Abstract
The nuclear hormone receptor retinoic acid receptor–related orphan receptor γt (RORγt) is required for the generation of T helper 17 cells expressing the proinflammatory cytokine interleukin (IL)-17. In vivo, however, less than half of RORγt+ T cells express IL-17. We report here that RORγt+ Tαβ cells include Foxp3+ cells that coexist with IL-17–producing RORγt+ Tαβ cells in all tissues examined. The Foxp3+ RORγt+ Tαβ express IL-10 and CCL20, and function as regulatory T cells. Furthermore, the ratio of Foxp3+ to IL-17–producing RORγt+ Tαβ cells remains remarkably constant in mice enduring infection and inflammation. This equilibrium is tuned in favor of IL-10 production by Foxp3 and CCL20, and in favor of IL-17 production by IL-6 and IL-23. In the lung and skin, the largest population of RORγt+ T cells express the γδ T cell receptor and produce the highest levels of IL-17 independently of IL-6. Thus, potentially antagonistic proinflammatory IL-17–producing and regulatory Foxp3+ RORγt+ T cells coexist and are tightly controlled, suggesting that a perturbed equilibrium in RORγt+ T cells might lead to decreased immunoreactivity or, in contrast, to pathological inflammation.
T cells adapt immune responses not only to the antigens, but also to the type of pathogen they face. Cell-based pathogens, such as viruses, intracellular bacteria, and tumors evoke a CD4+ Th1 type of response characterized by the production of IFN-γ. In contrast, extracellular-based pathogens, such as worms and allergens, evoke a Th2 type of response characterized by the production of IL-4, IL-5, and IL-13 (1). Recently, some bacteria and fungi have been found to evoke a Th17 type of response characterized by the production of IL-17 (or IL-17A), IL-17F, IL-21, IL-22, IL-6, and TNF-α, as well as IL-23 by APCs (2–6). The proinflammatory Th17 response induces the recruitment of neutrophils and is involved in autoimmune inflammatory diseases. However, the reactivity of T cells is modulated by T regulatory (T reg) cells. These include so-called natural Foxp3+ T reg cells that are induced in the thymus (7) as well as adaptive Foxp3+ or Foxp3− T reg cells that are induced in the periphery and produce TGF-β and/or IL-10 (8, 9).
The generation of these distinct T cell effector types is under control of specific cytokines and transcription factors (2). In mice, TGF-β is required for the generation of both Th17 and T reg cells. The switch to the Th17 pathway is induced by IL-6 and/or IL-21 (4–6, 10–12), and requires IL-23 for full effector maturation (2, 3). However, TGF-β together with IL-6 induces CD4+ T cells to produce both IL-17 and IL-10, a population that is not able to induce inflammation in mice (13). In contrast, IL-23 generates proinflammatory cells that produce only IL-17. In accordance with these findings, spontaneous colitis is induced in mice deficient for IL-10 (14), and the inflammatory disease is prevented by concomitant deficiency in IL-23 (15). The immunosuppressive IL-10 can be produced by all types of T cells, including a subset of T reg cells (16), and is induced by IL-27 in Th1 and Th2 cells (17–19). TGF-β alone induces the T reg cell pathway (20) and is strongly promoted in the gut-associated lymphoid tissues by all-trans retinoic acid (RA) (21, 22). The generation of T reg cells requires the forkhead/winged-helix transcription factor Foxp3 (23–25). Mice deficient in Foxp3 lack natural T reg cells (24) and are highly susceptible to inflammatory disease (26).
The development of inflammatory pathologies, such as experimental autoimmune encephalomyelitis (27, 28), an animal model of multiple sclerosis, collagen-induced arthritis (CIA) (29), and inflammatory bowel disease (15, 30), depends on Th17 cells and IL-23. In humans, a coding variant of Il23 confers protection to Crohn's disease (31). The Th17–IL-23 pathway in also involved in resistance to a growing list of bacterial and fungal pathogens in mice (11, 32–35), and Th17 cells are readily isolated from patients with Candida infection (36). However, a comprehensive picture of the class of pathogens targeted by this pathway remains to be drawn. T reg cells, on the other hand, and IL-10 in particular, have been shown to limit inflammation and adaptive immunity to a variety of pathogens (37), as well as to promote long-term T cell memory (38). Collectively, these studies demonstrate the essential role of the Th17 and the T reg cells in immunity, immunoregulation, and immunopathology. They also suggest that a balanced expansion of these Th subsets during immune responses might ensure efficient yet restrained immunity that limits damage to self.
The nuclear hormone receptor RA receptor–related orphan receptor γt (RORγt) is a marker for Th17 cells and is required for their generation (39). Using an enhanced GFP (EGFP) reporter mouse, we found that RORγt+ Tαβ cells included IL-10–producing Foxp3+ T reg cells. These cells coexisted with IL-17–producing RORγt+ Tαβ cells in all tissues examined in an equilibrium regulated by Foxp3 and CCL20 in favor of Foxp3+ cells, and by IL-6 and IL-23 in favor of IL-17–producing cells. Foxp3 was shown to bind to RORγt, suggesting that it directly regulates RORγt activity. The equilibrium within RORγt+ Tαβ cells was maintained during massive inflammation of the intestine or the lungs, but it was perturbed in cancer. We suggest that the balanced expansion of RORγt+ Tαβ cell subsets during inflammation might ensure efficient yet regulated immunity, and that perturbation of this equilibrium might lead to inadequate immune reactivity to tumors, pathogens, or self.
RESULTS
Diversity and distribution of RORγt+ T cells in vivo
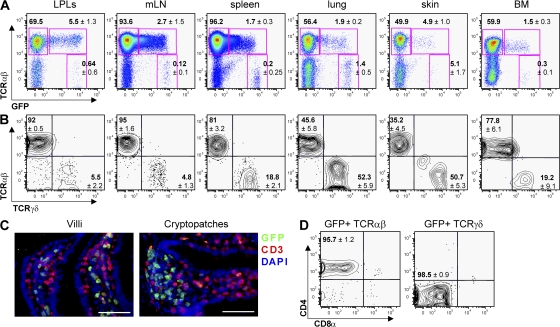
To optimize the visualization of RORγt+ cells, we have generated transgenic Rorc(γt)-GfpTG mice expressing EGFP under control of the Rorc(γt) locus on a bacterial artificial chromosome (BAC) (40). An estimated 5–10 copies of the BAC transgene were integrated, as assessed by quantitative PCR. The expression pattern of EGFP in Rorc(γt)-GfpTG mice was identical to the one described previously in Rorc(γt)+/GFP mice carrying one allele of Rorc(γt) inserted with EGFP (39, 41). Faithful expression of GFP was further confirmed by immunofluorescence histology of GFP and endogenous RORγt in various organs from Rorc(γt)-GfpTG mice. In all tissues analyzed, we found a strict correlation between GFP and RORγt protein expression (not depicted). High expression of GFP was found in fetal lymphoid tissue inducer (LTi) cells, LTi-like cells in the adult intestine, in immature CD4+CD8+ thymocytes (not depicted), and in a subpopulation of peripheral T cells (Fig. 1 A). The highest frequency of RORγt+ T cells was observed in the intestine, as previously reported (39), which were localized in the lamina propria of the villi, whereas CD3− RORγt+ cells, i.e., LTi-like cells, were clustered in cryptopatches that are precursor structures to isolated lymphoid follicles (Fig. 1 C) (42). RORγt+ T cells were also found in all organs or tissues tested, including the LNs, spleen, lung, skin, and bone marrow (Fig. 1 A). Assessing the homogeneity of these populations within different organs, we observed that up to 50% of RORγt+ T cells in the lung and skin expressed the γδ TCR instead of the αβ TCR (Fig. 1 B). RORγt+ Tγδ cells expressed the highest levels of EGFP (Fig. 1 A) and none of the CD4 or CD8 coreceptors (Fig. 1 D).
Figure 1.
Diversity and distribution of RORγt+ T cells in vivo. (A and B) Flow cytometry analysis of cells isolated from the organs of 8–12-wk-old Rorc(γt)-GfpTG mice. Plots are gated on CD3+ cells (A) or GFP+ CD3+ cells (B). Numbers indicate mean percent cells in quadrants ± SD obtained with at least three Rorc(γt)-GfpTG mice. LPLs, lamina propria lymphocytes isolated from small intestine; mLN, mesenteric LNs; BM, bone marrow. (C) Immunofluorescence histology of RORγt+ cells in the small intestine of Rorc(γt)-GfpTG mice. Most RORγt+ cells in villi are T cells, whereas RORγt+ cells in cryptopatches located between crypts are CD3− LTi cells. Bar, 50 μm. (D) Expression of CD4 and CD8α by spleen GFP+TCR-β+ and lung or GFP+TCR-δ+ cells.
