Abstract
Myxoma virus (MYX) is a leporipoxvirus of rabbits that induces a lethal syndrome characterized by disseminated tumorlike lesions, generalized immunosuppression, and secondary gram-negative bacterial infection. A MYX deletion mutant (vMYX-GF- delta M11L) was constructed to remove the entire myxoma growth factor (MGF) coding sequence and that for the C-terminal five amino acids of the partially overlapping upstream gene, M11L. Unexpectedly, this deletion completely abrogates the capacity of MYX to cause the characteristic disease symptoms of myxomatosis. Upon inoculation of rabbits with vMYX-GF- delta M11L, recipient animals developed only a benign, localized nodule reminiscent of a Shope fibroma virus-induced tumor in which a single primary lesion appeared at the site of injection and then completely regressed within 14 days, leaving the animals resistant to challenge with wild-type MYX. No evidence of the purulent conjunctivitis and rhinitis that always accompany wild-type MYX infection was observed. To ascertain whether the attenuation observed in vMYX-GF- delta M11L was due to a combined effect of the MGF deletion and alteration of the upstream M11L gene, two additional MYX recombinants were constructed: an MGF- virus (vMYX-GF-) containing an intact M11L gene and an M11L- virus (vMYX-M11L-) containing an intact MGF gene. Infection with vMYX-GF- resulted in moderated symptoms of myxomatosis, but all clinical stages of the disease were still detectable. In contrast, disruption of M11L alone dramatically reduced the virus virulence, resulting in a nonlethal syndrome whose clinical course was nevertheless distinct from that of vMYX-GF- delta M11L. Upon inoculation with vMYX-M11L-, rabbits developed primary and secondary tumors which were larger and more circumscribed than those of wild-type MYX recipients. Whereas wild-type MYX infection always includes severe, purulent conjunctivitis and rhinitis, vMYX-M11L- recipients remained healthy and displayed only minimal signs of respiratory distress. By about 30 days after infection, the tumors induced by vMYX-M11L- had completely regressed and these animals were immune to challenge with wild-type MYX. Histological analysis indicated that tumors induced by vMYX-M11L- are much more heavily infiltrated with macrophages and heterophils and that the sites of viral replication are more edematous and necrotic than those of wild-type infection, suggesting that the host was able to mount a more vigorous inflammatory response to vMYX-M11L- infection.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


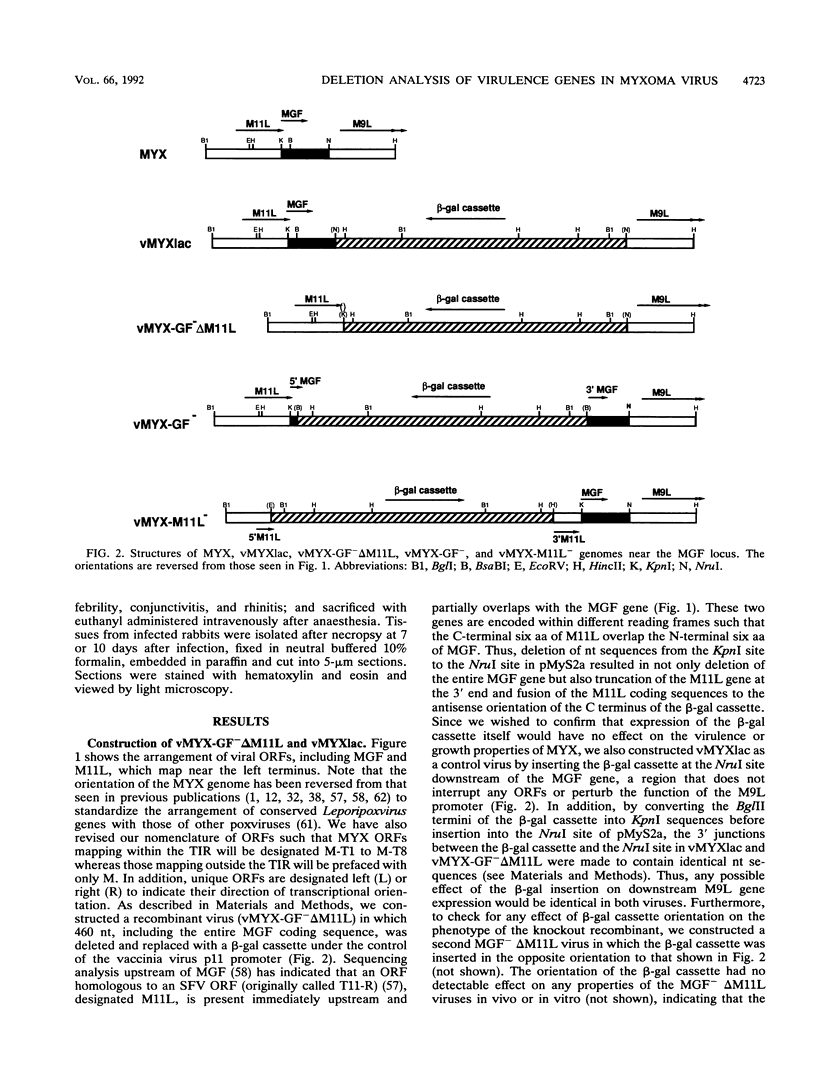


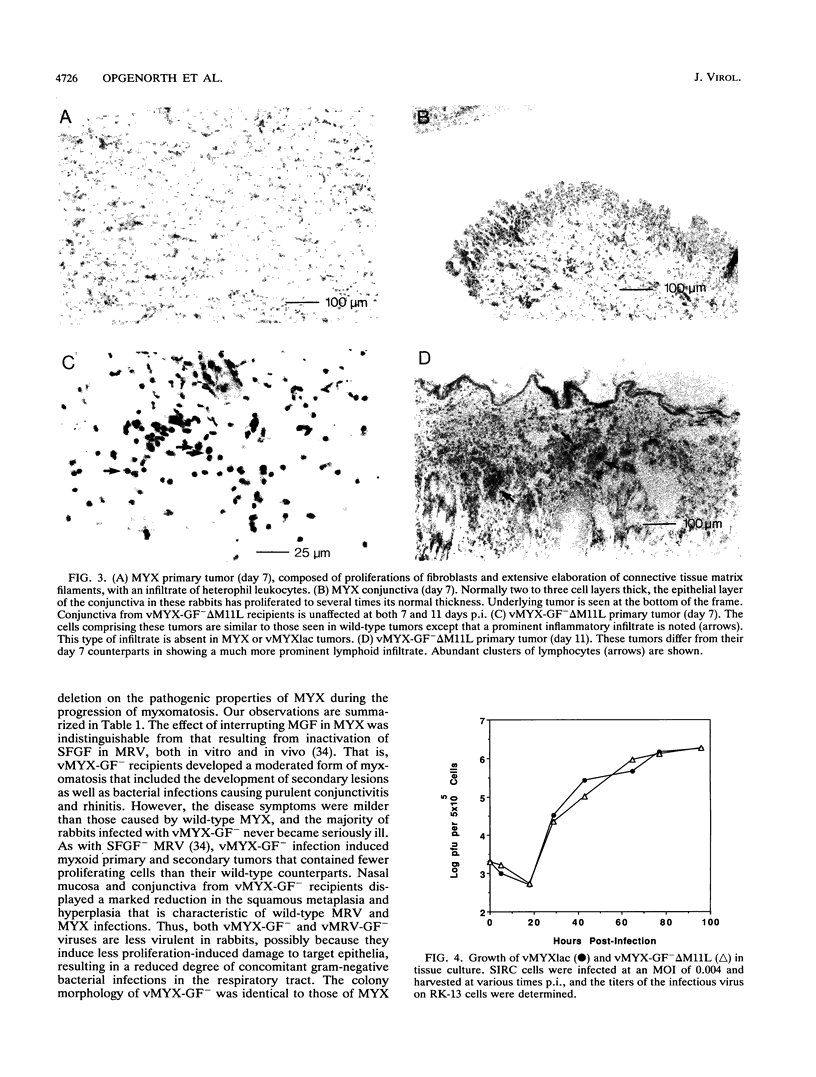



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block W., Upton C., McFadden G. Tumorigenic poxviruses: genomic organization of malignant rabbit virus, a recombinant between Shope fibroma virus and myxoma virus. Virology. 1985 Jan 15;140(1):113–124. doi: 10.1016/0042-6822(85)90450-7. [DOI] [PubMed] [Google Scholar]
