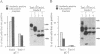Abstract
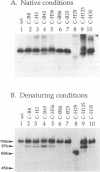
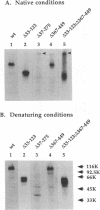
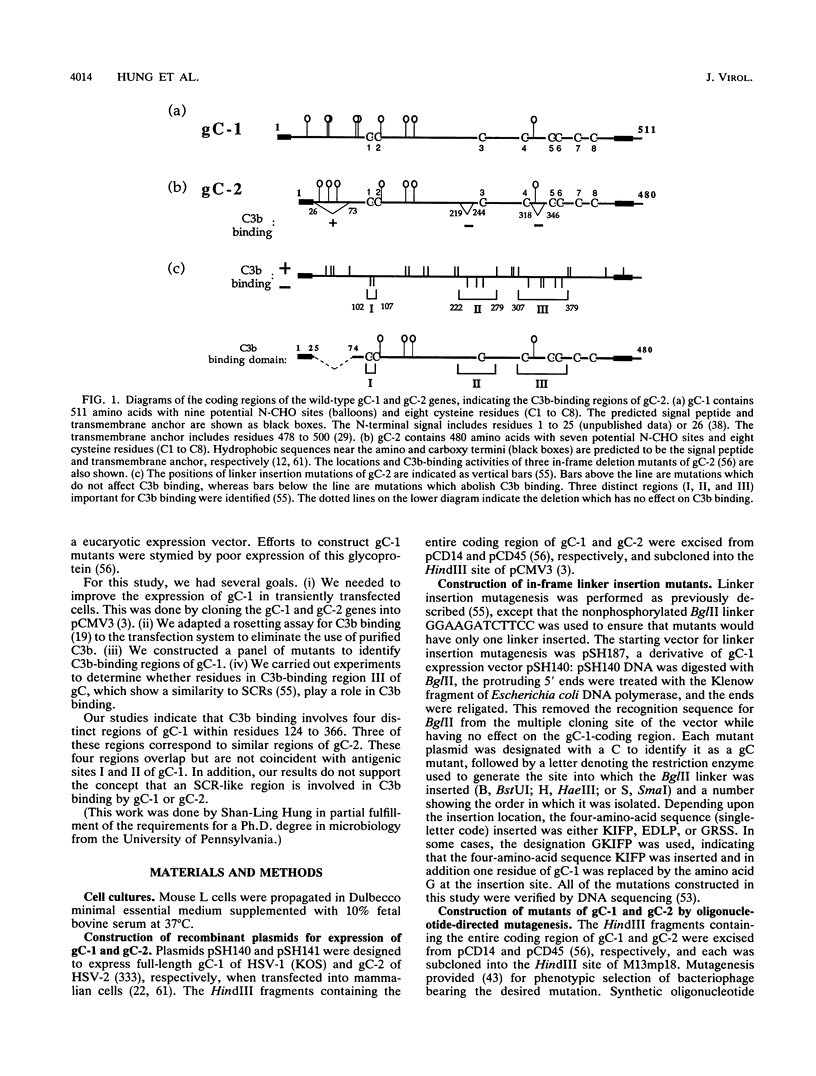
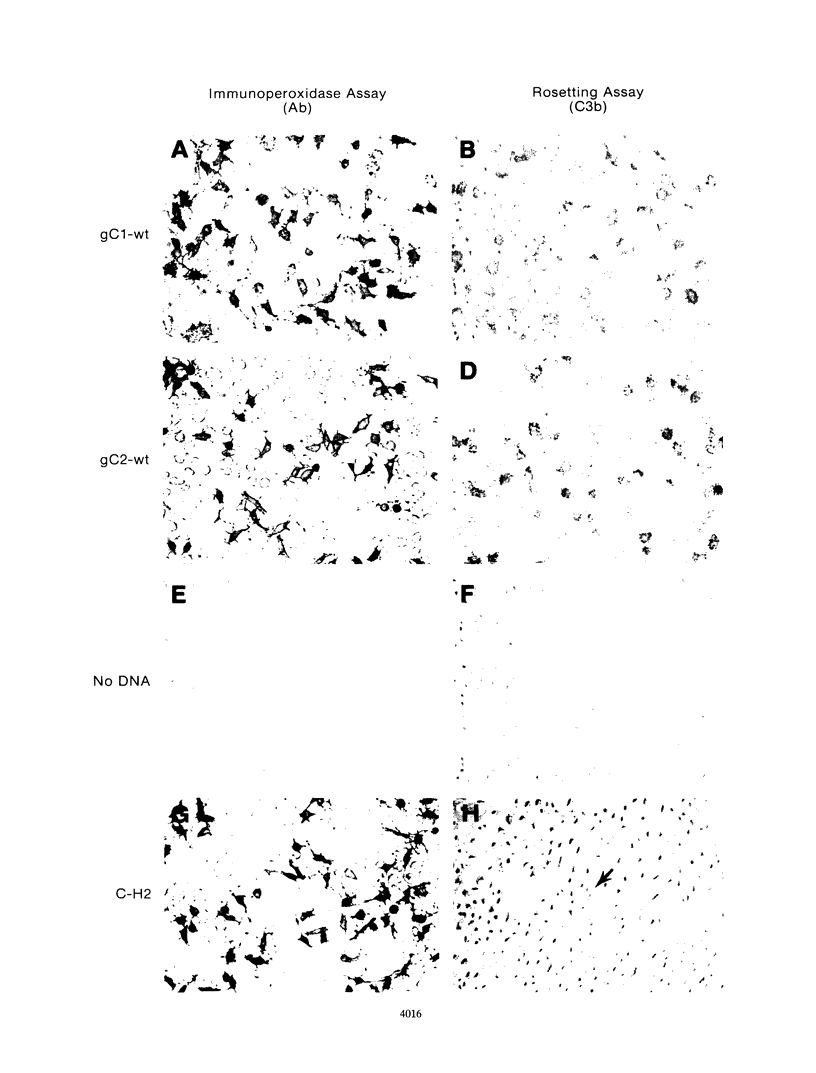
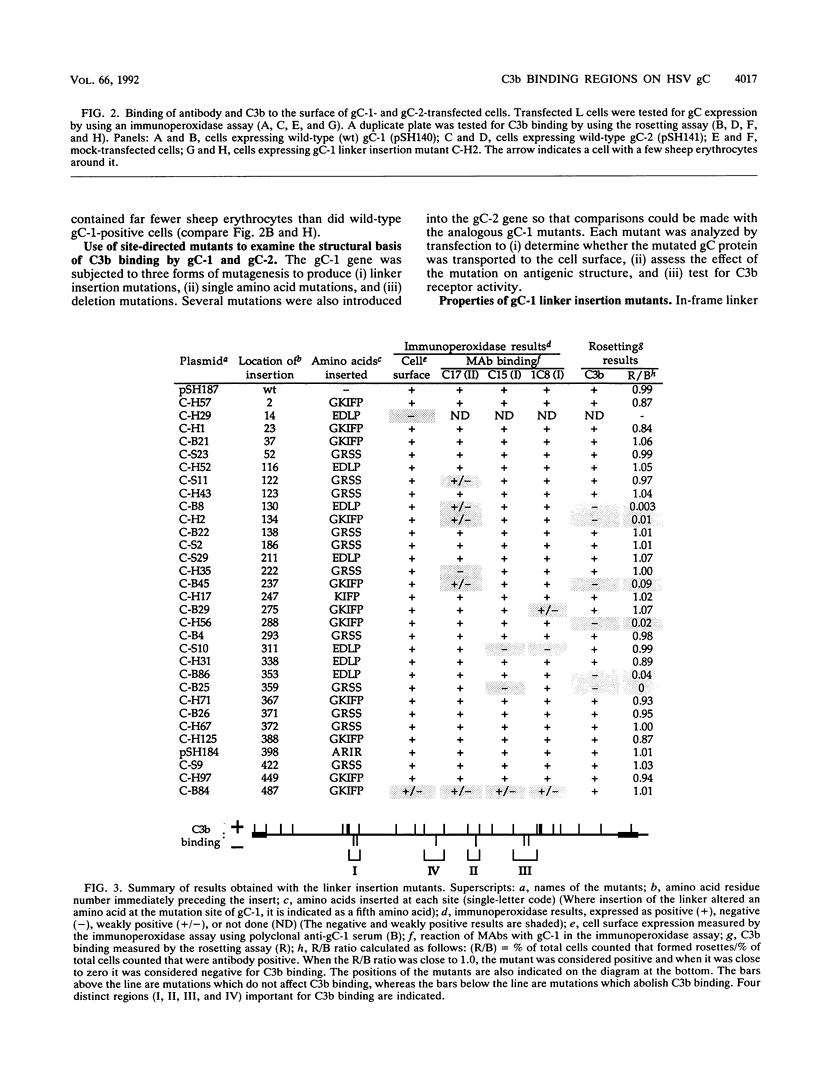
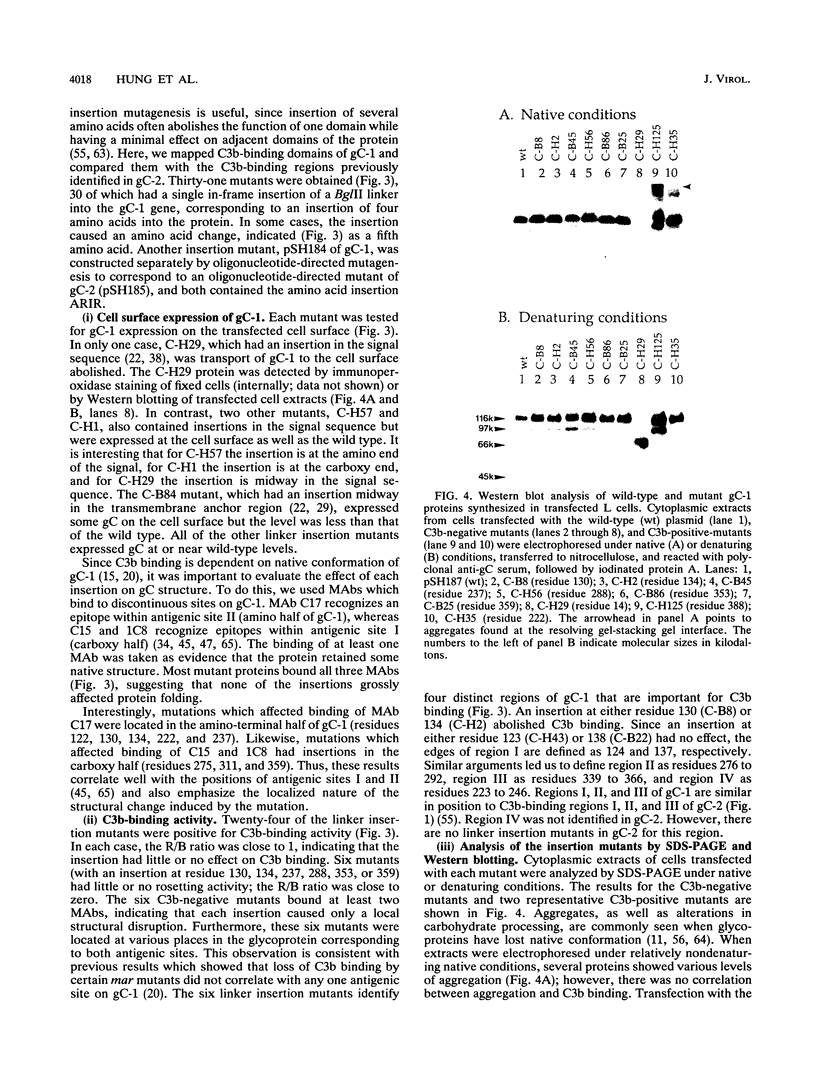
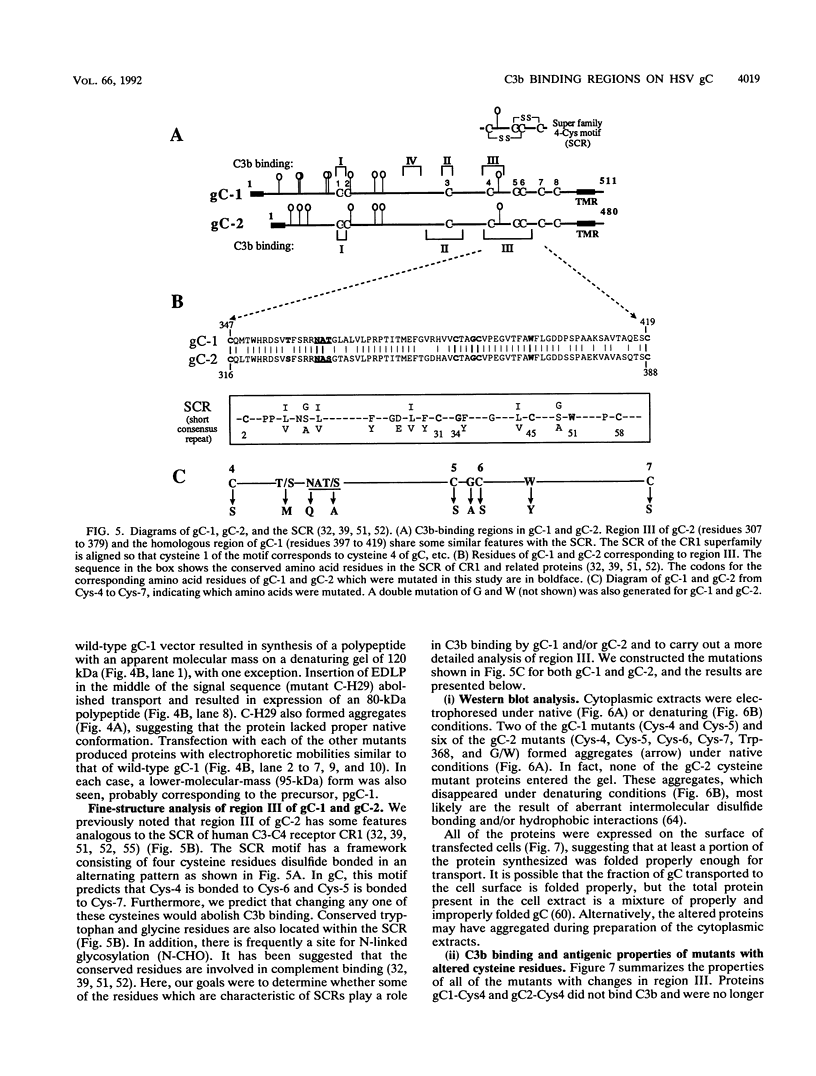
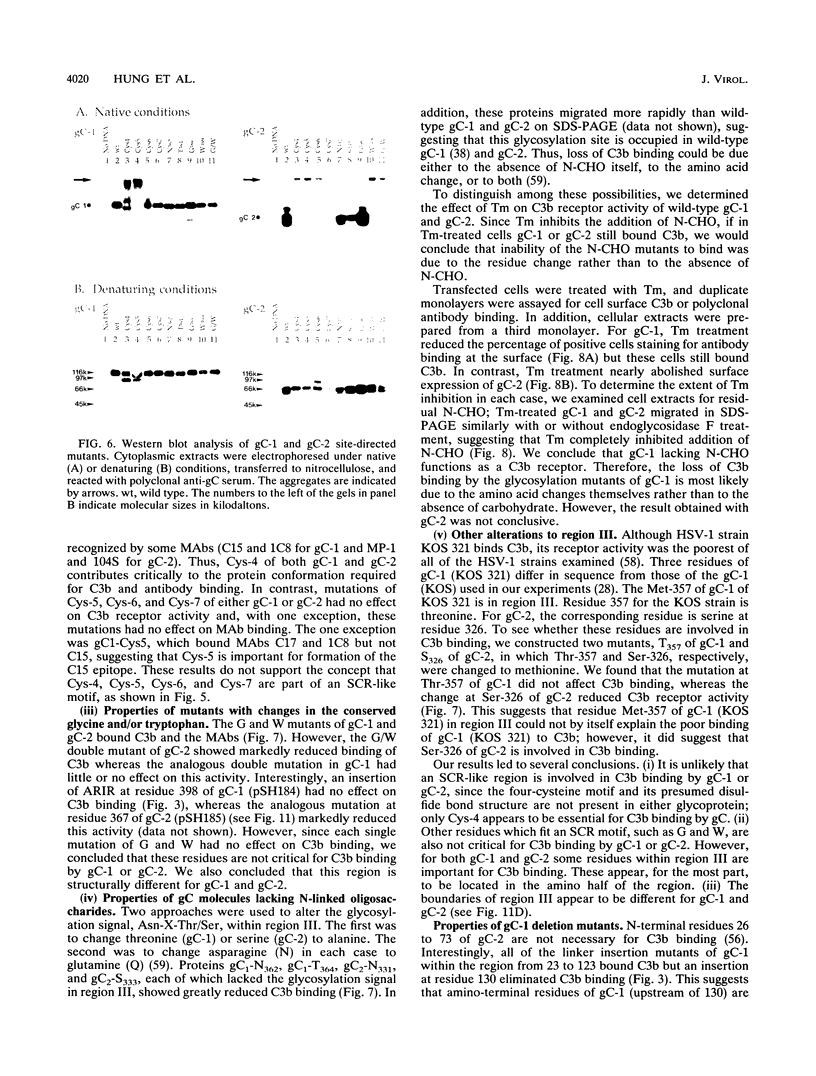
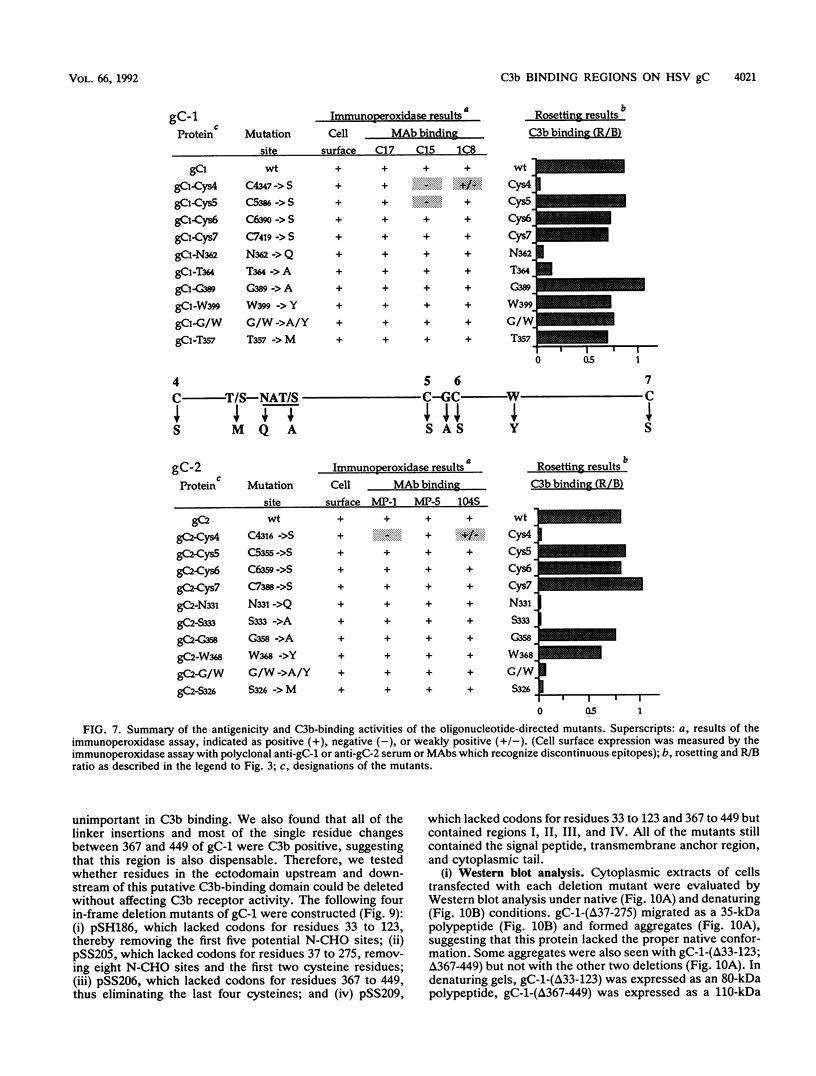
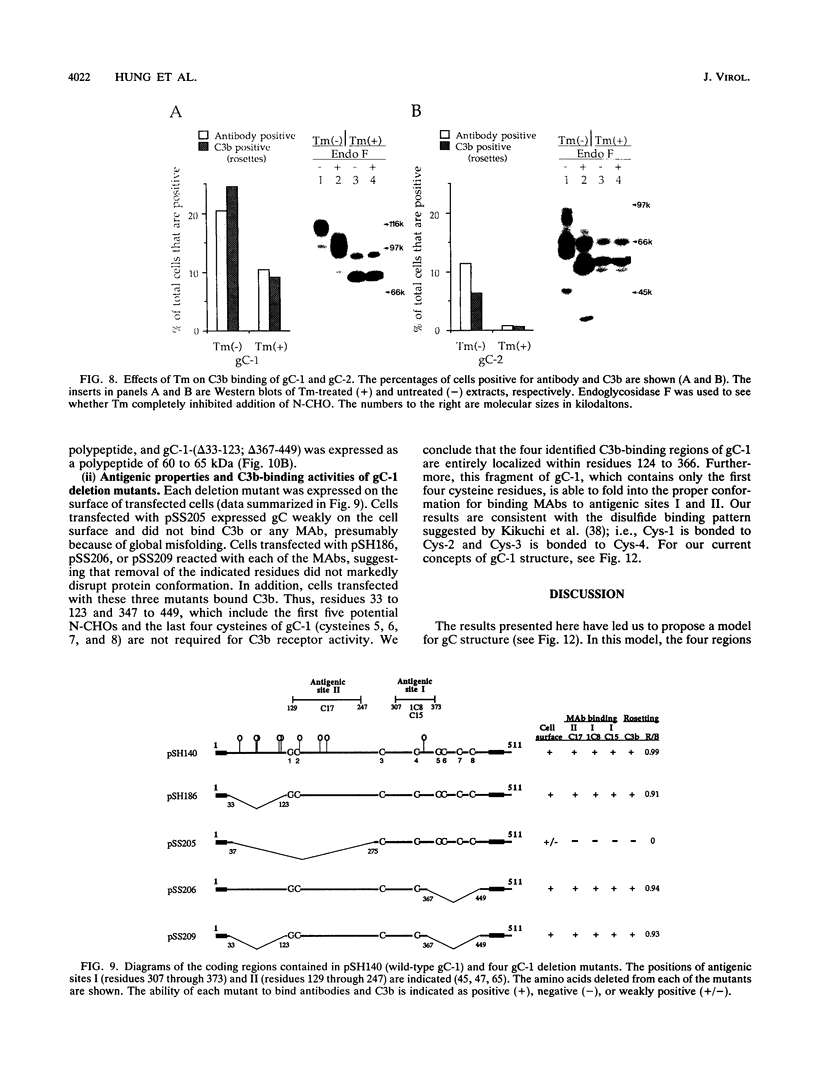
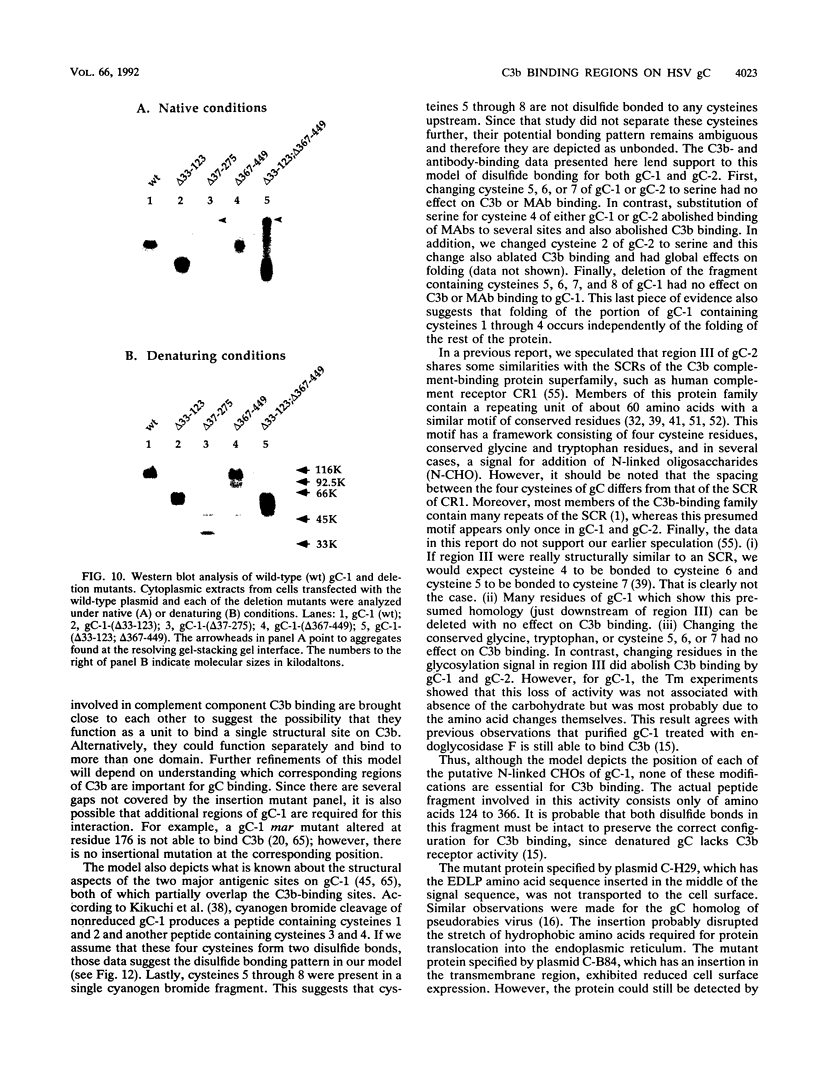
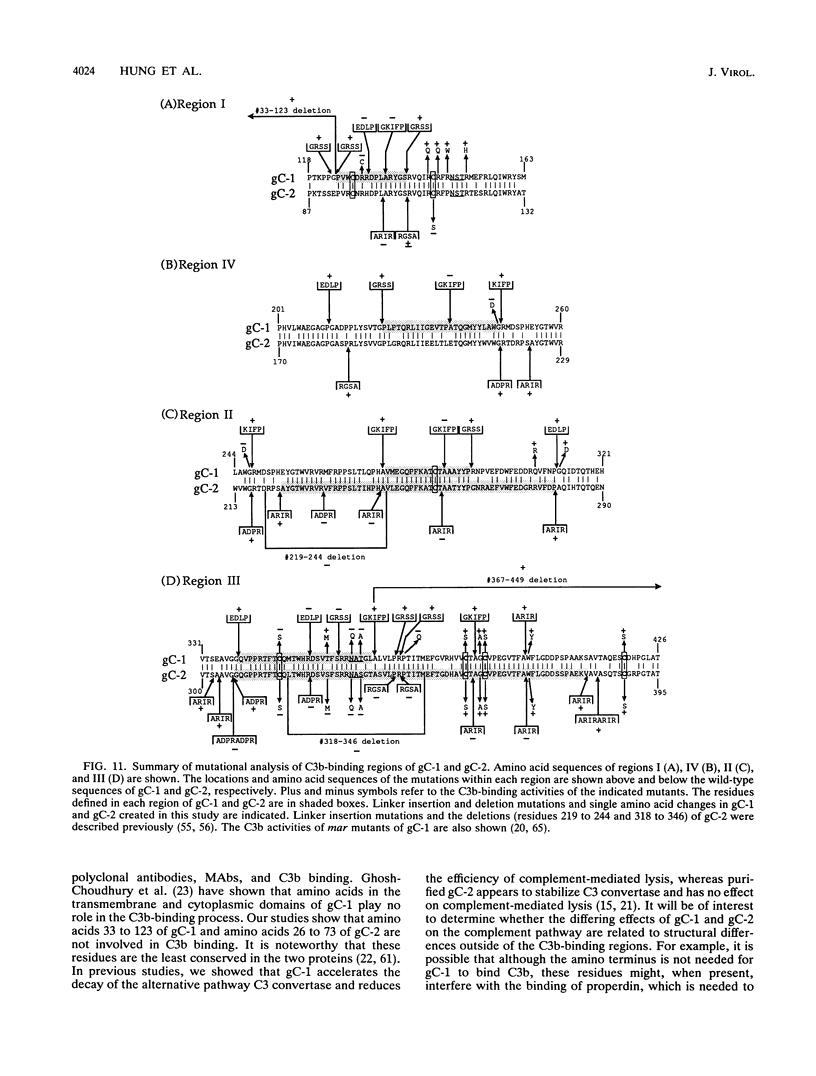
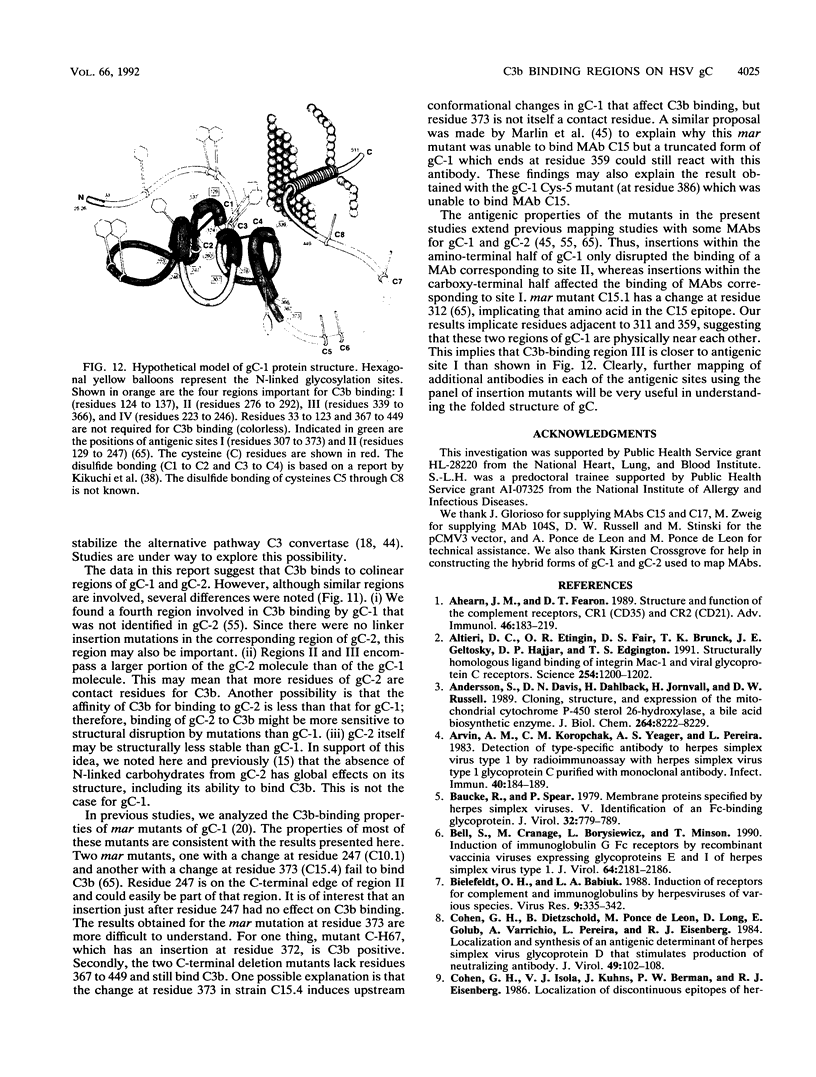
Glycoproteins C (gC) from herpes simplex virus type 1 (HSV-1) and HSV-2, gC-1 and gC-2, bind the human complement fragment C3b, although the two glycoproteins differ in their abilities to act as C3b receptors on infected cells and in their effects on the alternative complement pathway. Previously, we identified three regions of gC-2 (I, II, and III) which are important for C3b binding. In this study, our goal was to identify C3b-binding sites on gC-1 and to continue our analysis of gC-2. We constructed a large panel of mutants by using the cloned gC-1 and gC-2 genes. Most of the mutant proteins were transported to the surface of transiently transfected L cells and reacted with one or more monoclonal antibodies to discontinuous epitopes. By using 31 linker insertion mutants spread across the coding region of gC-1, we identified four regions in the ectodomain of gC-1 which are important for C3b binding, three of which are similar in position to C3b-binding regions I, II, and III of gC-2. Region III shares some similarities with the short consensus repeat found in CR1, the human complement receptor. These were, in part, the targets for construction of 20 single amino acid changes in region III of gC-1 and gC-2. These mutants identified similarities and differences in the C3b-binding properties of gC-1 and gC-2 and suggest that the amino half of region III is