RORγt+ T cells include both Th17 and IL-10–producing Foxp3+ T reg cells
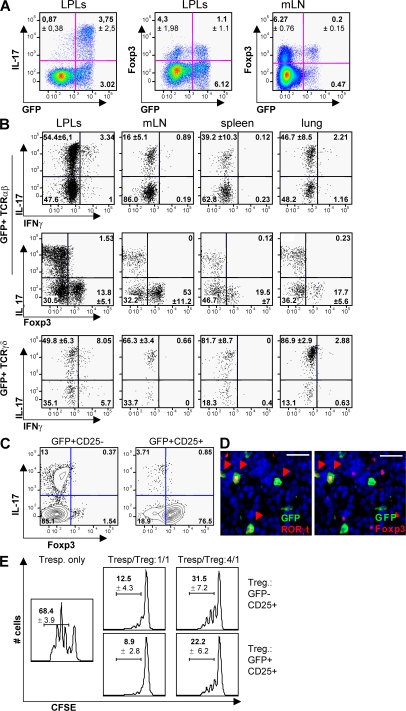
We assessed whether RORγt was a marker for IL-17–producing cells in both Tαβ and Tγδ populations, and stained RORγt+ T cells for IL-17, IFN-γ, and Foxp3. Surprisingly, even though a majority of IL-17–producing cells expressed RORγt (Fig. 2 A), only 15–50% of RORγt+ Tαβ cells expressed IL-17, depending on the organ examined, and few but detectable numbers of RORγt+ Tαβ cells produced both IL-17 and IFN-γ (Fig. 2 B). In contrast, a majority (50–90%) of RORγt+ Tγδ cells expressed high levels of IL-17. Even more surprising, 15–50% of IL-17− RORγt+ Tαβ cells expressed the T reg cell markers Foxp3 and CD25 (Fig. 2, B and C), with the highest incidence in LNs, even though Foxp3+ RORγt+ Tαβ cells represented only a minority of the total population of Foxp3+ T cells (Fig. 2 A). No RORγt+ Tαβ cells were found to express both IL-17 and Foxp3, and no RORγt+ Tγδ cells expressed Foxp3 (not depicted). The coexpression of RORγt and Foxp3 protein at the single cell level was confirmed by immunofluorescence histology of the LNs (Fig. 2 D). Furthermore, Foxp3+ RORγt+ Tαβ cells were functionally T reg cells, as they suppressed in vitro proliferation of activated CD4+ T cells (Fig. 2 E).
Figure 2.
RORγt+ T cells include Th17 and Foxp3+ T reg cells. (A and B) Flow cytometry analysis of cells isolated from Rorc(γt)-GfpTG mice. Cells were restimulated in vitro with PMA/ionomycin for 5 h and subjected to intracellular staining for GFP, IL-17, Foxp3, and/or IFN-γ. Plots are gated on TCR-β+ cells (A) and GFP+TCR-β+ or GFP+TCR-δ+ cells (B). Numbers indicate mean percent cells in quadrants ± SD obtained with at least three Rorc(γt)-GfpTG mice. (C) CD4+ T cells isolated from the spleen and mesenteric LNs of Rorc(γt)-GfpTG mice were sorted into two populations based on their expression of GFP and CD25. Sorted cells were subsequently restimulated in vitro and stained for IL-17 and Foxp3. Results are representative of three independent experiments. (D) Immunofluorescence histology staining for GFP, RORγt, Foxp3, and nuclei (DAPI) of a mesenteric LN isolated from a Rorc(γt)-GfpTG mouse. Arrowheads indicate cells expressing RORγt and/or Foxp3. Bar, 20 μm. (E) Proliferation assay of CD4+ T cells isolated from the spleen and mesenteric LNs of Rorc(γt)-GfpTG mice and sorted into two populations based on their expression of GFP and CD25. CFSE-labeled CD25− responder T cells (2.5 × 104) were cultured alone (left) or together with either GFP−CD25+ T cells or GFP+CD25+ T cells (2.5 × 104 or 0.625 × 104 cells). Cells were cultured in duplicates for 3 d in the presence of 105 irradiated spleen cells and anti-CD3ε antibody. Numbers indicate frequency of proliferating cells (± SD) obtained from three independent experiments.
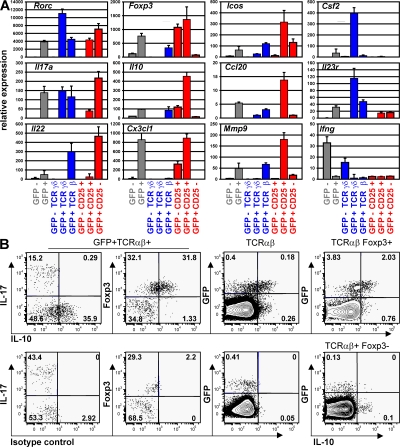
We next measured the expression, by the different subsets of RORγt+ T cells isolated by FACS (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20080034/DC1), of a restricted panel of genes involved in the Th17 and T reg cell pathways using quantitative qRT-PCR arrays. As compared with RORγt− T cells, RORγt+ T cells expressed higher levels of Rorc, Il17a, Il17f, il22, and Il23r, as expected, as well as of Il10, Icos, Ccl20, Cx3cl1, and Mmp9 (Fig. S2). Expression of these genes was further examined in the individual RORγt+ T cell subsets. As cells could not be fixed and stained for Foxp3 expression before isolation for RNA recovery, Foxp3+ RORγt+ T cells were isolated on the basis of CD25 expression. As expected, CD25− RORγt+ Tαβ cells enriched in IL-17–producing cells (Fig. 2 C), as well as RORγt+ Tγδ cells, expressed high levels of Il17a, Il17f, and Il22 transcripts (Fig. 3 A and not depicted). CD25+ RORγt+ Tαβ cells expressed detectable levels of Il17a and Il17f transcripts, probably due to the presence of a small population of IL-17–producing CD25+ cells (Fig. 2 C). In addition, RORγt+ Tγδ cells, but not RORγt− Tγδ cells, expressed Csf2, which codes for GM-CSF involved in the mobilization of granulocytes and monocytes.
Figure 3.
RORγt+ T cells express genes involved in the Th17 or T reg cell pathway. (A) Cells isolated from the spleen and mesenteric LNs of Rorc(γt)-GfpTG mice were sorted into eight distinct populations based on their expression of GFP, CD3, TCR-β, TCR-δ, CD4, and CD25 (Fig. S1), and gene expression was assessed using real-time PCR. Ct values were normalized to the mean Ct of five housekeeping genes. Data are the mean of two or three independent experiments. (B) Foxp3+ RORγt+ T cells express IL-10. Cells isolated from LNs of Rorc(γt)-GfpTG mice were restimulated in vitro with PMA/ionomycin for 5 h and subjected to intracellular staining for GFP, IL-17, Foxp3, and IL-10 or an isotype control. Numbers indicate percent cells in quadrants. Results are representative of at least three individual experiments.
CD25+ (Foxp3+) RORγt+ Tαβ cells specifically expressed high levels of the expected Foxp3 transcript, as well as Il10, Icos, Ccl20, Cx3cl1, and Mmp9. IL-10 expression was confirmed at the protein level, and Foxp3+, but not Foxp3− RORγt+ Tαβ, cells represented a major fraction of total IL-10–producing cells in LNs (Fig. 3 B), and to a lesser extent in the gut (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20080034/DC1). The transcript coding for CCL20 was specifically expressed by Foxp3+ RORγt+ Tαβ cells and barely detected in other T cell populations. Furthermore, CCR6, the receptor for CCL20, was expressed by both CD25+ and CD25− RORγt+ Tαβ cells (Fig. S4), suggesting that CD25+ RORγt+ Tαβ cells can regulate the recruitment of RORγt+ Tαβ cells. Interestingly, ICOS (43), CCL20 (13, 44), and MMP-9 (45, 46) have been associated with Th17 cells previously, but not with T reg cells, whereas CX3CL1 is normally expressed by activated endothelial cells (47).
