Abstract
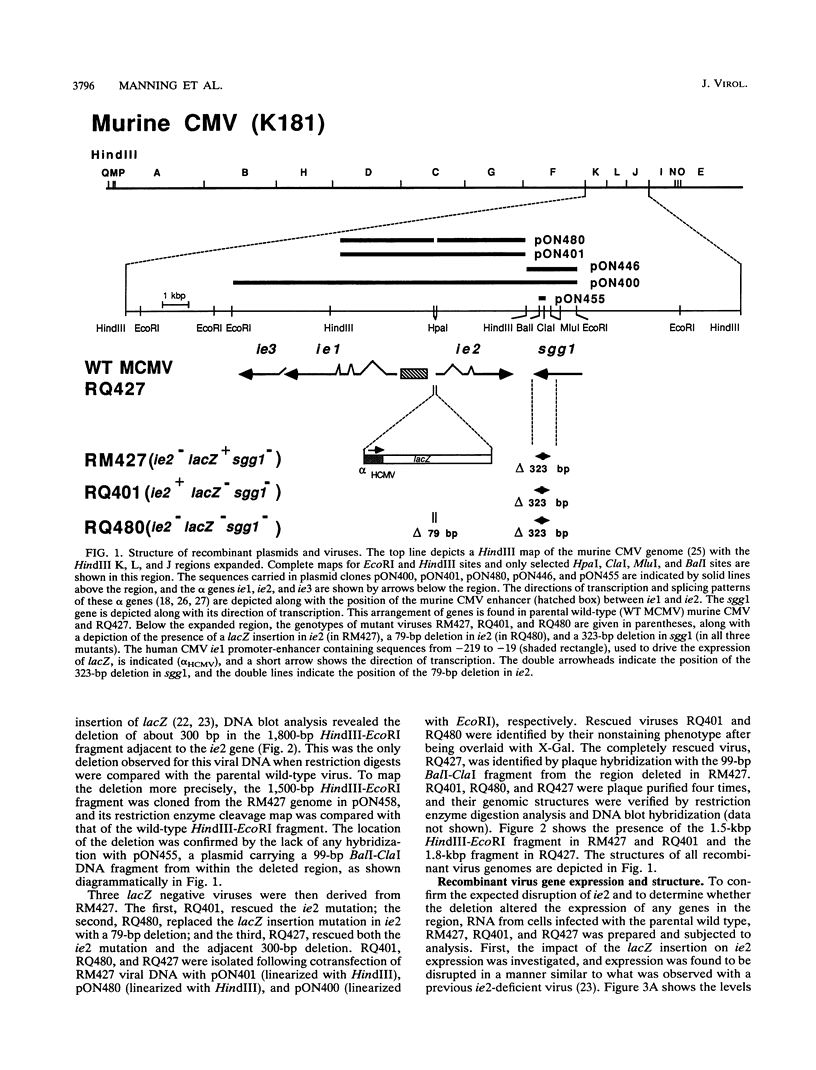
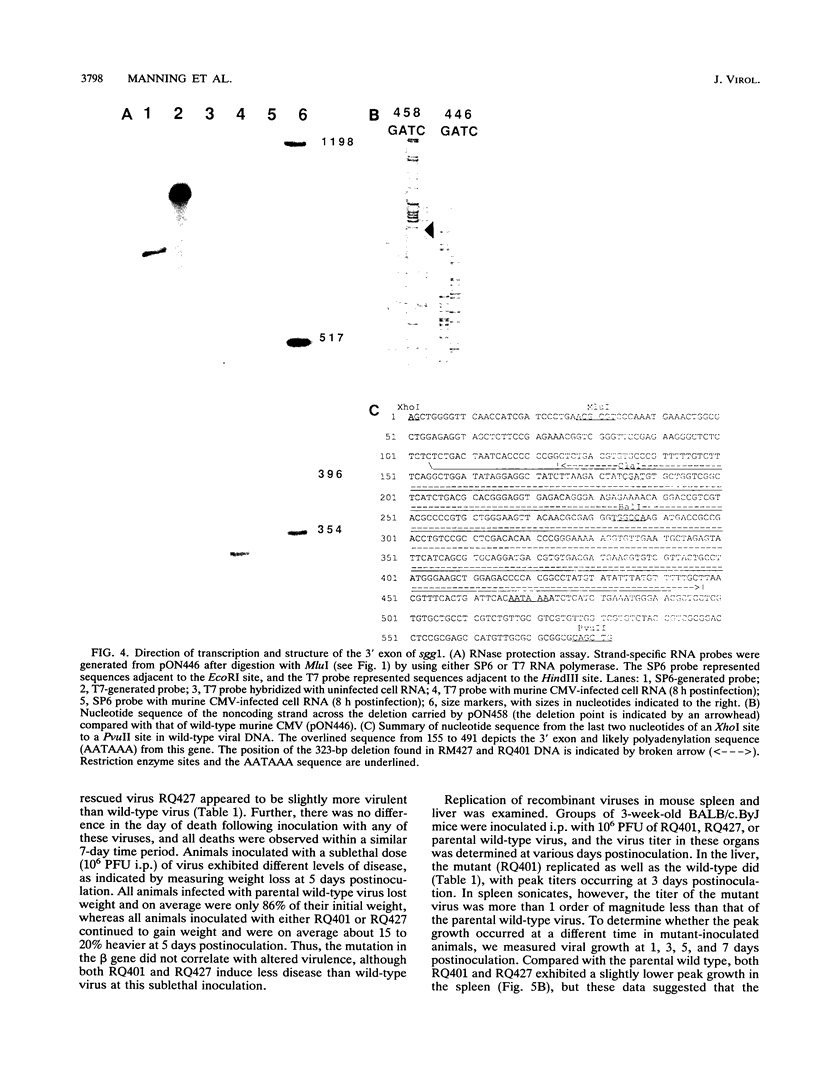
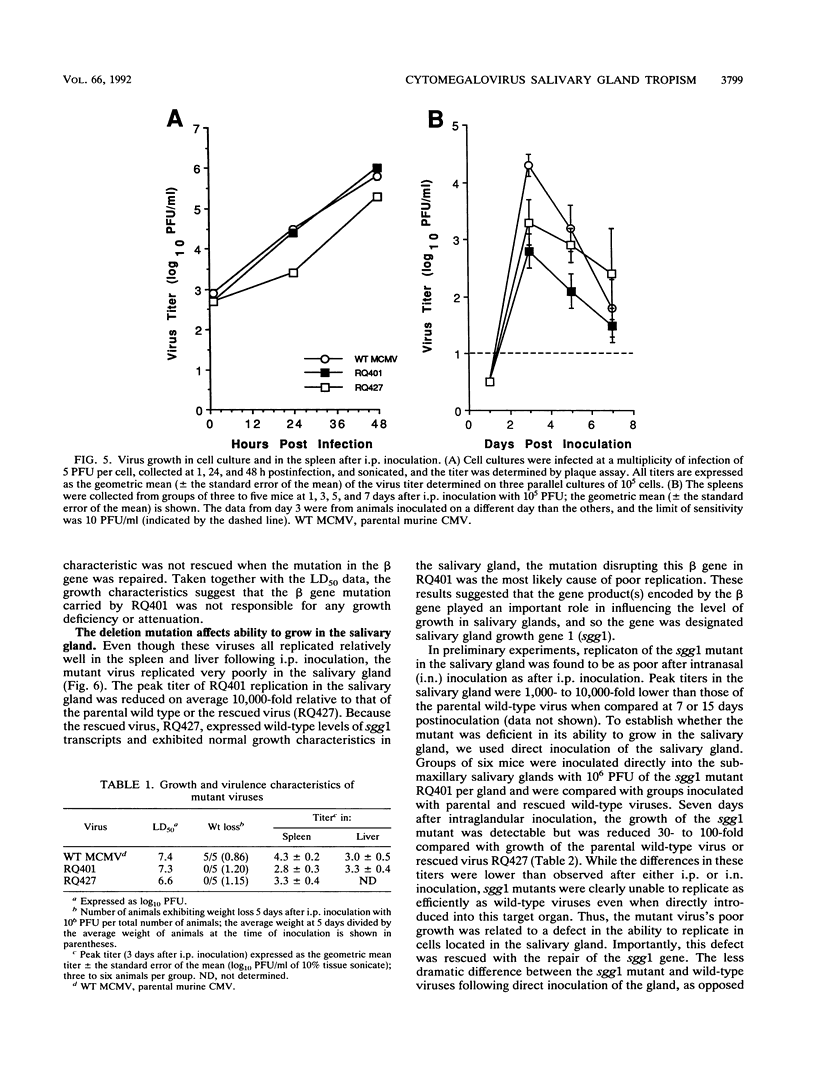
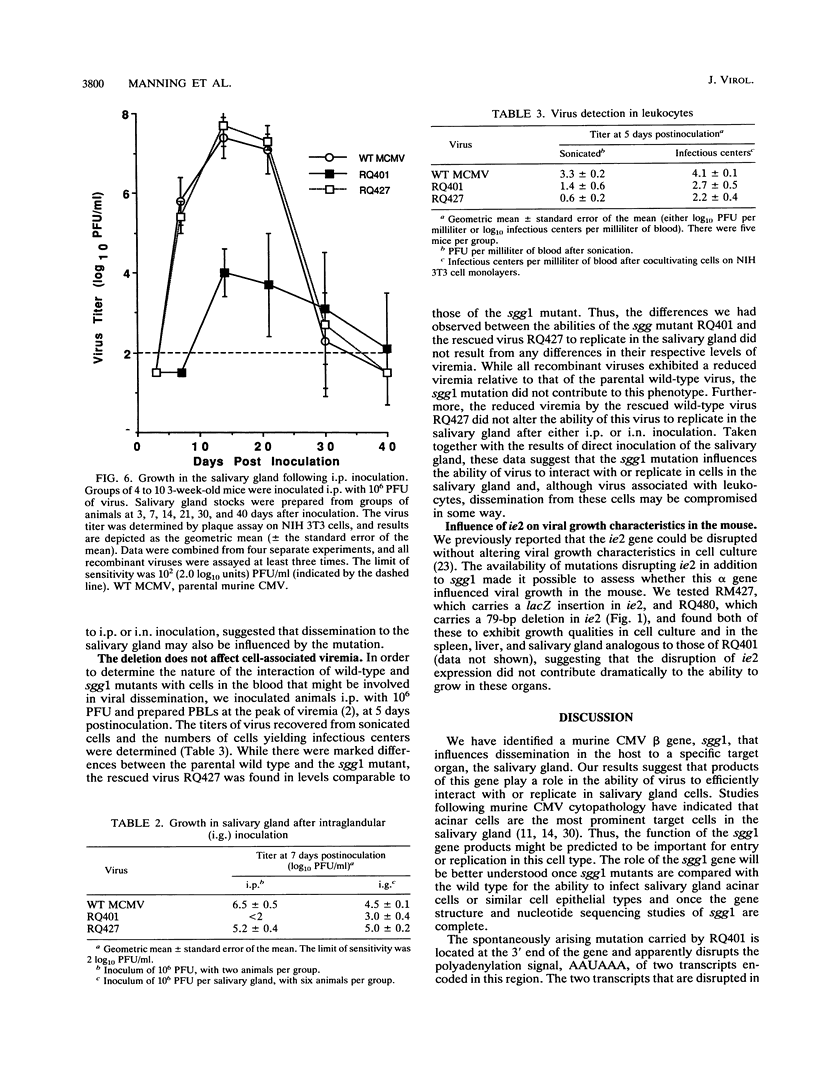
Murine cytomegalovirus carrying a deletion mutation disrupting the expression of a gene dispensable for growth in cultured cells was found to disseminate poorly in the mouse. The mutation resulted in a dramatic decrease in the expression of a 1.5-kb major and a 1.8-kb minor beta transcript from a region adjacent to the ie2 gene in the viral genome. Nucleotide sequence determination indicated that 323 bp, including a predicted polyadenylation signal, was deleted from this beta gene. In cultured cells, the plaque morphology and growth characteristics of the mutant were similar to those of parental or rescued wild-type viruses. Following intraperitoneal inoculation of BALB/c mice, growth of the mutant in the salivary gland was dramatically reduced 10,000-fold, while growth in the liver and spleen was not dramatically affected. The beta gene was thus denoted sgg1 (salivary gland growth gene 1). Neither intranasal infection nor direct inoculation into the salivary glands completely overcame the restriction of growth in this organ, suggesting that the sgg1 gene encoded a determinant of tissue tropism. To investigate the impact of the sgg1 mutation on virus dissemination via the blood, the virus titer in peripheral blood leukocytes was determined. No difference was found between the sgg1 mutant and rescued wild-type virus. Thus, murine cytomegalovirus sgg1 gene products appear to be involved in entry or replication of virus in salivary gland cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. E., Shellam G. R. Genetic control of murine cytomegalovirus infection: virus titres in resistant and susceptible strains of mice. Arch Virol. 1984;81(1-2):139–150. doi: 10.1007/BF01309303. [DOI] [PubMed] [Google Scholar]