more important for C3b binding. However, our results do not support the concept of a structural relationship between the short consensus repeat of CR1 and gC, since mutations of some of the conserved residues, including three of four cysteines in region III, had no effect on C3b binding. Finally, we constructed four deletion mutants of gC-1, including one which lacked residues 33 to 123, as well as residues 367 to 449. This severely truncated molecule, lacking four cysteines and five potential N-linked glycosylation sites, was transported to the cell surface and retained its ability to bind monoclonal antibodies as well as C3b. Thus, the four distinct C3b-binding regions of gC-1 and several epitopes within two different antigenic sites are localized within residues 124 to 366.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn J. M., Fearon D. T. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21). Adv Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- Altieri D. C., Etingin O. R., Fair D. S., Brunck T. K., Geltosky J. E., Hajjar D. P., Edgington T. S. Structurally homologous ligand binding of integrin Mac-1 and viral glycoprotein C receptors. Science. 1991 Nov 22;254(5035):1200–1202. doi: 10.1126/science.1957171. [DOI] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Arvin A. M., Koropchak C. M., Yeager A. S., Pereira L. Detection of type-specific antibody to herpes simplex virus type 1 by radioimmunoassay with herpes simplex virus type 1 glycoprotein C purified with monoclonal antibody. Infect Immun. 1983 Apr;40(1):184–189. doi: 10.1128/iai.40.1.184-189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S., Cranage M., Borysiewicz L., Minson T. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J Virol. 