Generation and regulation of IL-17+ or Foxp3+ RORγt+ cells
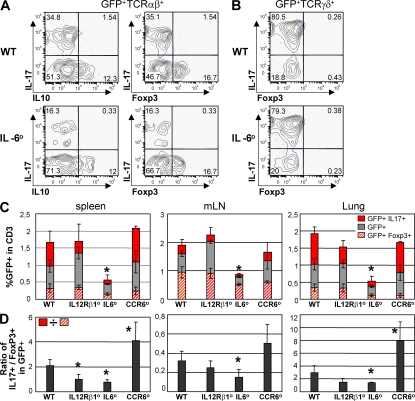
We further assessed how the generation of IL-17–producing or Foxp3+ RORγt+ T cells in vivo was affected by the absence of factors shown to be required for the generation of Th17 or T reg cells, such as IL-6 (4–6, 10–12) and Foxp3 (24, 26). In IL-6–deficient mice, the number and proportion of RORγt+ Tαβ cells, in particular of IL-17–producing RORγt+ Tαβ cells, were significantly decreased, whereas the proportion of IL-10–producing Foxp3+ RORγt+ T cells was unaffected (Fig. 4, A, C, and D). This confirms the requirement of IL-6 for the generation of Th17 cells, but also shows that IL-6 is not required for the generation of IL-10–producing RORγt+ T cells, even though in vitro it acts in synergy with TGF-β to induce IL-10–producing CD4+ T cells (13, 17). In contrast, IL-17–producing RORγt+ Tγδ cells were unaffected by the absence of IL-6 (Fig. 4 B). Furthermore, in IL-12Rβ1–deficient mice, unresponsive to both IL-12 and IL-23 (2, 3), the proportion of IL-17–producing RORγt+ Tαβ cells was slightly decreased (Fig. 4, C and D), in accordance with the important but not essential role of IL-23 in the generation of Th17 cells (2, 3).
Figure 4.
The role of IL-6, IL-12Rβ1, and CCR6 in the generation of RORγt+ T cell subsets. (A and B) Cells were isolated from the spleen of Rorc(γt)-GfpTG (WT) or Il6−/−× Rorc(γt)-GfpTG (IL-6°) mice, restimulated in vitro with PMA/ionomycin for 5 h and subjected to intracellular staining for GFP, IL-17, Foxp3, and IL-10. Plots are gated on GFP+TCR-β+ cells (A) or GFP+TCR-δ+ cells (B). Numbers indicate percent cells in quadrants and are representative of two independent experiments. (C) Cells were isolated from the spleen, mesenteric LN, and lung of Rorc(γt)-GfpTG (WT), Il12rb1−/−× Rorc(γt)-GfpTG (IL12Rβ1°), Ccr6−/− × Rorc(γt)-GfpTG (CCR6°), or Il6−/−× Rorc(γt)-GfpTG mice (IL-6°) and processed as in A and B. Histograms report percent GFP+TCR-β+ cell subsets in total T cells. Each bar also shows the percentages of IL-17+ and Foxp3+ cells within the GFP+TCR-β+ cell population. (D) Histograms report the ratio of the frequencies of IL-17–producing GFP+TCR-β+ cells to Foxp3+ GFP+TCR-β+ cells as indicated in the figure. Three to five mice were analyzed per group. *, P < 0.05 as compared with WT.
It has recently been shown that Th17 cells isolated from SKG mice, which spontaneously develop arthritis, as well as human Th17 cells, express CCR6 (44, 48, 49).We therefore assessed the impact of CCR6 on the generation of IL-17–producing or Foxp3+ RORγt+ T cells. In CCR6-deficient mice (50), contrary to IL6- or IL12Rβ1-deficient mice, the proportion of IL-17–producing RORγt+ Tαβ cells was significantly increased (Fig. 4, C and D). As CD25+ (Foxp3+) RORγt+ Tαβ cells specifically express Ccl20 transcripts (Fig. 3 A), these cells might regulate the generation of IL-17–producing RORγt+ Tαβ cells through CCR6.
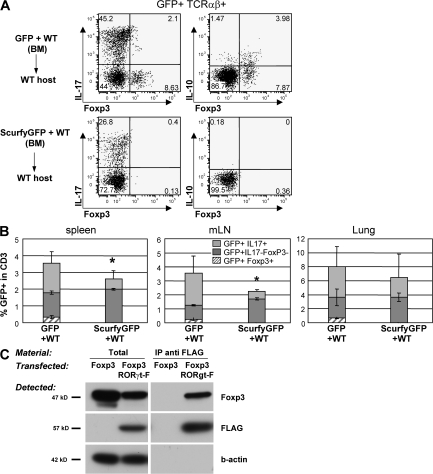
We next assessed whether Foxp3 regulates the generation of IL-17– or IL-10–producing RORγt+ Tαβ cells. Mice bearing the Scurfy mutation in the Foxp3 gene (Foxp3sf mice) lack T reg cells (24, 26). In these mice, however, the analysis of RORγt+ T cells was complicated by generalized autoimmunity, and the development of immature RORγt+ CD4−CD8− thymocytes was abnormal (not depicted). Therefore, mixed bone marrow chimeras were generated by adoptive transfer of bone marrow from Foxp3sf × Rorc(γt)-GfpTG and WT mice into irradiated lymphopenic hosts (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20080034/DC1). In such chimeras, RORγt+ T cells do not express Foxp3, but cells from WT mice protected the host from a scurfy phenotype (not depicted). Foxp3sf RORγt+ Tαβ cells failed to generate IL-10–producing cells and generated a reduced proportion of IL-17–producing cells as compared with Foxp3wt RORγt+ Tαβ cells in control chimeras (Fig. 5, A and B). This shows that Foxp3 protein or Foxp3+ cells are required for the generation of IL-10–producing RORγt+ Tαβ cells and, surprisingly, also favor the differentiation of IL-17–producing RORγt+ Tαβ cells.
Figure 5.
Regulation of RORγt+ T cells by Foxp3. (A and B) Bone marrow cells isolated from CD45.2+Rorc(γt)-GfpTG (WT) or Foxp3sf × Rorc(γt)-GfpTG (Scurfy) mice were mixed with bone marrow cells isolated from CD45.1+ C57BL/6 mice and injected into irradiated lymphopenic mice. 6 wk after transfer, cells were isolated from the spleen, LN, and lung and restimulated in vitro with PMA/ionomycin for 5 h and subjected to intracellular staining for GFP, IL-17, Foxp3, and IL-10. (A) Spleen cells are gated on GFP+TCR-β+ cells. Numbers indicate percent cells in quadrants. Reconstitutions with CD45.1+ and CD45.2+ cells were comparable in all chimeras. Results are representative of two independent experiments. (B) Histograms report percent GFP+TCR-β+ cell subsets in total CD45.2+ T cells (see Fig. 4 C). *, P < 0.05 as compared with WT. (C) Immunoprecipitation of RORγt and Foxp3. Foxp3 was expressed in HEK293T cells alone or together with N-terminally FLAG-tagged RORγt. Total cell lysates or immunoprecipitates (IP) using anti-FLAG antibody were blotted and probed with antibodies to Foxp3, FLAG, or β-actin. Results are representative of two independent experiments.
To test whether Foxp3 regulates RORγt+ Tαβ cells through direct interaction with RORγt, N-terminally FLAG-tagged RORγt was expressed in HEK293T cells together with Foxp3. Foxp3 was specifically coimmunoprecipitated with FLAG-RORγt, indicating that the two factors interact (Fig. 5 C). Collectively, these data suggest that Foxp3 induces the generation of IL-10–producing RORγt+ T cells and regulates RORγt activity directly. On the other hand, RORγt is required for the generation of Th17 cells (39), but not for the generation of “mainstream” Foxp3+ and/or IL-10–producing T cells. In Rorc(γt)-CreTG × Rosa-Stopfl-Dta mice, which lack RORγt+ T cells due to their specific expression of the lethal diphtheria toxin subunit A (51), no or few IL-17–producing cells were detected (not depicted), and the proportion of total Foxp3+ or IL-10–producing cells was unaffected (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20080034/DC1).