- Bale J. F., Jr, O'Neil M. E. Detection of murine cytomegalovirus DNA in circulating leukocytes harvested during acute infection of mice. J Virol. 1989 Jun;63(6):2667–2673. doi: 10.1128/jvi.63.6.2667-2673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler B., Keil G. M., Weiland F., Koszinowski U. H. Characterization of the murine cytomegalovirus early transcription unit e1 that is induced by immediate-early proteins. J Virol. 1990 May;64(5):1907–1919. doi: 10.1128/jvi.64.5.1907-1919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmer J. E., Mackenzie J. S., Stanley N. F. Resistance to murine cytomegalovirus linked to the major histocompatibility complex of the mouse. J Gen Virol. 1977 Oct;37(1):107–114. doi: 10.1099/0022-1317-37-1-107. [DOI] [PubMed] [Google Scholar]
- Cherrington J. M., Mocarski E. S. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J Virol. 1989 Mar;63(3):1435–1440. doi: 10.1128/jvi.63.3.1435-1440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Kern E. R., Whitley R. J., Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990 Nov 30;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Dankner W. M., McCutchan J. A., Richman D. D., Hirata K., Spector S. A. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J Infect Dis. 1990 Jan;161(1):31–36. doi: 10.1093/infdis/161.1.31. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late gene expression: gamma genes are controlled by posttranscriptional events. J Virol. 1986 Mar;57(3):864–874. doi: 10.1128/jvi.57.3.864-874.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E., Mackenzie J. S., Stanley N. F. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect Immun. 1981 Apr;32(1):277–286. doi: 10.1128/iai.32.1.277-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson D., Strano A. J. Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am J Pathol. 1972 Jul;68(1):183–202. [PMC free article] [PubMed] [Google Scholar]
