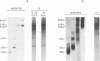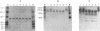Abstract
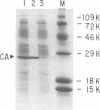
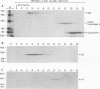
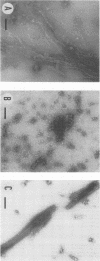
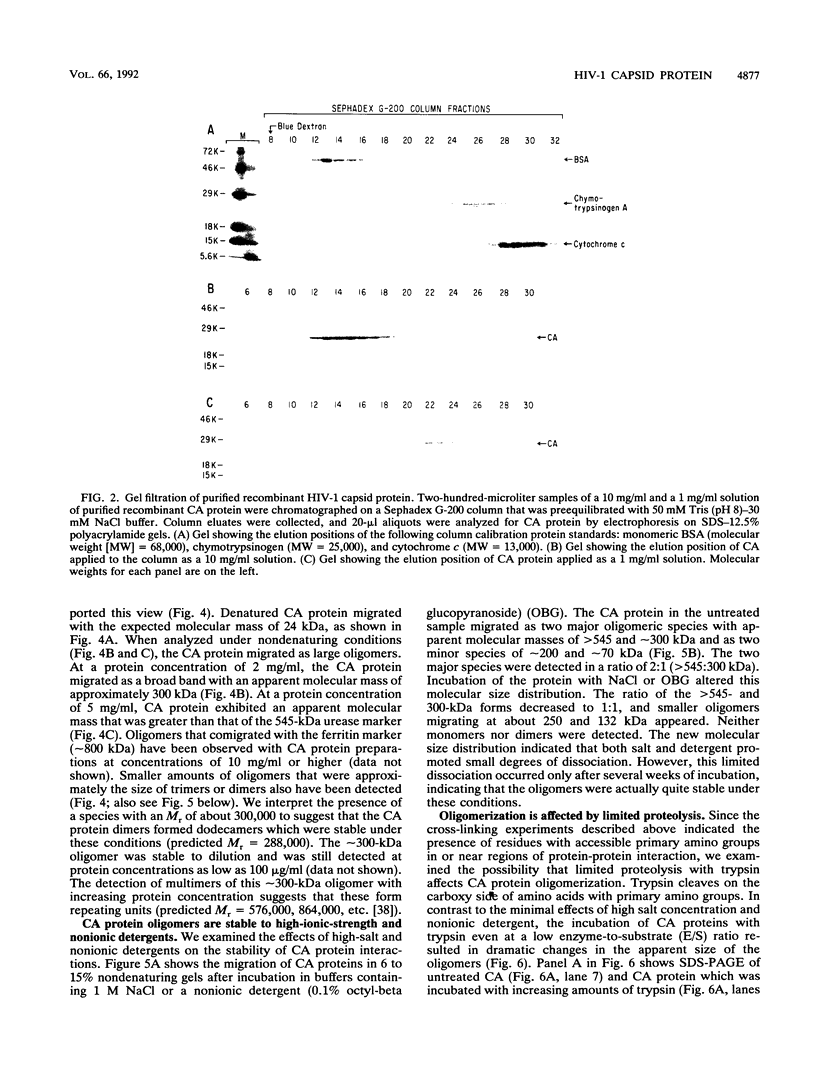
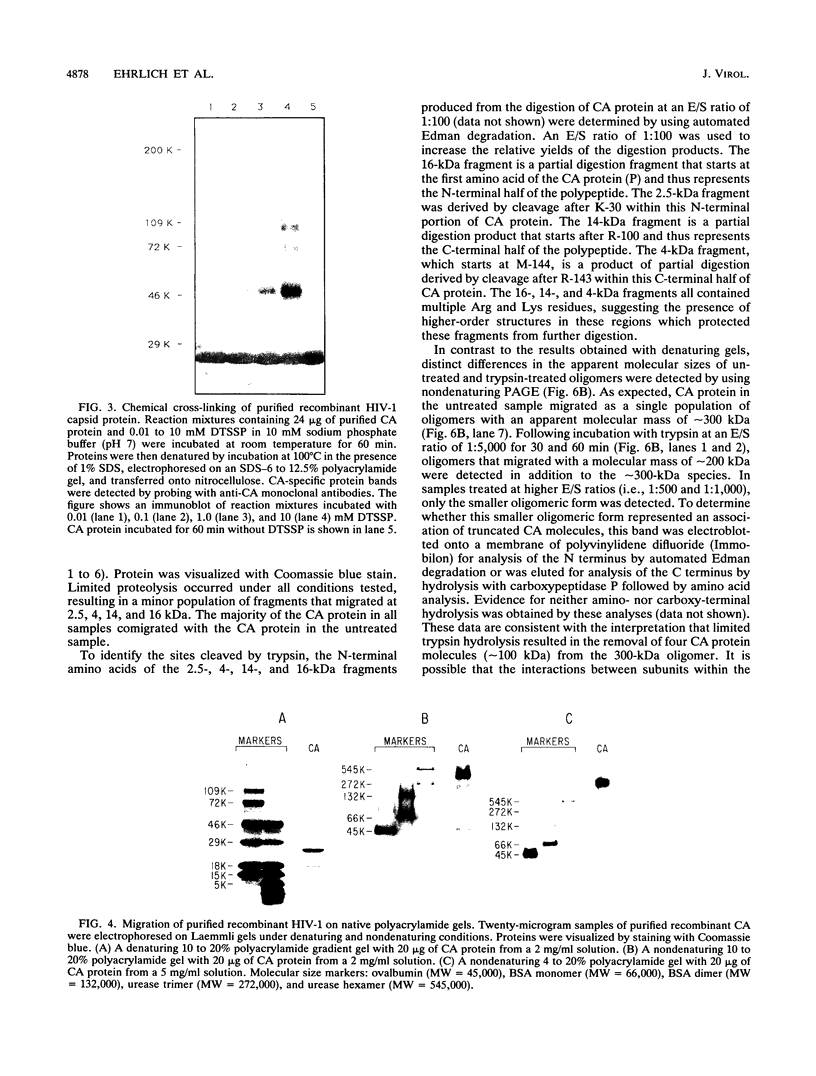
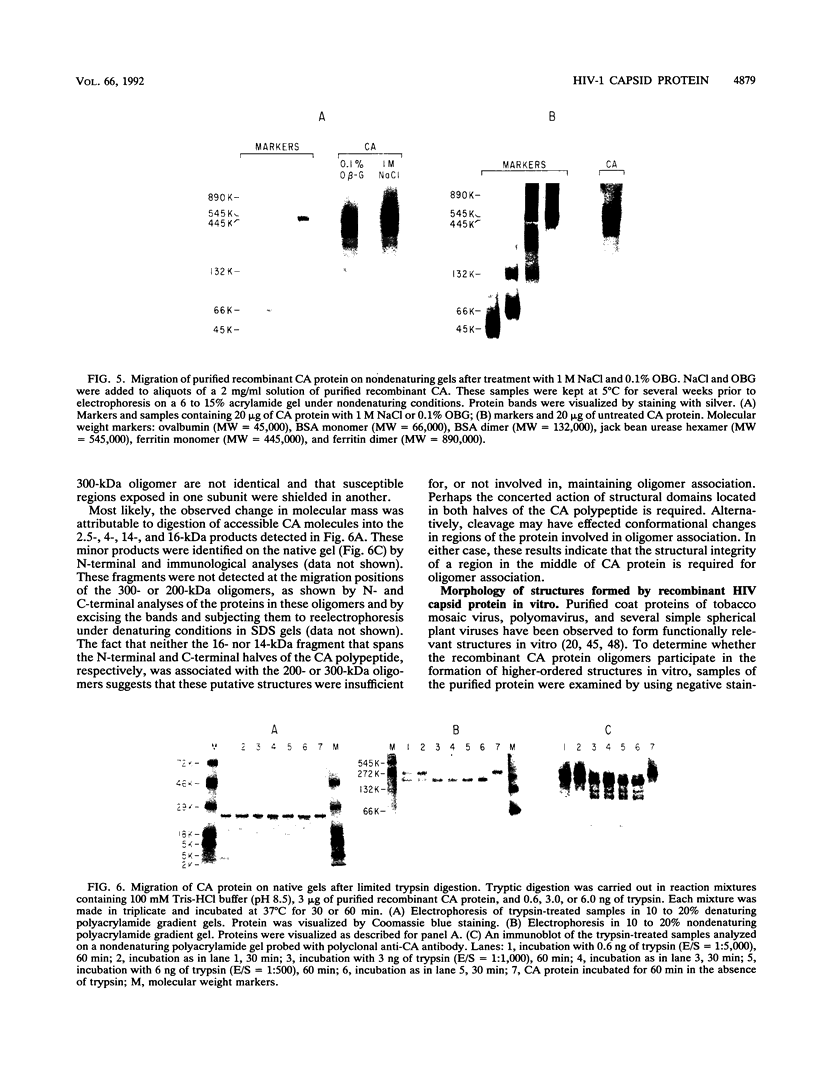
The capsid protein (CA) (p24) of human immunodeficiency virus (HIV) type 1 expressed in Escherichia coli and purified to greater than 90% homogeneity was used to examine assembly in vitro and to probe the nature of interactions involved in the formation of capsid structures. The protein was detected in dimeric and oligomeric forms as indicated by molecular size measurements by gel filtration column chromatography, sedimentation through sucrose, and nondenaturing gel electrophoresis. Chemical cross-linking of CA molecules was observed with several homobifunctional reagents. Oligomer size was dependent on cross-linker concentration and exhibited a nonrandom pattern in which dimers and tetramers were more abundant than trimers and pentamers. Oligomers as large as dodecamers were detected in native polyacrylamide gels. These were stable in solutions of high ionic strength or in the presence of nonionic detergent, indicating that strong interactions were involved in oligomer stabilization. Limited tryptic digestion converted the putative dodecamers to octamers, suggesting that a region involved in CA protein multimerization was exposed in the structure. This region was mapped to the central portion of the protein. The recombinant CA proteins assembled in vitro into long rodlike structures and were disassembled into small irregular spheres by alterations in ionic strength and pH. The observation that assembly and disassembly of purified HIV type 1 CA protein can be induced in vitro suggests an approach for identifying possible control mechanisms involved in HIV viral core assembly.
