Abstract
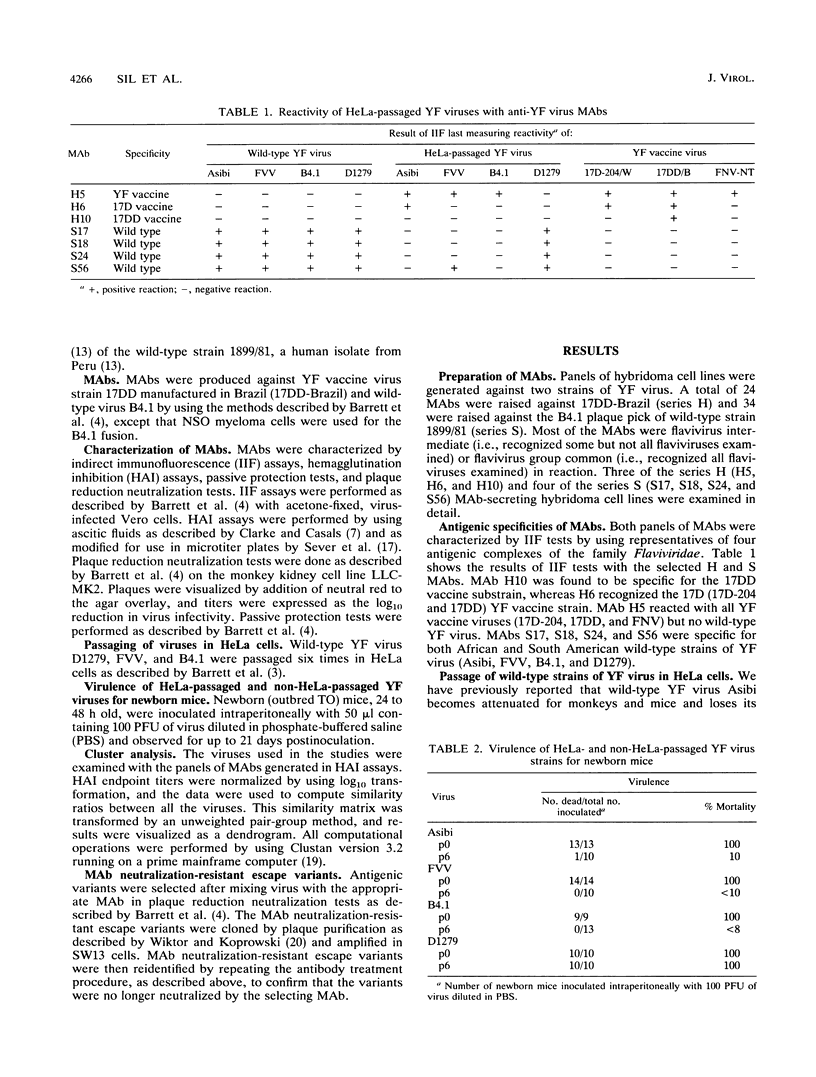
Monoclonal antibodies (MAbs) have been prepared against vaccine and wild-type strains of yellow fever (YF) virus, and envelope protein epitopes specific for vaccine (MAbs H5 and H6) and wild-type (MAbs S17, S18, S24, and S56) strains of YF virus have been identified. Wild-type YF virus FVV, Dakar 1279, and B4.1 were each given six passages in HeLa cells. FVV and B4.1 were attenuated for newborn mice following passage in HeLa cells, whereas Dakar 1279 was not. Examination of the envelope proteins of the viruses with 87 MAbs showed that attenuated viruses gained only the vaccine epitope recognized by MAb H5 and lost wild-type epitopes recognized by MAbs S17, S18, and S24 whereas the nonattenuated Dakar 1279 HeLa p6 virus did not gain the vaccine epitope, retained the wild-type epitopes, and showed no other physical epitope alterations. MAb neutralization-resistant (MAbr) escape variants generated by using wild-type-specific MAbs S18 and S24 were found to lose the epitopes recognized by MAbs S18 and S24 and to acquire the epitope recognized by vaccine-specific MAb H5. In addition, the MAbr variants became attenuated for mice. Thus, the data presented in this paper indicate that acquisition of vaccine epitopes and loss of wild-type epitopes on the envelope protein are directly involved in the attenuation process of YF virus and suggest that the envelope protein is one of the genes encoding determinants of YF virus pathogenicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. D., Mathews J. H., Miller B. R., Medlen A. R., Ledger T. N., Roehrig J. T. Identification of monoclonal antibodies that distinguish between 17D-204 and other strains of yellow fever virus. J Gen Virol. 1990 Jan;71(Pt 1):13–18. doi: 10.1099/0022-1317-71-1-13. [DOI] [PubMed] [Google Scholar]
- Barrett A. D., Monath T. P., Cropp C. B., Adkins J. A., Ledger T. N., Gould E. A., Schlesinger J. J., Kinney R. M., Trent D. W. Attenuation of wild-type yellow fever virus by passage in HeLa cells. J Gen Virol. 1990 Oct;71(Pt 10):2301–2306. doi: 10.1099/0022-1317-71-10-2301. [DOI] [PubMed] [Google Scholar]
- Barrett A. D., Pryde A., Medlen A. R., Ledger T. N., Whitby J. E., Gibson C. A., DeSilva M., Groves D. J., Langley D. J., Minor P. D. Examination of the envelope glycoprotein of yellow fever vaccine viruses with monoclonal antibodies. Vaccine. 1989 Aug;7(4):333–336. doi: 10.1016/0264-410x(89)90196-5. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Cammack N., Gould E. A. Topographical analysis of epitope relationships on the envelope glycoprotein of yellow fever 17D vaccine and the wild type Asibi parent virus. Virology. 1986 Apr 30;150(2):333–341. doi: 10.1016/0042-6822(86)90298-9. [DOI] [PubMed] [Google Scholar]
- Chen W. R., Tesh R. B., Rico-Hesse R. Genetic variation of Japanese encephalitis virus in nature. J Gen Virol. 1990 Dec;71(Pt 12):2915–2922. doi: 10.1099/0022-1317-71-12-2915. [DOI] [PubMed] [Google Scholar]