Equilibrium between IL-17+ or Foxp3+ RORγt+ cells during inflammation
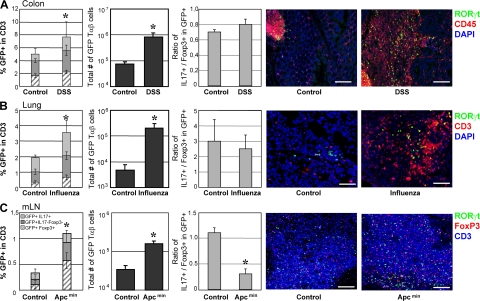
Given the prominent role of IL-17–producing cells in inflammation and of IL-10–producing cells in immune regulation, we assessed if and how the relative frequencies of the different RORγt+ T cell subsets were modified in mice enduring severe intestinal inflammation, virus infection, or cancer. Dextran sodium sulfate (DSS) was administered orally to Rorc(γt)-GfpTG mice until the development of chronic intestinal inflammation (Fig. 6 A). A high number of T cells was recovered in DSS-treated mice and both the frequency and the number of RORγt+ Tαβ cells increased, but the ratio of IL-17–producing to Foxp3+ RORγt+ T cells remained constant. Similar results were obtained in mice exposed to intranasal infection with influenza A virus, even though their lungs sustained heavy leukocyte infiltration (Fig. 6 B). In contrast, in Rorc(γt)-GfpTG x Apcmin/+ mice bearing colonic and ileal polyps and tumors (52), no significant infiltration of leukocyte and RORγt+ Tαβ cells was observed in the colon and the terminal ileum (Fig. S7, available at http://www.jem.org/cgi/content/full/jem.20080034/DC1), whereas the mesenteric LNs were largely hyperplastic. The ratio of IL-17–producing to Foxp3+ RORγt+ T cell in these LNs was significantly decreased, even though the frequency and total number of RORγt+ Tαβ cells were increased (Fig. 6 C). Similar results have recently been obtained comparing Th17 and Foxp3 cells in mice injected with B16 melanoma (53). These data indicate that the relative frequency of IL-17–producing or Foxp3+ RORγt+ Tαβ cells can remain constant during severe inflammation or infection. However, chronic tumors can alter this balance in favor of the generation or recruitment of Foxp3+ RORγt+ Tαβ cells producing the immunosuppressive IL-10.
Figure 6.
Equilibrium in RORγt+ T cell subsets during pathology. (A) Rorc(γt)-GfpTG mice were treated with DSS in the drinking water for 6 d, followed by water for 10 d. This protocol was repeated for a total of three cycles. After the last cycle, cells isolated from the colon were restimulated in vitro with PMA/ionomycin for 5 h and subjected to intracellular staining for GFP, IL-17, and Foxp3. Histograms (from left to right) report percent GFP+TCR-β+ cell subsets in total T cells (see Fig. 4 C), total numbers of RORγt+ Tαβ cells present in the organ, and the ratio of IL-17–producing to Foxp3+ cells within RORγt+ Tαβ cells (see Fig. 4 D). Right panels show immunofluorescence histology of a colon from a healthy or a treated Rorc(γt)-GfpTG mouse. Bar, 100 μm. (B) Rorc(γt)-GfpTG mice were infected intranasally with 100 PFUs of influenza A virus for 7 d. Cells were then isolated from the lung and processed as in A. Right panels show immunofluorescence histology of a lung from healthy or an infected Rorc(γt)-GfpTG mouse. Bar, 50 μm. (C) Cells were isolated from the mesenteric LNs of a 4-mo-old Rorc(γt)-GfpTG × Apcmin/+ mouse and processed as in A. Right panels show immunofluorescence histology of a mesenteric LN from a normal or a tumor-bearing mouse. Bar, 100 μm. Data shown are representative of at least three independent experiments. Three to four mice were analyzed per group. *, P < 0.05 as compared with control (mock-treated or WT mice).
Generation of Th17, Foxp3+, or IL-10–producing RORγt+ T cells in vitro
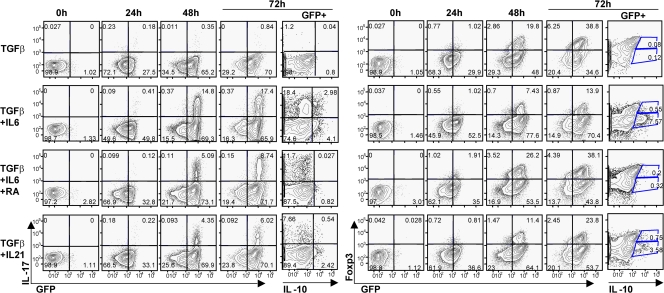
Many steps in the differentiation pathway of Th17 cells have been inferred from in vitro studies. We therefore assessed if the distinct subsets of RORγt+ Tαβ cells we have identified in vivo were derived in vitro from naive CD4+ T cells. TGF-β together with IL-6 or IL-21 induced the generation of Th17 cells from naive CD4+ T cells (4–6, 10–12) after massive up-regulation of RORγt expression after 1–2 d of culture (Fig. 7) (39). In contrast, TGF-β alone induces the generation of T reg cells (20). However, we also observed that most cells up-regulated of RORγt after 1–2 d of culture in the presence of TGF-β alone, and all Foxp3+ cells coexpressed RORγt after 2–3 d. Up-regulation of RORγt was specific to these conditions because stimulation under Th1 conditions did not induce RORγt+ expression (Fig. S8, available at http://www.jem.org/cgi/content/full/jem.20080034/DC1). Expression of RORγt in the culture of naive CD4+ T cells in the presence of TGF-β has been shown before at the population level (39), but not in individual cells expressing Foxp3. In contrast to their in vivo counterparts, however, few in vitro–derived Foxp3+ RORγt+ Tαβ cells expressed IL-10, which was rather expressed by RORγt− Tαβ cells. IL-10 was not induced by TGF-β alone but required the addition of IL-6, as found previously (13, 17), or the addition of IL-21. IL-10 production was inhibited in the presence of RA, even though RA favored the generation of Foxp3+ RORγt+ Tαβ cells and decreased the generation of IL-17–producing cells in the presence of TGF-β and IL-6. A combination of TGF-β with IL-6 (with or without RA) or IL-21 generated IL-17–producing as well as IL-10–producing or Foxp3+ RORγt+ Tαβ cells, showing that the engagement of these pathways is not mutually exclusive at the cell population level, in agreement with recent reports (13, 17). RORγt+ Tαβ cells producing both IL-17 and IL-10 could be generated in the presence of TGF-β and IL-6, but Foxp3+ RORγt+ T cells produced neither IL-10 nor IL-17 (not depicted). Collectively, these data show that even though naive CD4+ T cells differentiate in vitro into IL-17–producing, IL-10–producing, or Foxp3+ RORγt+ Tαβ cells, substantial differences exist with the in vivo RORγt+ Tαβ cell subsets in the phenotype of the cells producing IL-10, and the proportion of Foxp3+ cells expressing RORγt.
Figure 7.
Generation and differentiation of RORγt+ Tαβ cells in vitro. MACS-sorted naive (CD62L+) CD4+ T cells from the spleens of Rorc(γt)-GfpTG mice were stimulated in duplicates with anti-CD3ε and anti-CD28 in the presence of blocking anti–IFN-γ and anti–IL-4 antibodies and the indicated cytokines or RA. After different periods of time, cells were restimulated with PMA/ionomycin for 5 h and analyzed by flow cytometry for the expression of GFP, Foxp3, IL-17, and IL-10. All plots are gated on TCR-β+ cells, except plots for IL-10 that are gated on GFP+TCR-β+ cells. Numbers indicate percent cells in quadrants. Data are representative of three independent experiments.
DISCUSSION
In this study, we have shown that RORγt+ T cells include both IL-17– and IL-10–producing Foxp3+ T reg cells, which are endowed with proinflammatory and regulatory functions, respectively. In addition, RORγt+ T cells include Tγδ cells producing high levels of IL-17 in an IL-6–independent pathway. The relative frequency of IL-17– or IL-10–producing RORγt+ Tαβ cells is maintained constantly during infection or inflammation, suggesting that a robust mechanism maintains an equilibrium between these two effector arms. However, this equilibrium is perturbed in mice harboring intestinal tumors. IL-6, Foxp3, and CCR6, and to a lesser extent IL-23, are essential regulators of this equilibrium.
Ivanov et al. (39) reported that RORγt+ T cells or IL-17–producing cells were only present in the intestinal lamina propria. These authors therefore suggested that the intestinal flora induces the differentiation of RORγt+ IL-17–producing T cells. We show now that although these cells are more abundant in the intestine, sizeable populations of RORγt+ T cells were found in all organs examined, including the LNs, spleen, lung, skin, and bone marrow. This extended distribution of RORγt+ T cells was not due to infection, as specific pathogen-free mice were used in all experiments. This discrepancy might be explained by the sensitivity of the reporter mice that have been used. Ivanov et al. (39) used a knock-in mouse encoding one copy of the Egfp reporter gene inserted into the Rorc(γt) locus (41), whereas we used a transgenic mouse encoding 5–10 copies of a BAC carrying a similar Egfp insertion and therefore induces a brighter reporter expression. The more extended distribution of RORγt+ IL-17–producing T cells, in particular in the LNs and spleen, might better explain the occurrence of Th17-dependent inflammatory disease in the brain or joints of mice enduring experimental autoimmune encephalomyelitis or CIA (27–29, 39).