Full text
PDF



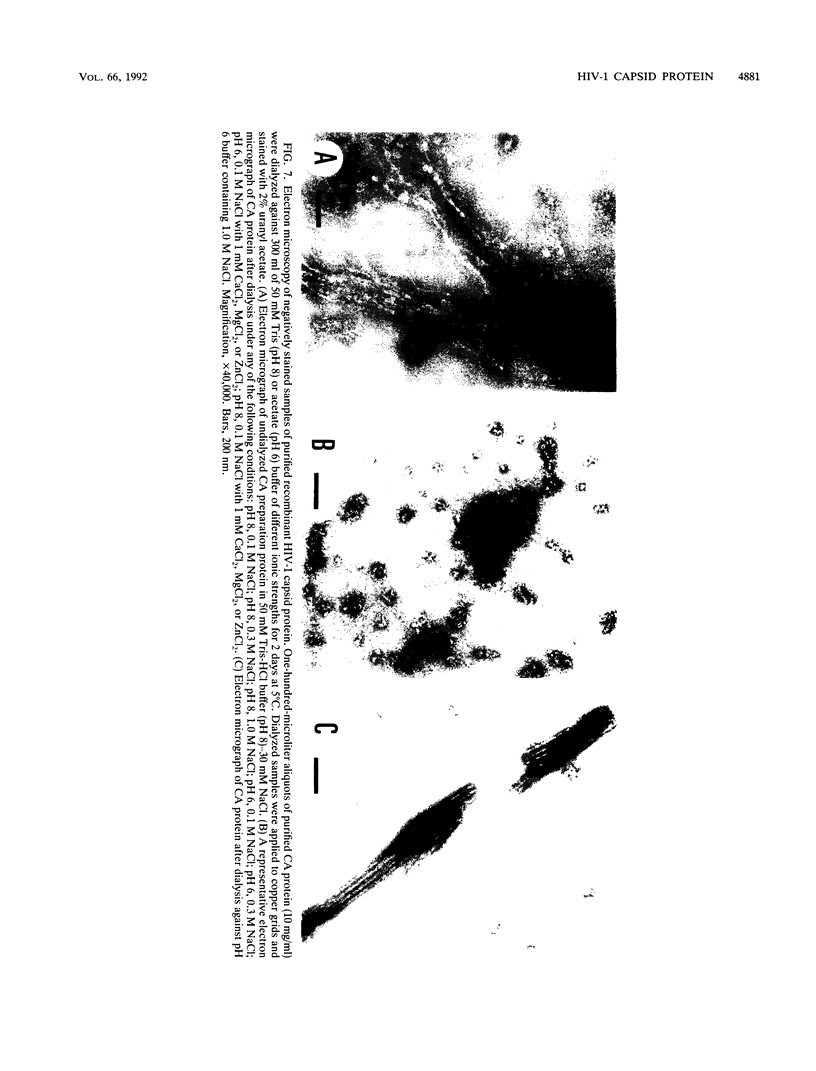


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdella P. M., Smith P. K., Royer G. P. A new cleavable reagent for cross-linking and reversible immobilization of proteins. Biochem Biophys Res Commun. 1979 Apr 13;87(3):734–742. doi: 10.1016/0006-291x(79)92020-5. [DOI] [PubMed] [Google Scholar]
- Argos P. A possible homology between immunodeficiency virus p24 core protein and picornaviral VP2 coat protein: prediction of HIV p24 antigenic sites. EMBO J. 1989 Mar;8(3):779–785. doi: 10.1002/j.1460-2075.1989.tb03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N., Holladay L. A., Mitchell W. M. Physical and chemical properties of Moloney murine leukemia virus p30 protein: a major core structural component exhibiting high helicity and self-association. J Mol Biol. 1976 Oct 25;107(2):131–143. doi: 10.1016/s0022-2836(76)80022-8. [DOI] [PubMed] [Google Scholar]
- Cann A. J., Karn J. Molecular biology of HIV: new insights into the virus life-cycle. AIDS. 1989;3 (Suppl 1):S19–S34. [PubMed] [Google Scholar]
- Chrystie I. L., Almeida J. D. Further studies of HIV morphology by negative staining. AIDS. 1988 Dec;2(6):459–464. doi: 10.1097/00002030-198812000-00008. [DOI] [PubMed] [Google Scholar]
- Cremers A. F., Oostergetel G. T., Schilstra M. J., Mellema J. E. An electron microscopic investigation of the structure of alfalfa mosaic virus. J Mol Biol. 1981 Jan 25;145(3):545–561. doi: 10.1016/0022-2836(81)90544-1. [DOI] [PubMed] [Google Scholar]
- Debouck C., Gorniak J. G., Strickler J. E., Meek T. D., Metcalf B. W., Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Doms R. W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991 Apr;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L. S., Krausslich H. G., Wimmer E., Carter C. A. Expression in Escherichia coli and purification of human immunodeficiency virus type 1 capsid protein (p24). AIDS Res Hum Retroviruses. 1990 Oct;6(10):1169–1175. doi: 10.1089/aid.1990.6.1169. [DOI] [PubMed] [Google Scholar]