1990 May;64(5):2181–2186. doi: 10.1128/jvi.64.5.2181-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C., Sodora D. L., Long D., Levin J. Z., Eisenberg R. J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988 Jun;62(6):1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowbenko D. J., Lasky L. A. Extensive homology between the herpes simplex virus type 2 glycoprotein F gene and the herpes simplex virus type 1 glycoprotein C gene. J Virol. 1984 Oct;52(1):154–163. doi: 10.1128/jvi.52.1.154-163.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper K. G., Costa R. H., Lee G. T., Spear P. G., Wagner E. K. Molecular basis of the glycoprotein-C-negative phenotype of herpes simplex virus type 1 macroplaque strain. J Virol. 1984 Sep;51(3):578–585. doi: 10.1128/jvi.51.3.578-585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin G., Frank I., Friedman H. M. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J Virol. 1990 Jun;64(6):2725–2731. doi: 10.1128/jvi.64.6.2725-2731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Hastings J. C., Cohen G. H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987 Dec;3(6):423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Keeler C. L., Jr, Robbins A. K., Ryan J. P., Whealy M. E. An amino-terminal deletion mutation of pseudorabies virus glycoprotein gIII affects protein localization and RNA accumulation. J Virol. 1988 Oct;62(10):3565–3573. doi: 10.1128/jvi.62.10.3565-3573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Friedman H. M., Hajjar D. P. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990 May 18;61(4):657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975 Oct 1;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Glorioso J. C., Cohen G. H., Hastings J. C., Harris S. L., Eisenberg R. J. Binding of complement component C3b to glycoprotein gC of herpes simplex virus type 1: mapping of gC-binding sites and demonstration of conserved C3b binding in low-passage clinical isolates. J Virol. 1986 Nov;60(2):470–475. doi: 10.1128/jvi.60.