Foxp3+ RORγt+ Tαβ cells were an important fraction of RORγt+ T cells. They produced the immunosuppressive IL-10 and functioned as genuine T reg cells in vitro. Furthermore, these cells, but not IL-17–producing RORγt+ T cells, also expressed high levels of the transcript coding for CCL20. This transcript was undetected in RORγt− T cells, suggesting that Foxp3+ RORγt+ T cells were the main source of CCL20 in T cells, even though it might be expressed by a small subset of RORγt− T cells. In accordance with our results, McGeachy et al. (13) have reported that CD4+ T cells cultured in the presence of TGF-β and IL-6, which produce both IL-17 and IL-10, express Ccl20, in contrast to cells cultured with IL-23 alone, which produce only IL-17. CCL20 is the ligand for CCR6, suggesting that Foxp3+ RORγt+ T cells regulate the recruitment of CCR6+ T cells. Th17 cells isolated from SKG mice, which spontaneously develop arthritis, as well as human Th17 cells, express CCR6 (44, 48, 49). CCR6 is also expressed by a subset of “memory-like” CD25+ T reg cells that produce elevated levels of Il10 transcripts (54). We found that 35–50% of RORγt+ Tαβ cells expressed CCR6, regardless of their expression of CD25 (or Foxp3). Importantly, CCR6-deficient mice generated an increased ratio of IL-17– to IL-10–producing RORγt+ Tαβ cells as compared with WT mice. These results suggest that Foxp3+ RORγt+ T cells producing IL-10 and CCL20 recruit CCR6+ RORγt+ T cells and negatively regulate their differentiation into IL-17–producing cells.
Foxp3+ RORγt+ T cells also expressed elevated levels of the costimulatory receptor gene Icos, shown to be essential for the induction of CIA and the optimal generation of Th17 cells (43, 55). The expression of ICOS is also induced by IL-23 in human cells (56). Furthermore, Foxp3+ RORγt+ T cells specifically expressed the metalloproteinase gene Mmp9, induced by IL-23 in a mouse tumor model (57), by IL-17 in the lung (45) and by IL-17R in inflamed joints (46), as well as elevated levels of the fractalkine gene Cx3cl1. The functional consequences of elevated expression of Icos, Mmp9, and Cx3cl1 by Foxp3+ RORγt+ remain to be explored, but this expression pattern appears paradoxical as the product of these genes might promote rather than modulate inflammation through the recruitment and activation of effectors, as well as through tissue remodeling through MMP-9. It might be suggested that the role of Foxp3+ RORγt+ T cells is not merely to regulate inflammation through IL-10, but also to participate in the recruitment of inflammatory cells such as Th17 cells to an immunosuppressive environment. On the other hand, we cannot formally exclude that Foxp3+ RORγt+ T cells contain distinct functional subsets of IL-10–producing and/or CCL20-, ICOS-, MMP-9–, and CX3CL1-expressing cells.
Of note, 30–50% of RORγt+ Tαβ cells did not express IL-17, IL-10, or Foxp3. We could not purify these cells from IL-17 producers for transcript analysis, as no markers were found to distinguish these cells before intracellular cytokine staining. It is possible that non–IL-17 and non–IL-10 producers contained uncommitted RORγt+ T cells, i.e., not yet committed to either the Th17 or the T reg cell pathway. They could also represent a third committed subpopulation that remains to be characterized. In favor of the first hypothesis, only few IFN-γ– and no IL-4–producing cells (not depicted) could be detected in RORγt+ T cells.
RORγt+ Tγδ cells have been reported previously to represent 50% of total Tγδ cells in the lamina propria of the small intestine, a majority of them expressing IL-17 (39). In the CIA model, 60–80% of Vγ4+ Tγδ cells and an equivalent number of CD4+ Tαβ cells produce IL-17 in the draining LNs (58). Furthermore, Tγδ cells are a primary source of IL-17 in the lung before and during infection with Mycobacterium tuberculosis (59) or Mycobacterium bovis (60), and Vδ1+ Tγδ cells produce IL-17 during intraperitoneal infection with Escherichia coli (61). In accordance with these data, we found that RORγt+ Tγδ cells were a major source of IL-17 in the lung and skin, as >80% of them produced IL-17, representing half of the total RORγt+ T cell population in these compartments. RORγt+ Tγδ cells expressed no Foxp3, but produced the highest levels of RORγt, as well of Il23r and csf2 coding for GM-CSF. The latter induces the differentiation of granulocytes and monocytes, as well as the production of proinflammatory cytokines such as TNF-α and IL-6 (62). In contrast to RORγt+ Tαβ cells, IL-17–producing RORγt+ Tγδ cells were not affected by the absence of IL-6 in deficient mice, indicating that RORγt+ Tγδ cells and IL-17–producing RORγt+ Tαβ cells are generated by distinct pathways. Collectively, these data suggest that RORγt+ Tγδ cells play an important role in inflammation to pathogens through the production of IL-17 and GM-CSF; however, their precise role in vivo remains to be assessed.
RORγt is required for the generation of Th17 cells and has been shown to induce Th17 cells when transduced into mouse naive CD4+ T cells (39). Furthermore, naive CD4+ T cells cultured in the presence of TGF-β and IL-6 or IL-21 generate Th17 cells (4–6, 10–12) and IL-17–producing RORγt+ T cells (Fig. 4). We find that a large fraction of RORγt+ Tαβ cells expressed Foxp3, in particular in the LNs, as well as the immunosuppressive IL-10, and functioned as T reg cells. It has been shown previously that culture conditions that favor the generation of Th17 cells from naive CD4+ T cells, i.e., in the presence of TGF-β and IL-6, also induce the generation of IL-10–producing cells (13, 17). But in contrast to the in vivo situation, IL-10 was produced by Foxp3− rather than Foxp3+ RORγt+ T cells, indicating that these culture systems might not faithfully replicate in vivo pathways. Interestingly, RA blocked the generation of IL-10–producing cells, whereas it favored the generation of Foxp3+ RORγt+ T cells. Furthermore, cultures of CD4+ T cells in the presence of TGF-β and IL-6 or IL-21 generated RORγt+ T cells expressing both IL-17 and IL-10, as observed previously (13, 17). However, RORγt+ T cells producing both cytokines could not be detected in vivo, exposing potential conflicts between in vivo and in vitro differentiation pathways.
Most cells cultured in the presence of TGF-β alone expressed RORγt after 1–2 d, but then differentiated into Foxp3+ RORγt+ T cells that did not express IL-17 after 2–3 d. It has been previously reported that TGF-β alone induces significant levels of Rorc(γt) transcripts but no detectable Il17 or Il23r transcripts (4–6). These data indicate that RORγt is necessary but not sufficient for the generation of Th17 cells from naive CD4+ T cells, in accordance with the observation that cells cultured in the presence of TGF-β and IL-6 express RORγt after 1–2 d, but express IL-17 only after 2–3 d of culture (Fig. 4) (39). Collectively, these data indicate that TGF-β is opening a RORγt-dependent differentiation pathway in CD4+ T cells that leads either to Th17 or T reg cells, depending on the presence of maturation and polarizing factors such as IL-6, IL-21, RA, IL-23, and possibly IL-10.
The ratio of Th17 to T reg cells within RORγt+ T cells varied between organs, with the highest proportions of Th17 cells in the intestine and lung, and of Foxp3+ RORγt+ T cells in the LNs. This ratio was tuned in favor of Th17 cells by IL-6 and IL-23, as the ratio of IL-17–producing to Foxp3+ RORγt+ T cells decreased in IL-6– or IL-12Rβ1–deficient mice. However, in IL-6–deficient mice, the total number of RORγt+ Tαβ cells was also significantly diminished, indicating that IL-6 is involved more generally in the generation of RORγt+ Tαβ cells, as observed previously (6, 39). Conversely, the ratio of Th17 to T reg cells within RORγt+ T cells was tuned in favor of T reg cells by Foxp3 and CCL20, as inferred from scurfy mice or CCR6-deficient mice. Foxp3 might regulate the fate of “uncommitted” RORγt+ T cells into IL-17– or IL-10–producing cells by directly modifying the molecular function of RORγt, as Foxp3 was coimmunoprecipitated with RORγt. Foxp3 might also act through Foxp3+ RORγt+ T cells or “mainstream” Foxp3+ T cells regulating RORγt+ T cells. Interestingly, cells derived from scurfy mice generated a decreased proportion of RORγt+ Tαβ cells and IL-17–producing RORγt+ Tαβ cells, indicating a more complex role for Foxp3 in the generation and regulation of RORγt+ Tαβ cells that remains to be elucidated.
