Abstract
Expression of the human cytomegalovirus (HCMV) (AD169) DNA polymerase gene under the control of the polyhedrin promoter of Autographa californica nuclear polyhedrosis virus in Spodoptera frugiperda (Sf9) cells has provided a source of highly active CMV DNA polymerase. In extracts from CMV-infected cells, the CMV DNA polymerase is found strongly associated with an additional polypeptide, ICP36. This protein has been identified as the CMV homolog of the herpes simplex virus type 1 UL42 gene product and may have a similar function. We have expressed HCMV DNA polymerase and ICP36 in the same system and demonstrated that they interact to form a stable complex. Moreover, ICP36 functions to stimulate the DNA polymerase activity in a template-dependent manner. We have compared the activity of the recombinant DNA polymerase in the presence and absence of ICP36 on a number of DNA templates and measured the effect of the polymerase inhibitors phosphonoformic acid and acyclovir triphosphate.
Full text
PDF


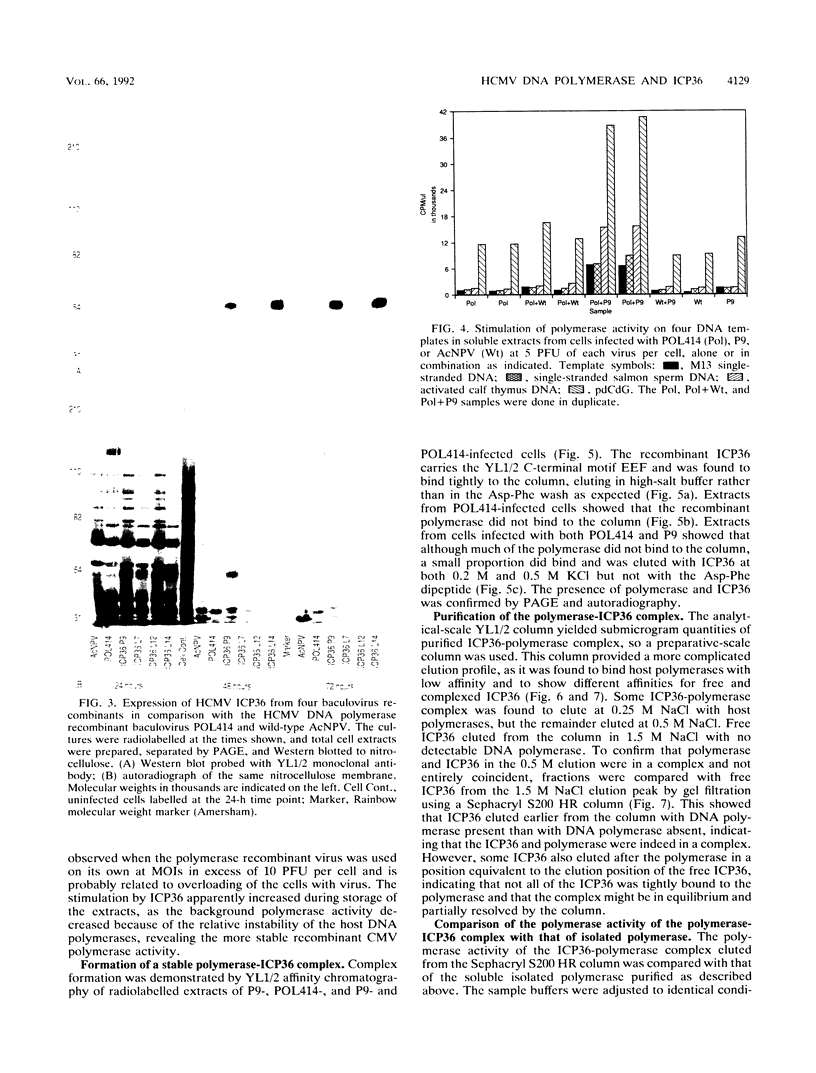




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks L. M., Vaughan P. J., Meredith D., Powell K. L. Monoclonal antibodies to herpes simplex virus thymidine kinase. J Gen Virol. 1984 Sep;65(Pt 9):1625–1630. doi: 10.1099/0022-1317-65-9-1625. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Balachandran N. Identification, characterization, and sequence analysis of a cDNA encoding a phosphoprotein of human herpesvirus 6. J Virol. 1991 Dec;65(12):7085–7085. doi: 10.1128/jvi.65.12.7085-.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. K., Balachandran N. Identification, characterization, and sequence analysis of a cDNA encoding a phosphoprotein of human herpesvirus 6. J Virol. 1991 Jun;65(6):2884–2894. doi: 10.1128/jvi.65.6.2884-2894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Ertl P. F., Thomas M. S., Powell K. L. High level expression of DNA polymerases from herpesviruses. J Gen Virol. 1991 Jul;72(Pt 7):1729–1734. doi: 10.1099/0022-1317-72-7-1729. [DOI] [PubMed] [Google Scholar]
- Gallo M. L., Dorsky D. I., Crumpacker C. S., Parris D. S. The essential 65-kilodalton DNA-binding protein of herpes simplex virus stimulates the virus-encoded DNA polymerase. J Virol. 1989 Dec;63(12):5023–5029. doi: 10.1128/jvi.63.12.5023-5029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich L. D., Schaffer P. A., Dorsky D. I., Crumpacker C. S., Parris D. S. Localization of the herpes simplex virus type 1 65-kilodalton DNA-binding protein and DNA polymerase in the presence and absence of viral DNA synthesis. J Virol. 1990 Dec;64(12):5738–5749. doi: 10.1128/jvi.64.12.5738-5749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J., Marcy A. I., Coen D. M., Challberg M. D. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J Virol. 1990 Dec;64(12):5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez T. R., Lehman I. R. Functional interaction between the herpes simplex-1 DNA polymerase and UL42 protein. J Biol Chem. 1990 Jul 5;265(19):11227–11232. [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y. Baculovirus vectors for expression of foreign genes. Adv Virus Res. 1988;35:177–192. doi: 10.1016/s0065-3527(08)60711-3. [DOI] [PubMed] [Google Scholar]