- Fukuyama K., Abdel-Meguid S. S., Johnson J. E., Rossmann M. G. Structure of a T = 1 aggregate of alfalfa mosaic virus coat protein seen at 4.5 A resolution. J Mol Biol. 1983 Jul 15;167(4):873–890. doi: 10.1016/s0022-2836(83)80116-8. [DOI] [PubMed] [Google Scholar]
- Garcea R. L., Salunke D. M., Caspar D. L. Site-directed mutation affecting polyomavirus capsid self-assembly in vitro. Nature. 1987 Sep 3;329(6134):86–87. doi: 10.1038/329086a0. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Gorelick R. J., Nigida S. M., Jr, Bess J. W., Jr, Arthur L. O., Henderson L. E., Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990 Jul;64(7):3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Dorfman T., Sodroski J. G., Haseltine W. A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C. Virus structure: high-resolution perspectives. Adv Virus Res. 1983;28:175–240. doi: 10.1016/s0065-3527(08)60724-1. [DOI] [PubMed] [Google Scholar]
- Hockley D. J., Wood R. D., Jacobs J. P., Garrett A. J. Electron microscopy of human immunodeficiency virus. J Gen Virol. 1988 Oct;69(Pt 10):2455–2469. doi: 10.1099/0022-1317-69-10-2455. [DOI] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Schneider H., Zybarth G., Carter C. A., Wimmer E. Processing of in vitro-synthesized gag precursor proteins of human immunodeficiency virus (HIV) type 1 by HIV proteinase generated in Escherichia coli. J Virol. 1988 Nov;62(11):4393–4397. doi: 10.1128/jvi.62.11.4393-4397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langedijk J. P., Schalken J. J., Tersmette M., Huisman J. G., Meloen R. H. Location of epitopes on the major core protein p24 of human immunodeficiency virus. J Gen Virol. 1990 Nov;71(Pt 11):2609–2614. doi: 10.1099/0022-1317-71-11-2609. [DOI] [PubMed] [Google Scholar]