A robust mechanism maintains an equilibrium between Th17 to T reg cells within RORγt+ T cells. During massive inflammation induced by DSS in the intestine, the total number of RORγt+ T cells increased 10-fold in the intestine and >10-fold in influenza A virus–infected lungs. However, the numbers of IL-17– and IL-10–producing (Foxp3+) RORγt+ T cells were increased in comparable proportions. Regulating IL-17 versus IL-10 production might promote inflammation while limiting “collateral” damage, a necessary compromise between effective immunity and tissue integrity. IL-10–deficient mice, which lack one of these effectors arms, generate increased proportions of IL-17–producing CD4+ T cells and develop spontaneous colitis in an IL-23–dependent pathway (15). In contrast, Apcmin/+ mice that developed large colon and ileal polyps and tumors by 3–4 mo of age generated an increased proportion of IL-10–producing RORγt+ T cells. Similar observations have been made in mice injected with the B16 melanoma, which develop an increased ratio of Foxp3+ cells versus IL-17–producing cells as early as 8 d after tumor injection (53). The mechanisms used by tumors to alter the equilibrium between IL-17– and IL-10–producing RORγt+ T cells remain to be investigated, but this phenomenon is reminiscent of the IL-10–mediated immunosuppression induced by diverse viruses and bacteria (63). In contrast, the equilibrium between IL-17– and IL-10–producing RORγt+ T cells might be disrupted in favor of Th17 cells in chronic inflammatory diseases such as rheumatoid arthritis, multiple sclerosis, or inflammatory bowel disease. Tools to control the fate of RORγt+ T cells might therefore prove useful either to boost or to limit IL-17–producing cells. Potential targets include RORγt as well as any factors that regulate the differentiation of RORγt+ T cells into IL-17– or IL-10–producing effectors.
MATERIALS AND METHODS
Mice.
BAC-transgenic Rorc(γt)-GfpTG or Rorc(γt)-CreTG mice were generated as described previously (40). The coding sequence for EGFP or Cre, including the stop codon, was inserted into exon 1 of Rorc(γt) in place of the endogenous ATG translation start codon, on a 200-kb BAC (Invitrogen) carrying at least 70 kb of sequence upstream of the Rorc(γt) translation start site. Transgenic mice used in transfer experiments were backcrossed eight times to C57BL/6 mice. CCR6-deficient (50), Rosa-Stopfl-Dta (51), and Rag2−/−Il2rb−/− (64) mice have been described previously. Foxp3sf, Il12rb1−/−, Il6−/−, and Apcmin/+ mice were obtained from The Jackson Laboratory. All mice were kept in specific pathogen–free conditions, and all animal experiments were approved by the committee on animal experimentation of the Institut Pasteur and by the French Ministry of Agriculture.
Antibodies.
The following mAbs were purchased from BD Biosciences: PE-conjugated anti–IL-17 (TC11-18H10), PerCP-conjugated anti-CD4 (RM4-5) and purified anti-CD3ε (145-2C11), and anti–IFN-γ (XMG1.2). The following were purchased from eBioscience: PE-conjugated anti–IL-10 (JES5-16E3), TCR-δ (GL3), TCR-β (H57-597), and rat IgG1 isotype control; APC-conjugated anti–IL-10 (JES5-16E3), CD45.2 (104), TCR-β (H57-597), and rat IgG2b isotype control; PE-Cy5–conjugated anti-Foxp3 (FJK-16S); PE-Cy7–conjugated anti-CD4 (GK1.5), CD8α (53-6.7), and streptavidin; biotinylated anti–TCR-δ (GL3), CD25 (PC61.5), and CD45.2 (104); APC-Alexa750–conjugated anti-CD3ε (17A2); Alexa647-conjugated anti–IFN-γ (XMG1.2); and purified anti-CD28 (37.51) and anti–IL-4 (11B11). PE-conjugated anti-CCR6 was from R&D Systems. Purified anti-GFP (A-11122) and FITC-conjugated anti–rabbit polyclonal antibodies were from Invitrogen. Anti-RORγ was described previously (41).
Flow cytometry.
Fragments of small intestine or colon were first incubated in PBS (Ca/Mg free) containing 15 ml of 1 mM DTT and 3 mM EDTA, and then for 40 min at 37°C in DMEM medium containing 1 mg/ml collagenase (Roche) and 1 U/ml DNase (Invitrogen). Lung and ear skin were directly incubated for 40 min to 1 h at 37°C in DMEM medium containing collagenase and DNase. Tissue suspensions were then pressed through a 100-μm mesh, pelleted, resuspended in a 40% isotonic Percoll solution (GE Healthcare), and underlaid with an 80% isotonic Percoll solution. Centrifugation for 20 min at 2,000 rpm yielded the mononuclear cells at the 40–80% interface. Cells were finally washed twice with PBS-F (PBS containing 2% FCS). Single cell suspensions were prepared from the thymus, spleen, and LNs by pressing the tissues through a 100-μm mesh and from the bone marrow by flushing the long bones with PBS. All cells were first preincubated with mAb 2.4G2 to block Fcγ receptors, and then washed and incubated with the indicated mAb conjugates for 40 min in a total volume of 100 μl PBS-F. For intracellular cytokine staining, cells were stimulated for 5 h in complete medium in the presence of 50 ng/ml phorbol 12-myristate 13-acetate and 500 ng/ml ionomycin (both from Sigma-Aldrich). For the last 2 h, 10 μg brefeldin A (Sigma-Aldrich) was added to the cultures. After surface staining for CD3, CD4, TCR-β, and/or TCR-δ, cells were fixed with fresh 2% paraformaldehyde in PBS for 20 min. Intracellular staining was performed in permeabilization-solution (1% saponin in FACS buffer) with anti–IL-17 (BD Biosciences), anti–IFN-γ, anti-Foxp3, and/or anti–IL-10. Polyclonal anti-GFP was used to increase GFP signal during intracellular staining. Cells were analyzed on a FACSCanto (BD Biosciences), followed by analysis with FlowJo software (Tristar).
In vitro T cell differentiation.
Spleen and mesenteric LN cells from Rorc(γt)-GfpTG mice were pooled and T cells were purified using a CD4+ T cell isolation kit (Miltenyi) and further sorted into naive CD4+CD62L+ cells using CD62L Microbeads (Miltenyi). 3 × 105 cells were stimulated for 1–3 d in complete DMEM with 5 μg/ml anti-CD3, 5 μg/ml anti-CD28, 10 μg/ml of neutralizing anti–IFN-γ, and 10 μg/ml anti–IL-4 in the presence of 10 ng/ml human TGF-β, 10 ng/ml mouse IL-6, 100 ng/ml mouse IL-21, and/or 100 nM RA (Sigma-Aldrich).
In vitro proliferation assay.
2.5 × 104 FACS-sorted CD4+CD25− T cells were labeled with CFSE using CellTrace Cell Proliferation kit (Invitrogen) and cultured alone or in the presence of 2.5 × 104 (or 0.625 × 104) of either GFP+ or GFP− CD4+CD25+ T cells sorted from the spleen and LNs of Rorc(γt)-GfpTG mice. T cells were stimulated in duplicates with irradiated splenic feeder cells (105 per well) and 1 μg/ml of soluble anti-CD3 in 96-well U-bottom plates for 96 h.
Gene expression analysis.
To obtain RNA for gene expression analysis by real-time RT-PCR, 500–5,000 cells were directly sorted into vials containing RLT buffer (QIAGEN) supplemented with β-mercaptoethanol, and total RNA was extracted using an RNeasy Micro kit (QIAGEN). The quality of total RNA was assessed using the 2100 Bioanalyzer system (Agilent Technologies). 250–500 pg of high quality total RNA was subjected to one linear mRNA amplification cycle using the MessageBooster kit for quantitative RT-PCR (Epicentre Biotechnologies). 50–100 ng of amplified mRNA was then converted to cDNA using the RT2 PCR Array first strand kit (SuperArray Bioscience Corporation). All procedures were performed according to the manufacturer's protocols. The expression of 84 different genes was measured using the mouse Th17 RT2 Profiler PCR Array (SuperArray Bioscience Corporation). Real-time PCR was performed on a PTC-200 thermocycler equipped with a Chromo4 detector (Bio-Rad Laboratories). Data were analyzed using Opticon Monitor software (Bio-Rad Laboratories).
