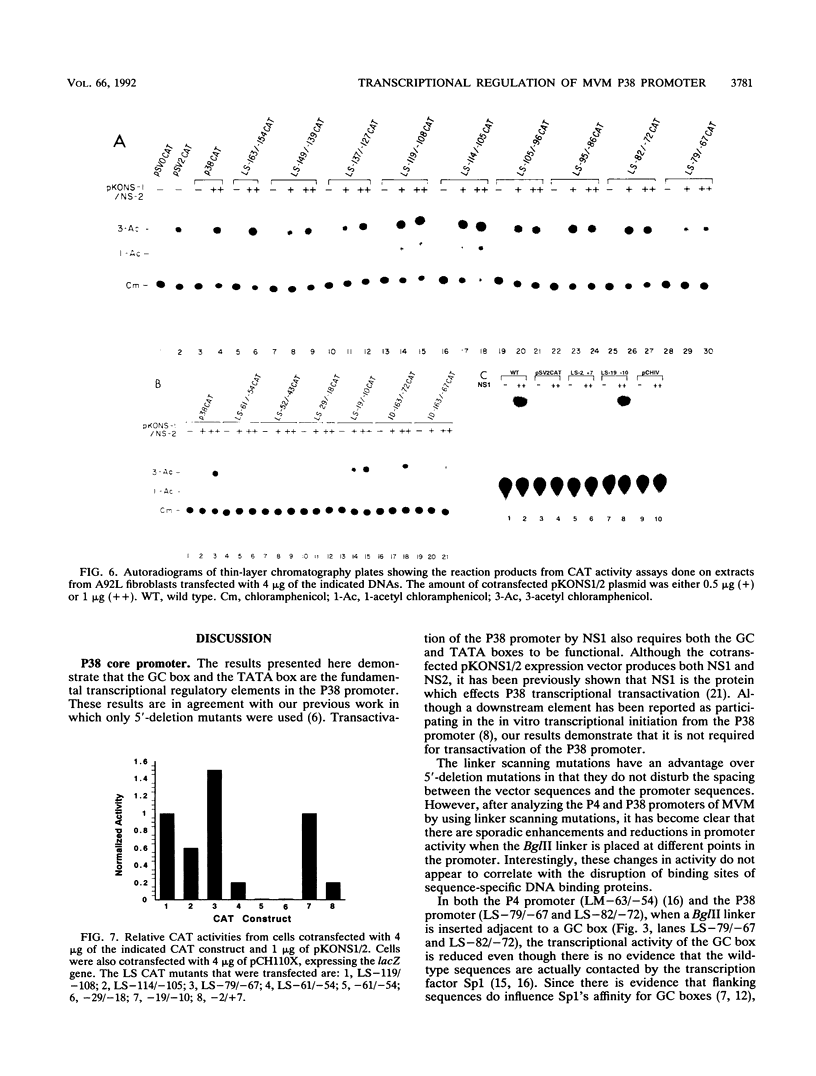
To further define the transcriptional regulation of the P38 promoter in the minute virus of mice (MVM) genome, we constructed a series of internal deletion and linker scanning mutations. The mutant P38 constructs were assayed for transcriptional activity in vitro by primer extension analysis with nuclear extracts from murine A92L fibroblasts. Mutations which disrupted the GC box and TATA box severely reduced transcription in vitro. DNase I footprinting analysis confirmed that the murine transcription factor Sp1 bound to the GC box; however, no factors were observed interacting with a putative transcriptional activation regulatory element, termed the TAR element. The linker scanning mutations were analyzed in vivo by using a chloramphenicol acetyltransferase expression assay system, in both the presence and absence of constructs expressing the viral nonstructural protein, NS1. The ability of NS1 to transactivate the P38 promoter (up to 1,000-fold) depended entirely on the presence of intact GC and TATA box sequences. Disruption of the TAR element by either linker insertion mutations or an internal deletion did not inhibit transactivation of the P38 promoter. These results suggest that NS1 transactivates the P38 promoter indirectly by interacting with one or more components of the P38 core-transcription complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn J. K., Gavin B. J., Kumar G., Ward D. C. Transcriptional analysis of minute virus of mice P4 promoter mutants. J Virol. 1989 Dec;63(12):5425–5439. doi: 10.1128/jvi.63.12.5425-5439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Perros M., Brandenburger A., Spegelaere P., Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990 Sep;9(9):2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin B. J., Ward D. C. Positive and negative regulation of the minute virus of mice P38 promoter. J Virol. 1990 May;64(5):2057–2063. doi: 10.1128/jvi.64.5.2057-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höller M., Westin G., Jiricny J., Schaffner W. Sp1 transcription factor binds DNA and activates transcription even when the binding site is CpG methylated. Genes Dev. 1988 Sep;2(9):1127–1135. doi: 10.1101/gad.2.9.1127. [DOI] [PubMed] [Google Scholar]
- Krauskopf A., Resnekov O., Aloni Y. A cis downstream element participates in regulation of in vitro transcription initiation from the P38 promoter of minute virus of mice. J Virol. 1990 Jan;64(1):354–360. doi: 10.1128/jvi.64.1.354-360.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Hai T. Y., SivaRaman L., Thimmappaya B., Hurst H. C., Jones N. C., Green M. R. A cellular protein, activating transcription factor, activates transcription of multiple E1A-inducible adenovirus early promoters. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8355–8359. doi: 10.1073/pnas.84.23.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Rhode S. L., 3rd Mutation of lysine 405 to serine in the parvovirus H-1 NS1 abolishes its functions for viral DNA replication, late promoter trans activation, and cytotoxicity. J Virol. 1990 Oct;64(10):4654–4660. doi: 10.1128/jvi.64.10.4654-4660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Interaction of a common cellular transcription factor, ATF, with regulatory elements in both E1a- and cyclic AMP-inducible promoters. Proc Natl Acad Sci U S A. 1988 May;85(10):3396–3400. doi: 10.1073/pnas.85.10.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Wold B., Dervan P. B. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science. 1989 Aug 18;245(4919):725–730. doi: 10.1126/science.2549631. [DOI] [PubMed] [Google Scholar]
- Means A. L., Farnham P. J. Transcription initiation from the dihydrofolate reductase promoter is positioned by HIP1 binding at the initiation site. Mol Cell Biol. 1990 Feb;10(2):653–661. doi: 10.1128/mcb.10.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitluk Z. W., Ward D. C. Unusual Sp1-GC box interaction in a parvovirus promoter. J Virol. 1991 Dec;65(12):6661–6670. doi: 10.1128/jvi.65.12.6661-6670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Richard S. M. Characterization of the trans-activation-responsive element of the parvovirus H-1 P38 promoter. J Virol. 1987 Sep;61(9):2807–2815. doi: 10.1128/jvi.61.9.2807-2815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Ciesiolka T., Gruss P. A nexus between Oct-4 and E1A: implications for gene regulation in embryonic stem cells. Cell. 1991 Jul 26;66(2):291–304. doi: 10.1016/0092-8674(91)90619-a. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis G. E., Labieniec-Pintel L., Clemens K. E., Pintel D. Generation and characterization of a temperature-sensitive mutation in the NS-1 gene of the autonomous parvovirus minute virus of mice. J Virol. 1988 Aug;62(8):2736–2744. doi: 10.1128/jvi.62.8.2736-2744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]