- Boursnell M. E., Foulds I. J., Campbell J. I., Binns M. M. Non-essential genes in the vaccinia virus HindIII K fragment: a gene related to serine protease inhibitors and a gene related to the 37K vaccinia virus major envelope antigen. J Gen Virol. 1988 Dec;69(Pt 12):2995–3003. doi: 10.1099/0022-1317-69-12-2995. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Twardzik D. R., Marquardt H., Todaro G. J. Vaccinia virus encodes a polypeptide homologous to epidermal growth factor and transforming growth factor. Nature. 1985 Feb 7;313(6002):491–492. doi: 10.1038/313491a0. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Cooper J. A., Twardzik D. R., Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol. 1988 Mar;62(3):866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988 May;164(1):182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Palumbo G. J. Poxvirus pathogenesis. Microbiol Rev. 1991 Mar;55(1):80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W. Epidermal growth factor and transforming growth factor alpha. Br Med Bull. 1989 Apr;45(2):401–424. doi: 10.1093/oxfordjournals.bmb.a072331. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S. P., Prior S. E., Barstow D. A., Minton N. P. The pMTL nic- cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene. 1988 Aug 15;68(1):139–149. doi: 10.1016/0378-1119(88)90606-3. [DOI] [PubMed] [Google Scholar]
- Chang W., Upton C., Hu S. L., Purchio A. F., McFadden G. The genome of Shope fibroma virus, a tumorigenic poxvirus, contains a growth factor gene with sequence similarity to those encoding epidermal growth factor and transforming growth factor alpha. Mol Cell Biol. 1987 Jan;7(1):535–540. doi: 10.1128/mcb.7.1.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange A. M., Reddy M., Scraba D., Upton C., McFadden G. Replication and resolution of cloned poxvirus telomeres in vivo generates linear minichromosomes with intact viral hairpin termini. J Virol. 1986 Aug;59(2):249–259. doi: 10.1128/jvi.59.2.249-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F., BURNET F. M. A short description of the poxvirus group (vaccinia and related viruses). Virology. 1957 Oct;4(2):305–314. doi: 10.1016/0042-6822(57)90065-x. [DOI] [PubMed] [Google Scholar]
- FENNER F., MARSHALL I. D. A comparison of the virulence for European rabbits (Oryctolagus cuniculus) of strains of myxoma virus recovered in the field in Australia, Europe and America. J Hyg (Lond) 1957 Jun;55(2):149–191. doi: 10.1017/s0022172400037098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. Wallace P. Rowe lecture. Poxviruses of laboratory animals. Lab Anim Sci. 1990 Sep;40(5):469–480. [PubMed] [Google Scholar]