Immunofluorescence histology.
Tissues were washed and fixed overnight at 4°C in a fresh solution of 4% paraformaldehyde (Sigma-Aldrich) in PBS. The samples were then washed for 1 d in PBS, incubated in a solution of 30% sucrose (Sigma-Aldrich) in PBS until the samples sank, embedded in OCT compound 4583 (Sakura Finetek), frozen in a bath of isopentane cooled with liquid nitrogen, and stocked at −80°C. Frozen blocs were cut at 8-μm thickness, and sections were collected onto Superfrost/Plus slides (VWR). Slides were dried for 1 h and processed for staining or stocked at −80°C. For staining, slides were first hydrated in PBS-XG (PBS containing 0.1% Triton X-100 and 1% normal goat serum; Sigma-Aldrich) for 5 min and blocked with 10% bovine serum in PBS-XG for 1 h at room temperature. Endogenous biotin was blocked with a biotin blocking kit (Vector Laboratories). Slides were then incubated with primary polyclonal antibody or conjugated mAb (in general 1/100) in PBS-XG overnight at 4°C, washed three times for 5 min with PBS-XG, incubated with secondary conjugated polyclonal antibody or streptavidin for 1 h at room temperature, washed once, incubated with DAPI (Sigma-Aldrich) for 5 min at room temperature, washed three times for 5 min, and mounted with Fluoromount-G (SouthernBiotech). Slides were examined under an AxioImager M1 fluorescence microscope (Carl Zeiss, Inc.) equipped with a CCD camera, and images were processed with AxioVision software (Carl Zeiss, Inc.).
Immunoprecipitation.
A FLAG-encoding sequence was added to the 5′ end of the Rorc(γt) cDNA by PCR and cloned into the pIRES2-EGFP vector (Clontech Laboratories, Inc.), and the FoxP3 cDNA was cloned into the pIRES-DsRed vector (Clontech Laboratories, Inc.). 4 μg of each construct was cotransfected into 1.106 293T cells using Lipofectamine 2000 (Invitrogen). After 48 h, immunoprecipitation was performed using the FLAG Tagged Protein Immunoprecipitation kit (Sigma-Aldrich) and resolved on a 10% gel bis-tris acrylamide gel.
DSS and influenza.
To induce colitis, Rorc(γt)-GfpTG mice were treated with 2.5% DSS (MP Biomedicals) in the drinking water for 6 d, followed by water for 10 d. This protocol was repeated for a total of three cycles. To infect lungs, Rorc(γt)-GfpTG mice were infected intranasally with 100 PFUs of influenza A virus (H3N2 strain Scotland/20/74), and mice were killed after 7 d.
Chimeras.
Bone marrow cells isolated from CD45.2+Rorc(γt)-GfpTG or Foxp3sf × Rorc(γt)-GfpTG mice were treated with a lineage depletion kit (Miltenyi). Purified cells were then mixed in a 1:1 ratio with lin− bone marrow cells from CD45.1+ C57BL/6 mice, and a total of 4 × 105 was injected into Rag2−/−Il2rb−/− mice (64) irradiated at 600 rads. After 4 wk, reconstitution of the mice was assessed in peripheral blood. Reconstituted mice were killed and analyzed 6 wk after transfer.
Online supplemental material.
In Fig. S1, the purification of the different subsets of RORγt+ T cells is shown. Fig. S2 shows that RORγt+ T cells express genes involved in the Th17 or T reg cell pathway. In Fig. S3, expression of IL-10 by intestinal RORγt+ Tαβ cells is depicted. Fig. S4 shows the expression of CCR6 by RORγt+ Tαβ cells. Fig. S5 shows the generation of mixed bone marrow chimeras, and Fig. S6 shows the generation of Foxp3+ T cells in mice ablated of RORγt+ cells. In Fig. S7, immunofluorescence histology of terminal ileum from a WT or a 4-mo-old Rorc(γt)-GfpTG x Apcmin/+ mouse developing polyps and carcinoma is shown. Figs. S1–S7 are available at http://www.jem.org/cgi/content/full/jem.20080034/DC1.
Supplementary Material
Acknowledgments
We thank L. Rogge, E. Bianchi, A. Bandeira, P. Vieira, C. Leclerc, D. Guy-Grand, and T. Sparwasser for discussions and critical reading of the manuscript; A. Freitas for Foxp3sf mice; M. Albert for Il6−/− mice; and L. Polomack and V. Balloy for technical assistance.
This work was supported by Institut Pasteur, CNRS, INSERM, Agence Nationale de la Recherche, Fondation de la Recherche Médicale, Mairie de Paris, a Marie Curie Excellence grant, and Deutsche Forschungsgemeinschaft (to M. Lochner).
The authors have no conflicting financial interests.
Abbreviations used: BAC, bacterial artificial chromosome; CIA, collagen-induced arthritis; DSS, dextran sodium sulfate; EGFP, enhanced GFP; LTi, lymphoid tissue inducer; RA, retinoic acid; RORγt, RA receptor–related orphan receptor γt; T reg, T regulatory.
L. Peduto and M. Cherrier contributed equally to this work.
References
- 1.Mosmann, T.R., and R.L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173. [DOI] [PubMed] [Google Scholar]
- 2.Weaver, C.T., R.D. Hatton, P.R. Mangan, and L.E. Harrington. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821–852. [DOI] [PubMed] [Google Scholar]
- 3.Kastelein, R.A., C.A. Hunter, and D.J. Cua. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25:221–242. [DOI] [PubMed] [Google Scholar]
- 4.Nurieva, R., X.O. Yang, G. Martinez, Y. Zhang, A.D. Panopoulos, L. Ma, K. Schluns, Q. Tian, S.S. Watowich, A.M. Jetten, and C. Dong. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 448:480–483. [DOI] [PubMed] [Google Scholar]
- 5.Korn, T., E. Bettelli, W. Gao, A. Awasthi, A. Jager, T.B. Strom, M. Oukka, and V.K. Kuchroo. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 448:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou, L., I.I. Ivanov, R. Spolski, R. Min, K. Shenderov, T. Egawa, D.E. Levy, W.J. Leonard, and D.R. Littman. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8:967–974. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. [DOI] [PubMed] [Google Scholar]
- 8.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 9.Groux, H. 2003. Type 1 T-regulatory cells: their role in the control of immune responses. Transplantation. 75:8S–12S. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 11.Mangan, P.R., L.E. Harrington, D.B. O'Quinn, W.S. Helms, D.C. Bullard, C.O. Elson, R.D. Hatton, S.M. Wahl, T.R. Schoeb, and C.T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234. [DOI] [PubMed] [Google Scholar]
- 12.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 13.McGeachy, M.J., K.S. Bak-Jensen, Y. Chen, C.M. Tato, W. Blumenschein, T. McClanahan, and D.J. Cua. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8:1390–1397. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 75:263–274. [DOI] [PubMed] [Google Scholar]
- 15.Yen, D., J. Cheung, H. Scheerens, F. Poulet, T. McClanahan, B. McKenzie, M.A. Kleinschek, A. Owyang, J. Mattson, W. Blumenschein, et al. 2006. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116:1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Garra, A., and P. Vieira. 2007. T(H)1 cells control themselves by producing interleukin-10. Nat. Rev. Immunol. 7:425–428. [DOI] [PubMed] [Google Scholar]
- 17.Stumhofer, J.S., J.S. Silver, A. Laurence, P.M. Porrett, T.H. Harris, L.A. Turka, M. Ernst, C.J. Saris, J.J. O'Shea, and C.A. Hunter. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8:1363–1371. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald, D.C., G.X. Zhang, M. El-Behi, Z. Fonseca-Kelly, H. Li, S. Yu, C.J. Saris, B. Gran, B. Ciric, and A. Rostami. 2007. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 8:1372–1379. [DOI] [PubMed] [Google Scholar]
- 19.Awasthi, A., Y. Carrier, J.P. Peron, E. Bettelli, M. Kamanaka, R.A. Flavell, V.K. Kuchroo, M. Oukka, and H.L. Weiner. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8:1380–1389. [DOI] [PubMed] [Google Scholar]
- 20.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mucida, D., Y. Park, G. Kim, O. Turovskaya, I. Scott, M. Kronenberg, and H. Cheroutre. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260. [DOI] [PubMed] [Google Scholar]
