Abstract
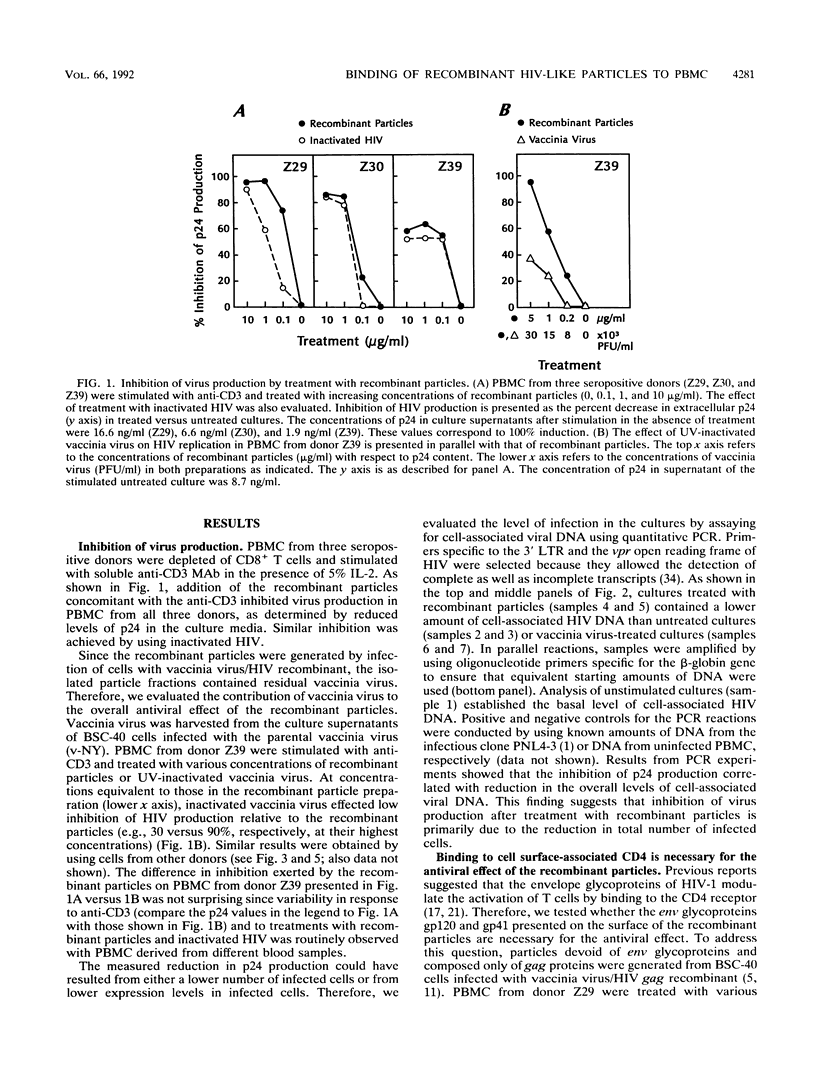
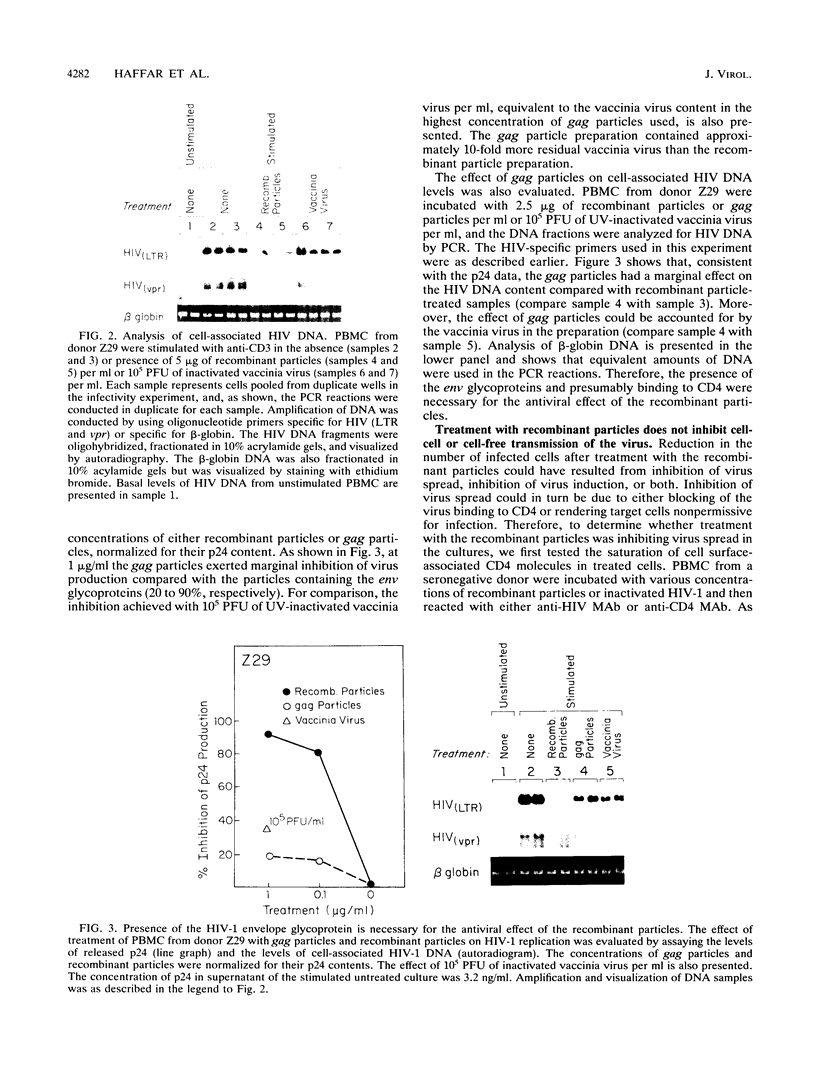
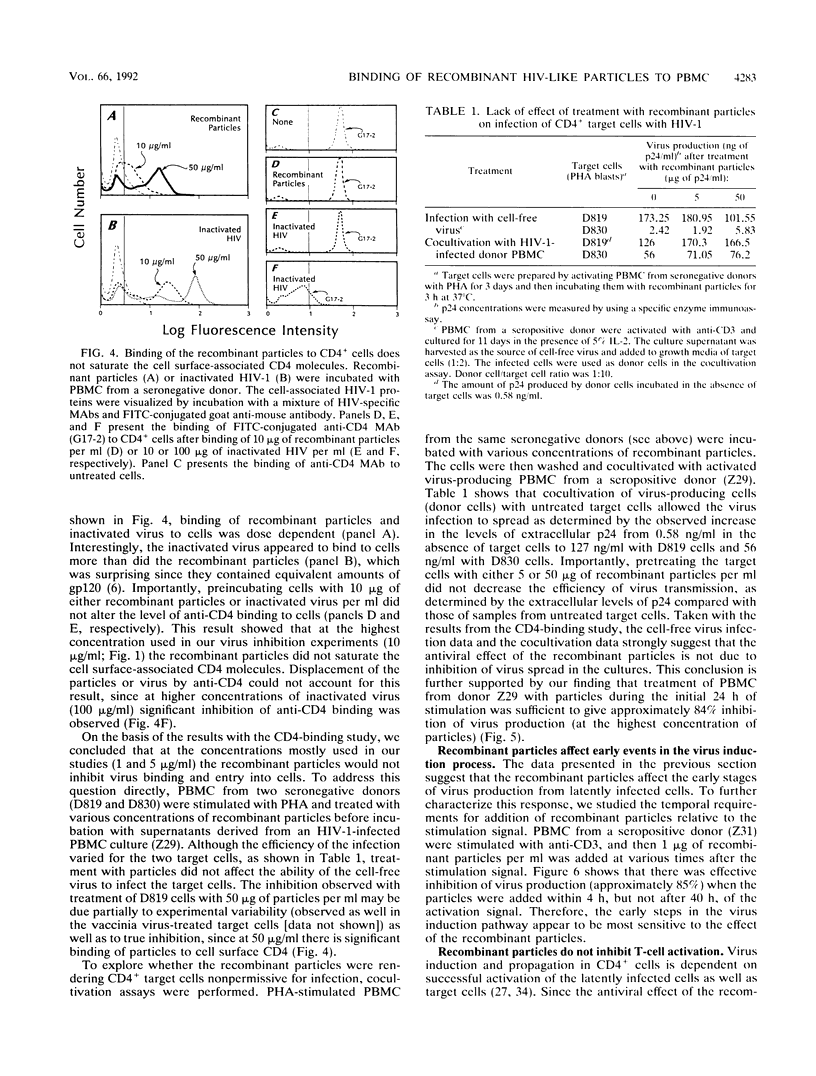
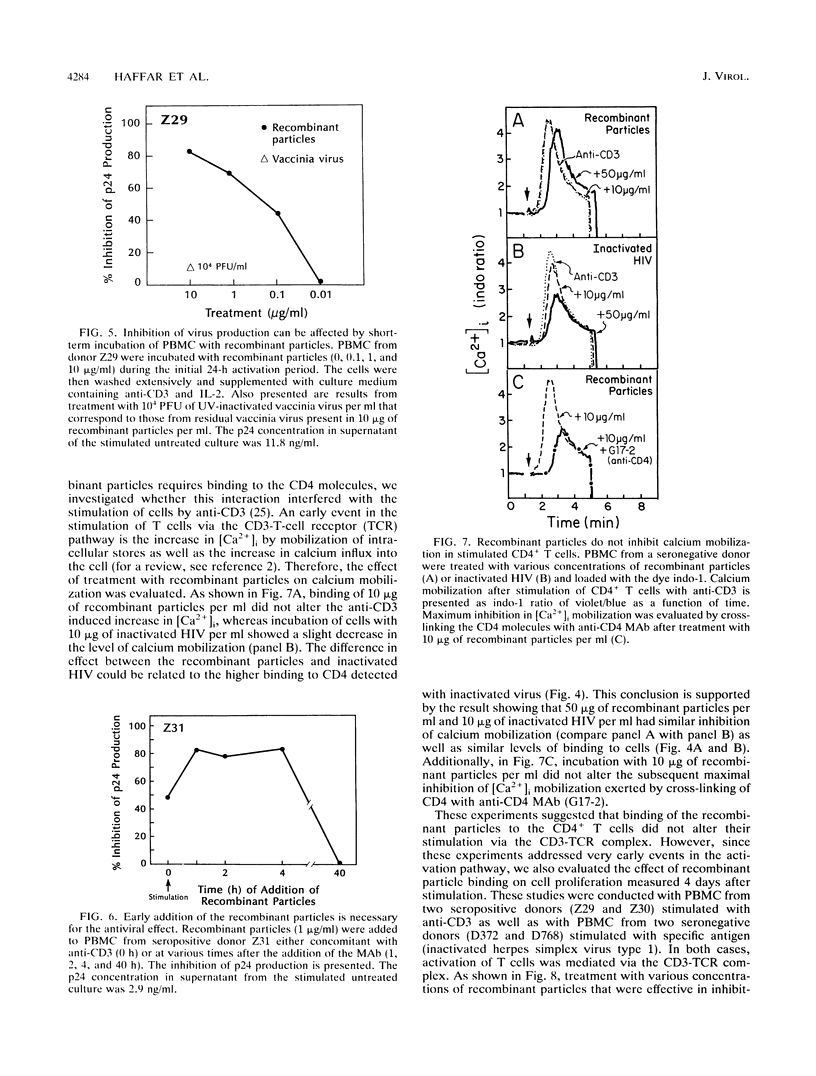
We have previously reported on the assembly of recombinant human immunodeficiency virus (HIV)-like particles that contain gag structural proteins and present env glycoproteins gp120 and gp41 on their surfaces (O. Haffar,. J. Garriques, B. Travis, P. Moran, J. Zarling, and S.-L. Hu, J. Virol. 64:2653-2659, 1990). On the basis of their structures, we hypothesized that the recombinant particles would interfere with virus infection and tested our hypothesis in vitro by using peripheral blood mononuclear cells (PBMC) from HIV type 1-seropositive donors. Addition of the recombinant particles to PBMC concomitant with stimulation by anti-CD3 inhibited virus production, as determined by reduced levels of p24 in the culture supernatants. This inhibition of p24 production correlated with lower levels of cell-associated viral DNA. Several lines of evidence suggested that the recombinant particles exerted their antiviral effects primarily by inhibiting virus production from latently infected cells and not by inhibiting subsequent virus spread. Importantly, CD4+ T-cell stimulation by specific antigen or by anti-CD3 was not inhibited by treatment with the recombinant particles. This apparent selective inhibition of virus replication in infected PBMC represents a novel property of the recombinant HIV-like particles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. Calcium and T lymphocyte activation. Cell. 1989 Oct 6;59(1):15–20. doi: 10.1016/0092-8674(89)90865-9. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gruters R. A., Otto S. A., Al B. J., Verhoeven A. J., Verweij C. L., Van Lier R. A., Miedema F. Non-mitogenic T cell activation signals are sufficient for induction of human immunodeficiency virus transcription. Eur J Immunol. 1991 Jan;21(1):167–172. doi: 10.1002/eji.1830210125. [DOI] [PubMed] [Google Scholar]
- Haffar O. K., Smithgall M. D., Moran P. A., Travis B. M., Zarling J. M., Hu S. L. HIV-specific humoral and cellular immunity in rabbits vaccinated with recombinant human immunodeficiency virus-like gag-env particles. Virology. 1991 Aug;183(2):487–495. doi: 10.1016/0042-6822(91)90978-k. [DOI] [PubMed] [Google Scholar]
- Haffar O., Garrigues J., Travis B., Moran P., Zarling J., Hu S. L. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990 Jun;64(6):2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan U., Thomas D., Alcami J., Bachelerie F., Israel N., Yssel H., Virelizier J. L., Arenzana-Seisdedos F. Stimulation of a human T-cell clone with anti-CD3 or tumor necrosis factor induces NF-kappa B translocation but not human immunodeficiency virus 1 enhancer-dependent transcription. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7861–7865. doi: 10.1073/pnas.87.20.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann B., Nishanian P., Baldwin R. L., Insixiengmay P., Nel A., Fahey J. L. HIV inhibits the early steps of lymphocyte activation, including initiation of inositol phospholipid metabolism. J Immunol. 1990 Dec 1;145(11):3699–3705. [PubMed] [Google Scholar]