- Heard H. K., O'Connor K., Strayer D. S. Molecular analysis of immunosuppression induced by virus replication in lymphocytes. J Immunol. 1990 May 15;144(10):3992–3999. [PubMed] [Google Scholar]
- Heath R. B. The pathogenesis of respiratory viral infection. Postgrad Med J. 1979 Feb;55(640):122–127. doi: 10.1136/pgmj.55.640.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A., Knight M. E. Pulmonary and systemic defenses against challenge with Staphylococcus aureus in mice with pneumonia due to influenza A virus. J Infect Dis. 1979 Jul;140(1):105–108. doi: 10.1093/infdis/140.1.105. [DOI] [PubMed] [Google Scholar]
- King C. S., Cooper J. A., Moss B., Twardzik D. R. Vaccinia virus growth factor stimulates tyrosine protein kinase activity of A431 cell epidermal growth factor receptors. Mol Cell Biol. 1986 Jan;6(1):332–336. doi: 10.1128/mcb.6.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990 Nov 9;250(4982):827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988 Sep 8;335(6186):176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes two proteins that are structurally related to members of the plasma serine protease inhibitor superfamily. J Virol. 1989 Feb;63(2):600–606. doi: 10.1128/jvi.63.2.600-606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence D. J., Gusterson B. A. The epidermal growth factor. A review of structural and functional relationships in the normal organism and in cancer cells. Tumour Biol. 1990;11(5):229–261. [PubMed] [Google Scholar]
- Lin Y. Z., Caporaso G., Chang P. Y., Ke X. H., Tam J. P. Synthesis of a biological active tumor growth factor from the predicted DNA sequence of Shope fibroma virus. Biochemistry. 1988 Jul 26;27(15):5640–5645. doi: 10.1021/bi00415a037. [DOI] [PubMed] [Google Scholar]
- Lin Y. Z., Ke X. H., Tam J. P. Synthesis and structure-activity study of myxoma virus growth factor. Biochemistry. 1991 Apr 2;30(13):3310–3314. doi: 10.1021/bi00227a020. [DOI] [PubMed] [Google Scholar]
- Opgenorth A., Strayer D., Upton C., McFadden G. Deletion of the growth factor gene related to EGF and TGF alpha reduces virulence of malignant rabbit fibroma virus. Virology. 1992 Jan;186(1):175–191. doi: 10.1016/0042-6822(92)90072-w. [DOI] [PubMed] [Google Scholar]
- Pickup D. J., Ink B. S., Hu W., Ray C. A., Joklik W. K. Hemorrhage in lesions caused by cowpox virus is induced by a viral protein that is related to plasma protein inhibitors of serine proteases. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7698–7702. doi: 10.1073/pnas.83.20.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. D., Archard L. C. Characterization and physical mapping of Molluscum contagiosum virus DNA and location of a sequence capable of encoding a conserved domain of epidermal growth factor. J Gen Virol. 1987 Mar;68(Pt 3):673–682. doi: 10.1099/0022-1317-68-3-673. [DOI] [PubMed] [Google Scholar]