- 22.Benson, M.J., K. Pino-Lagos, M. Rosemblatt, and R.J. Noelle. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams, L.M., and A.Y. Rudensky. 2007. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 8:277–284. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 25.Lahl, K., C. Loddenkemper, C. Drouin, J. Freyer, J. Arnason, G. Eberl, A. Hamann, H. Wagner, J. Huehn, and T. Sparwasser. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. [DOI] [PubMed] [Google Scholar]
- 27.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veldhoen, M., R.J. Hocking, R.A. Flavell, and B. Stockinger. 2006. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 7:1151–1156. [DOI] [PubMed] [Google Scholar]
- 29.Murphy, C.A., C.L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R.A. Kastelein, J.D. Sedgwick, and D.J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hue, S., P. Ahern, S. Buonocore, M.C. Kullberg, D.J. Cua, B.S. McKenzie, F. Powrie, and K.J. Maloy. 2006. Interleukin-23 drives innate and T cell–mediated intestinal inflammation. J. Exp. Med. 203:2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duerr, R.H., K.D. Taylor, S.R. Brant, J.D. Rioux, M.S. Silverberg, M.J. Daly, A.H. Steinhart, C. Abraham, M. Regueiro, A. Griffiths, et al. 2006. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 314:1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malley, R., A. Srivastava, M. Lipsitch, C.M. Thompson, C. Watkins, A. Tzianabos, and P.W. Anderson. 2006. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect. Immun. 74:2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Happel, K.I., P.J. Dubin, M. Zheng, N. Ghilardi, C. Lockhart, L.J. Quinton, A.R. Odden, J.E. Shellito, G.J. Bagby, S. Nelson, and J.K. Kolls. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khader, S.A., G.K. Bell, J.E. Pearl, J.J. Fountain, J. Rangel-Moreno, G.E. Cilley, F. Shen, S.M. Eaton, S.L. Gaffen, S.L. Swain, et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377. [DOI] [PubMed] [Google Scholar]
- 35.Zelante, T., A. De Luca, P. Bonifazi, C. Montagnoli, S. Bozza, S. Moretti, M.L. Belladonna, C. Vacca, C. Conte, P. Mosci, et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37:2695–2706. [DOI] [PubMed] [Google Scholar]
- 36.Acosta-Rodriguez, E.V., L. Rivino, J. Geginat, D. Jarrossay, M. Gattorno, A. Lanzavecchia, F. Sallusto, and G. Napolitani. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646. [DOI] [PubMed] [Google Scholar]
- 37.Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875–888. [DOI] [PubMed] [Google Scholar]
- 38.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov, I.I., B.S. McKenzie, L. Zhou, C.E. Tadokoro, A. Lepelley, J.J. Lafaille, D.J. Cua, and D.R. Littman. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 40.Sparwasser, T., S. Gong, J.Y. Li, and G. Eberl. 2004. General method for the modification of different BAC types and the rapid generation of BAC transgenic mice. Genesis. 38:39–50. [DOI] [PubMed] [Google Scholar]
- 41.Eberl, G., S. Marmon, M.J. Sunshine, P.D. Rennert, Y. Choi, and D.R. Littman. 2004. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5:64–73. [DOI] [PubMed] [Google Scholar]
- 42.Eberl, G., and D.R. Littman. 2004. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 305:248–251. [DOI] [PubMed] [Google Scholar]
- 43.Nurieva, R.I., P. Treuting, J. Duong, R.A. Flavell, and C. Dong. 2003. Inducible costimulator is essential for collagen-induced arthritis. J. Clin. Invest. 111:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Hirota, K., H. Yoshitomi, M. Hashimoto, S. Maeda, S. Teradaira, N. Sugimoto, T. Yamaguchi, T. Nomura, H. Ito, T. Nakamura, et al. 2007. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 204:2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prause, O., S. Bozinovski, G.P. Anderson, and A. Linden. 2004. Increased matrix metalloproteinase-9 concentration and activity after stimulation with interleukin-17 in mouse airways. Thorax. 59:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koenders, M.I., J.K. Kolls, B. Oppers-Walgreen, L. van den Bersselaar, L.A. Joosten, J.R. Schurr, P. Schwarzenberger, W.B. van den Berg, and E. Lubberts. 2005. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 52:3239–3247. [DOI] [PubMed] [Google Scholar]
- 47.Bazan, J.F., K.B. Bacon, G. Hardiman, W. Wang, K. Soo, D. Rossi, D.R. Greaves, A. Zlotnik, and T.J. Schall. 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature. 385:640–644. [DOI] [PubMed] [Google Scholar]
- 48.Annunziato, F., L. Cosmi, V. Santarlasci, L. Maggi, F. Liotta, B. Mazzinghi, E. Parente, L. Fili, S. Ferri, F. Frosali, et al. 2007. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204:1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson, N.J., K. Boniface, J.R. Chan, B.S. McKenzie, W.M. Blumenschein, J.D. Mattson, B. Basham, K. Smith, T. Chen, F. Morel, et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8:950–957. [DOI] [PubMed] [Google Scholar]
- 50.Varona, R., R. Villares, L. Carramolino, I. Goya, A. Zaballos, J. Gutierrez, M. Torres, A.C. Martinez, and G. Marquez. 2001. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J. Clin. Invest. 107:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brockschnieder, D., Y. Pechmann, E. Sonnenberg-Riethmacher, and D. Riethmacher. 2006. An improved mouse line for Cre-induced cell ablation due to diphtheria toxin A, expressed from the Rosa26 locus. Genesis. 44:322–327. [DOI] [PubMed] [Google Scholar]
- 52.Moser, A.R., E.M. Mattes, W.F. Dove, M.J. Lindstrom, J.D. Haag, and M.N. Gould. 1993. ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc. Natl. Acad. Sci. USA. 90:8977–8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kryczek, I., S. Wei, L. Zou, S. Altuwaijri, W. Szeliga, J. Kolls, A. Chang, and W. Zou. 2007. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol. 178:6730–6733. [DOI] [PubMed] [Google Scholar]
- 54.Kleinewietfeld, M., F. Puentes, G. Borsellino, L. Battistini, O. Rotzschke, and K. Falk. 2005. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 105:2877–2886. [DOI] [PubMed] [Google Scholar]
- 55.Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wassink, L., P.L. Vieira, H.H. Smits, G.A. Kingsbury, A.J. Coyle, M.L. Kapsenberg, and E.A. Wierenga. 2004. ICOS expression by activated human Th cells is enhanced by IL-12 and IL-23: increased ICOS expression enhances the effector function of both Th1 and Th2 cells. J. Immunol. 173:1779–1786. [DOI] [PubMed] [Google Scholar]
- 57.Langowski, J.L., X. Zhang, L. Wu, J.D. Mattson, T. Chen, K. Smith, B. Basham, T. McClanahan, R.A. Kastelein, and M. Oft. 2006. IL-23 promotes tumour incidence and growth. Nature. 442:461–465. [DOI] [PubMed] [Google Scholar]
- 58.Roark, C.L., J.D. French, M.A. Taylor, A.M. Bendele, W.K. Born, and R.L. O'Brien. 2007. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J. Immunol. 179:5576–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lockhart, E., A.M. Green, and J.L. Flynn. 2006. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177:4662–4669. [DOI] [PubMed] [Google Scholar]
- 60.Umemura, M., A. Yahagi, S. Hamada, M.D. Begum, H. Watanabe, K. Kawakami, T. Suda, K. Sudo, S. Nakae, Y. Iwakura, and G. Matsuzaki. 2007. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 178:3786–3796. [DOI] [PubMed] [Google Scholar]
- 61.Shibata, K., H. Yamada, H. Hara, K. Kishihara, and Y. Yoshikai. 2007. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178:4466–4472. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton, J.A., and G.P. Anderson. 2004. GM-CSF biology. Growth Factors. 22:225–231. [DOI] [PubMed] [Google Scholar]
- 63.Redpath, S., P. Ghazal, and N.R. Gascoigne. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9:86–92. [DOI] [PubMed] [Google Scholar]
- 64.Vosshenrich, C.A., S. Lesjean-Pottier, M. Hasan, O. Richard-Le Goff, E. Corcuff, O. Mandelboim, and J.P. Di Santo. 2007. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J. Exp. Med. 204:2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.