- Hu S. L., Kosowski S. G., Dalrymple J. M. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature. 1986 Apr 10;320(6062):537–540. doi: 10.1038/320537a0. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Travis B. M., Garrigues J., Zarling J. M., Sridhar P., Dykers T., Eichberg J. W., Alpers C. Processing, assembly, and immunogenicity of human immunodeficiency virus core antigens expressed by recombinant vaccinia virus. Virology. 1990 Nov;179(1):321–329. doi: 10.1016/0042-6822(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Kanner S. B., Kavanagh T. J., Grossmann A., Hu S. L., Bolen J. B., Rabinovitch P. S., Ledbetter J. A. Sulfhydryl oxidation down-regulates T-cell signaling and inhibits tyrosine phosphorylation of phospholipase C gamma 1. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):300–304. doi: 10.1073/pnas.89.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S., Garcia J., Pearson L., Soultanakis E., Dasgupta A., Gaynor R. Multiple transcriptional regulatory domains in the human immunodeficiency virus type 1 long terminal repeat are involved in basal and E1A/E1B-induced promoter activity. J Virol. 1989 Nov;63(11):4616–4625. doi: 10.1128/jvi.63.11.4616-4625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Gentry L. E., June C. H., Rabinovitch P. S., Purchio A. F. Stimulation of T cells through the CD3/T-cell receptor complex: role of cytoplasmic calcium, protein kinase C translocation, and phosphorylation of pp60c-src in the activation pathway. Mol Cell Biol. 1987 Feb;7(2):650–656. doi: 10.1128/mcb.7.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Rabinovitch P. S., Grossmann A., Tsu T. T., Imboden J. B. Signal transduction through CD4 receptors: stimulatory vs. inhibitory activity is regulated by CD4 proximity to the CD3/T cell receptor. Eur J Immunol. 1988 Apr;18(4):525–532. doi: 10.1002/eji.1830180406. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A., Kinney-Thomas E., Hu S. L. Effects of anti-gp120 monoclonal antibodies on CD4 receptor binding by the env protein of human immunodeficiency virus type 1. J Virol. 1988 Oct;62(10):3695–3702. doi: 10.1128/jvi.62.10.3695-3702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. C., Touzjian N., Stenzel M., Dorfman T., Sodroski J. G., Haseltine W. A. The NF kappa B independent cis-acting sequences in HIV-1 LTR responsive to T-cell activation. J Acquir Immune Defic Syndr. 1991;4(2):173–177. [PubMed] [Google Scholar]
- Mittler R. S., Hoffmann M. K. Synergism between HIV gp120 and gp120-specific antibody in blocking human T cell activation. Science. 1989 Sep 22;245(4924):1380–1382. doi: 10.1126/science.2571187. [DOI] [PubMed] [Google Scholar]
- Oyaizu N., Chirmule N., Kalyanaraman V. S., Hall W. W., Pahwa R., Shuster M., Pahwa S. Human immunodeficiency virus type 1 envelope glycoprotein gp120 produces immune defects in CD4+ T lymphocytes by inhibiting interleukin 2 mRNA. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2379–2383. doi: 10.1073/pnas.87.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psallidopoulos M. C., Schnittman S. M., Thompson L. M., 3rd, Baseler M., Fauci A. S., Lane H. C., Salzman N. P. Integrated proviral human immunodeficiency virus type 1 is present in CD4+ peripheral blood lymphocytes in healthy seropositive individuals. J Virol. 1989 Nov;63(11):4626–4631. doi: 10.1128/jvi.63.11.4626-4631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Rosoff P. M., Burakoff S. J., Greenstein J. L. The role of the L3T4 molecule in mitogen and antigen-activated signal transduction. Cell. 1987 Jun 19;49(6):845–853. doi: 10.1016/0092-8674(87)90622-2. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax W. J., Willems H. W., Reekers P. P., Capel P. J., Koene R. A. Polymorphism in mitogenic effect of IgG1 monoclonal antibodies against T3 antigen on human T cells. Nature. 1983 Aug 4;304(5925):445–447. doi: 10.1038/304445a0. [DOI] [PubMed] [Google Scholar]
- Thomas E. K., Weber J. N., McClure J., Clapham P. R., Singhal M. C., Shriver M. K., Weiss R. A. Neutralizing monoclonal antibodies to the AIDS virus. AIDS. 1988 Feb;2(1):25–29. doi: 10.1097/00002030-198802000-00004. [DOI] [PubMed] [Google Scholar]
- Thompson P. A., Ledbetter J. A., Rapp U. R., Bolen J. B. The Raf-1 serine-threonine kinase is a substrate for the p56lck protein tyrosine kinase in human T-cells. Cell Growth Differ. 1991 Dec;2(12):609–617. [PubMed] [Google Scholar]
- Tong-Starkesen S. E., Luciw P. A., Peterlin B. M. Signaling through T lymphocyte surface proteins, TCR/CD3 and CD28, activates the HIV-1 long terminal repeat. J Immunol. 1989 Jan 15;142(2):702–707. [PubMed] [Google Scholar]
- Watson A. J., Klaniecki J., Hanson C. V. Psoralen/UV inactivation of HIV-1-infected cells for use in cytologic and immunologic procedures. AIDS Res Hum Retroviruses. 1990 Apr;6(4):503–513. doi: 10.1089/aid.1990.6.503. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Haffar O., Sias J., Richman D. D., Spina C. A., Myers D. E., Kuebelbeck V., Ledbetter J. A., Uckun F. M. Inhibition of HIV replication by pokeweed antiviral protein targeted to CD4+ cells by monoclonal antibodies. Nature. 1990 Sep 6;347(6288):92–95. doi: 10.1038/347092a0. [DOI] [PubMed] [Google Scholar]