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Friedman H. M., Cohen G. H., Eisenberg R. J., Hammer C. H., Frank M. M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986 Sep 1;137(5):1636–1641. [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Choudhury N., Butcher M., Ghosh H. P. Expression from cloned DNA of biologically active glycoprotein C of herpes simplex virus type 1 in mammalian cells. J Gen Virol. 1990 Mar;71(Pt 3):689–699. doi: 10.1099/0022-1317-71-3-689. [DOI] [PubMed] [Google Scholar]
- Hanke T., Graham F. L., Lulitanond V., Johnson D. C. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology. 1990 Aug;177(2):437–444. doi: 10.1016/0042-6822(90)90507-n. [DOI] [PubMed] [Google Scholar]
- Harris S. L., Frank I., Yee A., Cohen G. H., Eisenberg R. J., Friedman H. M. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990 Aug;162(2):331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- Herold B. C., WuDunn D., Soltys N., Spear P. G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991 Mar;65(3):1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka Y., Sakuma S., Kumano Y., Minagawa H., Mori R. Characterization of glycoprotein C-negative mutants of herpes simplex virus type 1 isolated from a patient with keratitis. Arch Virol. 1990;113(3-4):195–207. doi: 10.1007/BF01316673. [DOI] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Lerch R. J., Earhart K. The cytoplasmic domain of herpes simplex virus type 1 glycoprotein C is required for membrane anchoring. J Virol. 1988 May;62(5):1753–1761. doi: 10.1128/jvi.62.5.1753-1761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Sandri-Goldin R. M., Holland L. E., Marlin S. D., Levine M., Glorioso J. C. Physical mapping of the mutation in an antigenic variant of herpes simplex virus type 1 by use of an immunoreactive plaque assay. J Virol. 1983 May;46(2):649–652. doi: 10.1128/jvi.46.2.649-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Purifoy D. J., Glorioso J. C., Levine M. Molecular basis of the glycoprotein C-negative phenotypes of herpes simplex virus type 1 mutants selected with a virus-neutralizing monoclonal antibody. J Virol. 