- Ho D. Y., Mocarski E. S. Herpes simplex virus latent RNA (LAT) is not required for latent infection in the mouse. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7596–7600. doi: 10.1073/pnas.86.19.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B. The murine cytomegalovirus as a model for the study of viral pathogenesis and persistent infections. Arch Virol. 1979;62(1):1–29. doi: 10.1007/BF01314900. [DOI] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Association of virulence of murine cytomegalovirus with macrophage susceptibility and with virion-bound non-neutralizing antibody. J Gen Virol. 1985 Apr;66(Pt 4):879–882. doi: 10.1099/0022-1317-66-4-879. [DOI] [PubMed] [Google Scholar]
- Jordan M. C., Takagi J. L. Virulence characteristics of murine cytomegalovirus in cell and organ cultures. Infect Immun. 1983 Aug;41(2):841–843. doi: 10.1128/iai.41.2.841-843.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein D. A., Yu G. S., Jordan M. C. Lethal infection with murine cytomegalovirus after early viral replication in the spleen. J Infect Dis. 1983 Sep;148(3):406–411. doi: 10.1093/infdis/148.3.406. [DOI] [PubMed] [Google Scholar]
- Manley J. L. Polyadenylation of mRNA precursors. Biochim Biophys Acta. 1988 May 6;950(1):1–12. doi: 10.1016/0167-4781(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Manning W. C., Mocarski E. S. Insertional mutagenesis of the murine cytomegalovirus genome: one prominent alpha gene (ie2) is dispensable for growth. Virology. 1988 Dec;167(2):477–484. [PubMed] [Google Scholar]
- Mercer J. A., Marks J. R., Spector D. H. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith Strain). Virology. 1983 Aug;129(1):94–106. doi: 10.1016/0042-6822(83)90398-7. [DOI] [PubMed] [Google Scholar]