- Lightfoote M. M., Coligan J. E., Folks T. M., Fauci A. S., Martin M. A., Venkatesan S. Structural characterization of reverse transcriptase and endonuclease polypeptides of the acquired immunodeficiency syndrome retrovirus. J Virol. 1986 Nov;60(2):771–775. doi: 10.1128/jvi.60.2.771-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Malech H. L., Albert J. P. Negative staining of protein macromolecules: a simple rapid method. J Ultrastruct Res. 1979 Nov;69(2):191–195. doi: 10.1016/s0022-5320(79)90109-6. [DOI] [PubMed] [Google Scholar]
- Mastrangelo I. A., Hough P. V., Wall J. S., Dodson M., Dean F. B., Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989 Apr 20;338(6217):658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills H. R., Jones I. M. Expression and purification of p24, the core protein of HIV, using a baculovirus-insect cell expression system. AIDS. 1990 Nov;4(11):1125–1131. doi: 10.1097/00002030-199011000-00011. [DOI] [PubMed] [Google Scholar]
- Méric C., Goff S. P. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Virol. 1989 Apr;63(4):1558–1568. doi: 10.1128/jvi.63.4.1558-1568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin K., Zybarth G., Ehrlich L., DeCrombrugghe M., Wimmer E., Carter C. Deletion of sequences upstream of the proteinase improves the proteolytic processing of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4776–4780. doi: 10.1073/pnas.88.11.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C., Ho B. K., Chang T. W., Chang N. T. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol. 1989 Jun;63(6):2550–2556. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Cappiello D., Wilkowski C., Vogt V. M. Chemical crosslinking of proteins in avian sarcoma and leukemia viruses. Virology. 1980 Apr 15;102(1):205–210. doi: 10.1016/0042-6822(80)90081-1. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B. Localization of lipid-protein and protein-protein interactions within the murine retrovirus gag precursor by a novel peptide-mapping technique. J Biol Chem. 1983 Sep 25;258(18):11229–11235. [PubMed] [Google Scholar]