1986 May;58(2):281–289. doi: 10.1128/jvi.58.2.281-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourcade D., Holers V. M., Atkinson J. P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- Huemer H. P., Bröker M., Larcher C., Lambris J. D., Dierich M. P. The central segment of herpes simplex virus type 1 glycoprotein C (gC) is not involved in C3b binding: demonstration by using monoclonal antibodies and recombinant gC expressed in Escherichia coli. J Gen Virol. 1989 Jun;70(Pt 6):1571–1578. doi: 10.1099/0022-1317-70-6-1571. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987 Jul;61(7):2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., McDermott M. R., Chrisp C., Glorioso J. C. Pathogenicity in mice of herpes simplex virus type 2 mutants unable to express glycoprotein C. J Virol. 1986 Apr;58(1):36–42. doi: 10.1128/jvi.58.1.36-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G. E., Coligan J. E., Holland T. C., Levine M., Glorioso J. C., Nairn R. Biochemical characterization of peptides from herpes simplex virus glycoprotein gC: loss of CNBr fragments from the carboxy terminus of truncated, secreted gC molecules. J Virol. 1984 Dec;52(3):806–815. doi: 10.1128/jvi.52.3.806-815.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klickstein L. B., Wong W. W., Smith J. A., Weis J. H., Wilson J. G., Fearon D. T. Human C3b/C4b receptor (CR1). Demonstration of long homologous repeating domains that are composed of the short consensus repeats characteristics of C3/C4 binding proteins. J Exp Med. 1987 Apr 1;165(4):1095–1112. doi: 10.1084/jem.165.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousoulas K. G., Pellett P. E., Pereira L., Roizman B. Mutations affecting conformation or sequence of neutralizing epitopes identified by reactivity of viable plaques segregate from syn and ts domains of HSV-1(F) gB gene. Virology. 1984 Jun;135(2):379–394. doi: 10.1016/0042-6822(84)90194-6. [DOI] [PubMed] [Google Scholar]