- Messerle M., Bühler B., Keil G. M., Koszinowski U. H. Structural organization, expression, and functional characterization of the murine cytomegalovirus immediate-early gene 3. J Virol. 1992 Jan;66(1):27–36. doi: 10.1128/jvi.66.1.27-36.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerle M., Keil G. M., Koszinowski U. H. Structure and expression of murine cytomegalovirus immediate-early gene 2. J Virol. 1991 Mar;65(3):1638–1643. doi: 10.1128/jvi.65.3.1638-1643.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Misra V., Hudson J. B. Minor base sequence differences between the genomes of two strains of murine cytomegalovirus differing in virulence. Arch Virol. 1980;64(1):1–8. doi: 10.1007/BF01317385. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Jr, Abenes G. B., Manning W. C., Sambucetti L. C., Cherrington J. M. Molecular genetic analysis of cytomegalovirus gene regulation in growth, persistence and latency. Curr Top Microbiol Immunol. 1990;154:47–74. doi: 10.1007/978-3-642-74980-3_3. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Walker D. L. Virulence and attenuation of murine cytomegalovirus. Infect Immun. 1971 Feb;3(2):228–236. doi: 10.1128/iai.3.2.228-236.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Khan A. Resistance of peripheral autonomic neurons to in vivo productive infection by herpes simplex virus mutants deficient in thymidine kinase activity. Infect Immun. 1981 Nov;34(2):571–580. doi: 10.1128/iai.34.2.571-580.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Manischewitz J. F. Genetically determined resistance to lethal murine cytomegalovirus infection is mediated by interferon-dependent and -independent restriction of virus replication. J Virol. 1987 Jun;61(6):1875–1881. doi: 10.1128/jvi.61.6.1875-1881.