- Porter C. D., Blake N. W., Archard L. C. Structure and activity of epidermal-growth-factor-like peptides: induction of basal cell proliferation by a poxvirus gene product? Biochem Soc Trans. 1988 Oct;16(5):671–674. doi: 10.1042/bst0160671. [DOI] [PubMed] [Google Scholar]
- Russell R. J., Robbins S. J. Cloning and molecular characterization of the myxoma virus genome. Virology. 1989 May;170(1):147–159. doi: 10.1016/0042-6822(89)90362-0. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Kim N., Saeki T., Ciardiello F. Transforming growth factor-alpha: an oncodevelopmental growth factor. Cancer Cells. 1990 Dec;2(12):389–397. [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Wignall J. M., Din W. S., Farrah T., Upton C., McFadden G., Goodwin R. G. T2 open reading frame from the Shope fibroma virus encodes a soluble form of the TNF receptor. Biochem Biophys Res Commun. 1991 Apr 15;176(1):335–342. doi: 10.1016/0006-291x(91)90929-2. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Howard S. T., Chan Y. S. Vaccinia virus encodes a family of genes with homology to serine proteinase inhibitors. J Gen Virol. 1989 Sep;70(Pt 9):2333–2343. doi: 10.1099/0022-1317-70-9-2333. [DOI] [PubMed] [Google Scholar]
- Strayer D. S., Cabirac G., Sell S., Leibowitz J. L. Malignant rabbit fibroma virus: observations on the culture and histopathologic characteristics of a new virus-induced rabbit tumor. J Natl Cancer Inst. 1983 Jul;71(1):91–104. [PubMed] [Google Scholar]
- Strayer D. S., Laybourn K. A., Heard H. K. Determinants of the ability of malignant fibroma virus to induce immune dysfunction and tumor dissemination in vivo. Microb Pathog. 1990 Sep;9(3):173–189. doi: 10.1016/0882-4010(90)90020-q. [DOI] [PubMed] [Google Scholar]
- Strayer D. S., Leibowitz J. L. Virus-lymphocyte interactions during the course of immunosuppressive virus infection. J Gen Virol. 1987 Feb;68(Pt 2):463–472. doi: 10.1099/0022-1317-68-2-463. [DOI] [PubMed] [Google Scholar]
- Strayer D. S., Sell S. Immunohistology of malignant rabbit fibroma virus--a comparative study with rabbit myxoma virus. J Natl Cancer Inst. 1983 Jul;71(1):105–116. [PubMed] [Google Scholar]
- Strayer D. S., Sell S., Skaletsky E., Leibowitz J. L. Immunologic dysfunction during viral oncogenesis. I. Nonspecific immunosuppression caused by malignant rabbit fibroma virus. J Immunol. 1983 Nov;131(5):2595–2600. [PubMed] [Google Scholar]
- Strayer D. S., Skaletsky E., Leibowitz J. L., Dombrowski J. Growth of malignant rabbit fibroma virus in lymphoid cells. Virology. 1987 May;158(1):147–157. doi: 10.1016/0042-6822(87)90248-0. [DOI] [PubMed] [Google Scholar]
- Strayer D. S., Skaletsky E., Leibowitz J. L. In vitro growth of two related leporipoxviruses in lymphoid cells. Virology. 1985 Sep;145(2):330–334. doi: 10.1016/0042-6822(85)90167-9. [DOI] [PubMed] [Google Scholar]