- Krych M., Hourcade D., Atkinson J. P. Sites within the complement C3b/C4b receptor important for the specificity of ligand binding. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4353–4357. doi: 10.1073/pnas.88.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Gaither T. A., Cason J., O'Shea J. J., Lawley T. J. Characterization of the C3 receptor induced by herpes simplex virus type 1 infection of human epidermal, endothelial, and A431 cells. J Immunol. 1987 Feb 15;138(4):1137–1142. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lambris J. D. The multifunctional role of C3, the third component of complement. Immunol Today. 1988 Dec;9(12):387–393. doi: 10.1016/0167-5699(88)91240-6. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Holland T. C., Levine M., Glorioso J. C. Epitopes of herpes simplex virus type 1 glycoprotein gC are clustered in two distinct antigenic sites. J Virol. 1985 Jan;53(1):128–136. doi: 10.1128/jvi.53.1.128-136.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T. A., Odell C., Holers V. M., Spear P. G., Atkinson J. P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987 Nov 1;166(5):1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggeridge M. I., Wu T. T., Johnson D. C., Glorioso J. C., Eisenberg R. J., Cohen G. H. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology. 1990 Feb;174(2):375–387. doi: 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- Ohmann H. B., Babiuk L. A. Induction of receptors for complement and immunoglobulins by herpesviruses of various species. Virus Res. 1988 Mar;9(4):335–342. doi: 10.1016/0168-1702(88)90092-5. [DOI] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980 May;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Dondero D. V., Gallo D., Devlin V., Woodie J. D. Serological analysis of herpes simplex virus types 1 and 2 with monoclonal antibodies. Infect Immun. 