- Gould E. A., Buckley A., Cammack N., Barrett A. D., Clegg J. C., Ishak R., Varma M. G. Examination of the immunological relationships between flaviviruses using yellow fever virus monoclonal antibodies. J Gen Virol. 1985 Jul;66(Pt 7):1369–1382. doi: 10.1099/0022-1317-66-7-1369. [DOI] [PubMed] [Google Scholar]
- Gould E. A., Buckley A., Cane P. A., Higgs S., Cammack N. Use of a monoclonal antibody specific for wild-type yellow fever virus to identify a wild-type antigenic variant in 17D vaccine pools. J Gen Virol. 1989 Jul;70(Pt 7):1889–1894. doi: 10.1099/0022-1317-70-7-1889. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Dalrymple J. M., Strauss J. H., Rice C. M. Comparison of the virulent Asibi strain of yellow fever virus with the 17D vaccine strain derived from it. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2019–2023. doi: 10.1073/pnas.84.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Dalgarno L., Schlesinger J. J., Weir R. C. Location of a neutralization determinant in the E protein of yellow fever virus (17D vaccine strain). Virology. 1987 Dec;161(2):474–478. doi: 10.1016/0042-6822(87)90141-3. [DOI] [PubMed] [Google Scholar]
- Miller B. R., Adkins D. Biological characterization of plaque-size variants of yellow fever virus in mosquitoes and mice. Acta Virol. 1988 May;32(3):227–234. [PubMed] [Google Scholar]
- Méndez M. R., Calisher C. H., Kruger H., Sipan F., Sánchez S., Lazuick J. S. A continuing focus of yellow fever in the Apurimac River Valley, Ayacucho, Peru, and the first isolation of yellow fever virus in that country. Bull Pan Am Health Organ. 1984;18(2):172–179. [PubMed] [Google Scholar]
- SEVER J. L., LEY A. C., WOLMAN F., CAPLAN B. M., CROCKETT P. W., TURNER H. C. UTILIZATION OF DISPOSABLE PLASTIC PLATES, WITH A SEROLOGIC MICROTECHNIC. Am J Clin Pathol. 1964 Feb;41:167–170. doi: 10.1093/ajcp/41.2.167. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Monath T. P. Monoclonal antibodies distinguish between wild and vaccine strains of yellow fever virus by neutralization, hemagglutination inhibition, and immune precipitation of the virus envelope protein. Virology. 1983 Feb;125(1):8–17. doi: 10.1016/0042-6822(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Walsh E. E., Brandriss M. W. Analysis of 17D yellow fever virus envelope protein epitopes using monoclonal antibodies. J Gen Virol. 1984 Oct;65(Pt 10):1637–1644. doi: 10.1099/0022-1317-65-10-1637. [DOI] [PubMed] [Google Scholar]
- Theiler M., Smith H. H. THE USE OF YELLOW FEVER VIRUS MODIFIED BY IN VITRO CULTIVATION FOR HUMAN IMMUNIZATION. J Exp Med. 1937 May 31;65(6):787–800. doi: 10.1084/jem.65.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H. Antigenic variants of rabies virus. J Exp Med. 1980 Jul 1;152(1):99–112. doi: 10.1084/jem.152.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]