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath R. M., Graves M. C. Attenuated murine cytomegalovirus binds to N-acetylglucosamine, and shift to virulence may involve recognition of sialic acids. J Virol. 1990 Nov;64(11):5430–5440. doi: 10.1128/jvi.64.11.5430-5440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman R. L., Quirk M. R., Jordan M. C. Disseminated cytomegalovirus infection. Molecular analysis of virus and leukocyte interactions in viremia. J Clin Invest. 1988 Jan;81(1):75–81. doi: 10.1172/JCI113313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons C. C., Sweet C. Isolation and preliminary characterization of temperature-sensitive mutants of mouse cytomegalovirus of differing virulence for 1-week-old mice. J Gen Virol. 1989 Sep;70(Pt 9):2373–2381. doi: 10.1099/0022-1317-70-9-2373. [DOI] [PubMed] [Google Scholar]
- Sandford G. R., Burns W. H. Use of temperature-sensitive mutants of mouse cytomegalovirus as vaccines. J Infect Dis. 1988 Sep;158(3):596–601. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo A. A., Fitzgerald N. A., Simmons A., La Vista A. B., Shellam G. R. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J Exp Med. 1990 May 1;171(5):1469–1483. doi: 10.1084/jem.171.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Ressel S., Dunstan M. E. Herpes simplex virus thymidine kinase expression in trigeminal ganglion infection: correlation of enzyme activity with ganglion virus titer and evidence of in vivo complementation. Virology. 1981 Jul 15;112(1):328–341. doi: 10.1016/0042-6822(81)90638-3. [DOI] [PubMed] [Google Scholar]
- Tonari Y., Minamishima Y. Pathogenicity and immunogenicity of temperature-sensitive mutants of murine cytomegalovirus. J Gen Virol. 1983 Sep;64(Pt 9):1983–1990. doi: 10.1099/0022-1317-64-9-1983. [DOI] [PubMed] [Google Scholar]