1982 Jan;35(1):363–367. doi: 10.1128/iai.35.1.363-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. J., Haris P. I., Sim R. B., Chapman D. A study of the structure of human complement component factor H by Fourier transform infrared spectroscopy and secondary structure averaging methods. Biochemistry. 1988 May 31;27(11):4004–4012. doi: 10.1021/bi00411a017. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears A. E., McGwire B. S., Roizman B. Infection of polarized MDCK cells with herpes simplex virus 1: two asymmetrically distributed cell receptors interact with different viral proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5087–5091. doi: 10.1073/pnas.88.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel-Dugan C., Ponce de Leon M., Friedman H. M., Eisenberg R. J., Cohen G. H. Identification of C3b-binding regions on herpes simplex virus type 2 glycoprotein C. J Virol. 1990 May;64(5):1897–1906. doi: 10.1128/jvi.64.5.1897-1906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel-Dugan C., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Cohen G. H., Eisenberg R. J. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J Virol. 1988 Nov;62(11):4027–4036. doi: 10.1128/jvi.62.11.4027-4036.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley M. L., Friedman H. M. Binding of complement component C3b to glycoprotein C is modulated by sialic acid on herpes simplex virus type 1-infected cells. J Virol. 1985 Sep;55(3):857–861. doi: 10.1128/jvi.55.3.857-861.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley M. L., Hoxie J. A., Friedman H. M. Herpes simplex virus type 1 infection of endothelial, epithelial, and fibroblast cells induces a receptor for C3b. J Immunol. 1985 Apr;134(4):2673–2678. [PubMed] [Google Scholar]