- Strayer D. S., Skaletsky E., Sell S. Strain differences in Shope fibroma virus. An immunopathologic study. Am J Pathol. 1984 Aug;116(2):342–358. [PMC free article] [PubMed] [Google Scholar]
- Stroobant P., Rice A. P., Gullick W. J., Cheng D. J., Kerr I. M., Waterfield M. D. Purification and characterization of vaccinia virus growth factor. Cell. 1985 Aug;42(1):383–393. doi: 10.1016/s0092-8674(85)80133-1. [DOI] [PubMed] [Google Scholar]
- Traktman P. Poxviruses: an emerging portrait of biological strategy. Cell. 1990 Aug 24;62(4):621–626. doi: 10.1016/0092-8674(90)90106-o. [DOI] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. The molecular pathogenesis of poxviruses. Curr Top Microbiol Immunol. 1990;163:125–151. doi: 10.1007/978-3-642-75605-4_5. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Brown J. P., Ranchalis J. E., Todaro G. J., Moss B. Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5300–5304. doi: 10.1073/pnas.82.16.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C., Macen J. L., Maranchuk R. A., DeLange A. M., McFadden G. Tumorigenic poxviruses: fine analysis of the recombination junctions in malignant rabbit fibroma virus, a recombinant between Shope fibroma virus and myxoma virus. Virology. 1988 Sep;166(1):229–239. doi: 10.1016/0042-6822(88)90164-x. [DOI] [PubMed] [Google Scholar]
- Upton C., Macen J. L., McFadden G. Mapping and sequencing of a gene from myxoma virus that is related to those encoding epidermal growth factor and transforming growth factor alpha. J Virol. 1987 Apr;61(4):1271–1275. doi: 10.1128/jvi.61.4.1271-1275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C., Macen J. L., Schreiber M., McFadden G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991 Sep;184(1):370–382. doi: 10.1016/0042-6822(91)90853-4. [DOI] [PubMed] [Google Scholar]
- Upton C., Macen J. L., Wishart D. S., McFadden G. Myxoma virus and malignant rabbit fibroma virus encode a serpin-like protein important for virus virulence. Virology. 1990 Dec;179(2):618–631. doi: 10.1016/0042-6822(90)90129-f. [DOI] [PubMed] [Google Scholar]
- Upton C., Opgenorth A., Traktman P., McFadden G. Identification and DNA sequence of the Shope fibroma virus DNA topoisomerase gene. Virology. 1990 Jun;176(2):439–447. doi: 10.1016/0042-6822(90)90013-h. [DOI] [PubMed] [Google Scholar]
- Wills A., Delange A. M., Gregson C., Macaulay C., McFadden G. Physical characterization and molecular cloning of the Shope fibroma virus DNA genome. Virology. 1983 Oct 30;130(2):403–414. doi: 10.1016/0042-6822(83)90095-8. [DOI] [PubMed] [Google Scholar]
- Ye Y. K., Lin Y. Z., Tam J. P. Shope fibroma virus growth factor exhibits epidermal growth factor activities in newborn mice. Biochem Biophys Res Commun. 1988 Jul 29;154(2):497–501. doi: 10.1016/0006-291x(88)90167-2. [DOI] [PubMed] [Google Scholar]