- Kiehl A., Dorsky D. I. Cooperation of EBV DNA polymerase and EA-D(BMRF1) in vitro and colocalization in nuclei of infected cells. Virology. 1991 Sep;184(1):330–340. doi: 10.1016/0042-6822(91)90849-7. [DOI] [PubMed] [Google Scholar]
- Kitts P. A., Ayres M. D., Possee R. D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990 Oct 11;18(19):5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Li J. S., Zhou B. S., Dutschman G. E., Grill S. P., Tan R. S., Cheng Y. C. Association of Epstein-Barr virus early antigen diffuse component and virus-specified DNA polymerase activity. J Virol. 1987 Sep;61(9):2947–2949. doi: 10.1128/jvi.61.9.2947-2949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Huang Y. S., Huang E. S. Purification and characterization of varicella-zoster virus-induced DNA polymerase. J Virol. 1978 May;26(2):249–256. doi: 10.1128/jvi.26.2.249-256.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcy A. I., Olivo P. D., Challberg M. D., Coen D. M. Enzymatic activities of overexpressed herpes simplex virus DNA polymerase purified from recombinant baculovirus-infected insect cells. Nucleic Acids Res. 1990 Mar 11;18(5):1207–1215. doi: 10.1093/nar/18.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Rapp F. Varicella-zoster virus-induced DNA polymerase. J Gen Virol. 1977 Sep;36(3):515–524. doi: 10.1099/0022-1317-36-3-515. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Maeno K., Yoshida S. Characterization of human cytomegalovirus-induced DNA polymerase and the associated 3'-to-5', exonuclease. Virology. 1983 Jan 30;124(2):221–231. doi: 10.1016/0042-6822(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. Nonstructural proteins of herpes simplex virus. I. Purification of the induced DNA polymerase. J Virol. 1977 Nov;24(2):618–626. doi: 10.1128/jvi.24.2.618-626.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stammers D. K., Tisdale M., Court S., Parmar V., Bradley C., Ross C. K. Rapid purification and characterisation of HIV-1 reverse transcriptase and RNaseH engineered to incorporate a C-terminal tripeptide alpha-tubulin epitope. FEBS Lett. 1991 Jun 3;283(2):298–302. doi: 10.1016/0014-5793(91)80613-8. [DOI] [PubMed] [Google Scholar]
- Vaughan P. J., Purifoy D. J., Powell K. L. DNA-binding protein associated with herpes simplex virus DNA polymerase. J Virol. 1985 Feb;53(2):501–508. doi: 10.1128/jvi.53.2.501-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