- Sodora D. L., Cohen G. H., Eisenberg R. J. Influence of asparagine-linked oligosaccharides on antigenicity, processing, and cell surface expression of herpes simplex virus type 1 glycoprotein D. J Virol. 1989 Dec;63(12):5184–5193. doi: 10.1128/jvi.63.12.5184-5193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora D. L., Eisenberg R. J., Cohen G. H. Characterization of a recombinant herpes simplex virus which expresses a glycoprotein D lacking asparagine-linked oligosaccharides. J Virol. 1991 Aug;65(8):4432–4441. doi: 10.1128/jvi.65.8.4432-4441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain M. A., Peet R. W., Galloway D. A. Characterization of the gene encoding herpes simplex virus type 2 glycoprotein C and comparison with the type 1 counterpart. J Virol. 1985 Feb;53(2):561–569. doi: 10.1128/jvi.53.2.561-569.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal-Singer R., Seidel-Dugan C., Fries L., Huemer H. P., Eisenberg R. J., Cohen G. H., Friedman H. M. Herpes simplex virus glycoprotein C is a receptor for complement component iC3b. J Infect Dis. 1991 Oct;164(4):750–753. doi: 10.1093/infdis/164.4.750. [DOI] [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox W. C., Long D., Sodora D. L., Eisenberg R. J., Cohen G. H. The contribution of cysteine residues to antigenicity and extent of processing of herpes simplex virus type 1 glycoprotein D. J Virol. 1988 Jun;62(6):1941–1947. doi: 10.1128/jvi.62.6.1941-1947.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. T., Levine M., Homa F., Highlander S. L., Glorioso J. C. Characterization of the antigenic structure of herpes simplex virus type 1 glycoprotein C through DNA sequence analysis of monoclonal antibody-resistant mutants. J Virol. 1990 Feb;64(2):856–863. doi: 10.1128/jvi.64.2.856-863.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Mapping of the structural gene for the herpes simplex virus type 2 counterpart of herpes simplex virus type 1 glycoprotein C and identification of a type 2 mutant which does not express this glycoprotein. J Virol. 1984 Mar;49(3):741–747. doi: 10.1128/jvi.49.3.741-747.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]