- Pinter A., Fleissner E. Structural studies of retroviruses: characterization of oligomeric complexes of murine and feline leukemia virus envelope and core components formed upon cross-linking. J Virol. 1979 Apr;30(1):157–165. doi: 10.1128/jvi.30.1.157-165.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prongay A. J., Smith T. J., Rossmann M. G., Ehrlich L. S., Carter C. A., McClure J. Preparation and crystallization of a human immunodeficiency virus p24-Fab complex. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9980–9984. doi: 10.1073/pnas.87.24.9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra K., Salunke D. M., Caspar D. L., Schuster T. M. Disk aggregates of tobacco mosaic virus protein in solution: electron microscopy observations. Biochemistry. 1986 Oct 7;25(20):6276–6279. doi: 10.1021/bi00368a066. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. Antiviral agents targeted to interact with viral capsid proteins and a possible application to human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4625–4627. doi: 10.1073/pnas.85.13.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Polymorphism in the assembly of polyomavirus capsid protein VP1. Biophys J. 1989 Nov;56(5):887–900. doi: 10.1016/S0006-3495(89)82735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staros J. V. N-hydroxysulfosuccinimide active esters: bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry. 1982 Aug 17;21(17):3950–3955. doi: 10.1021/bi00260a008. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand ("immature") to a collapsed ("mature") form of the virus core. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]