Abstract
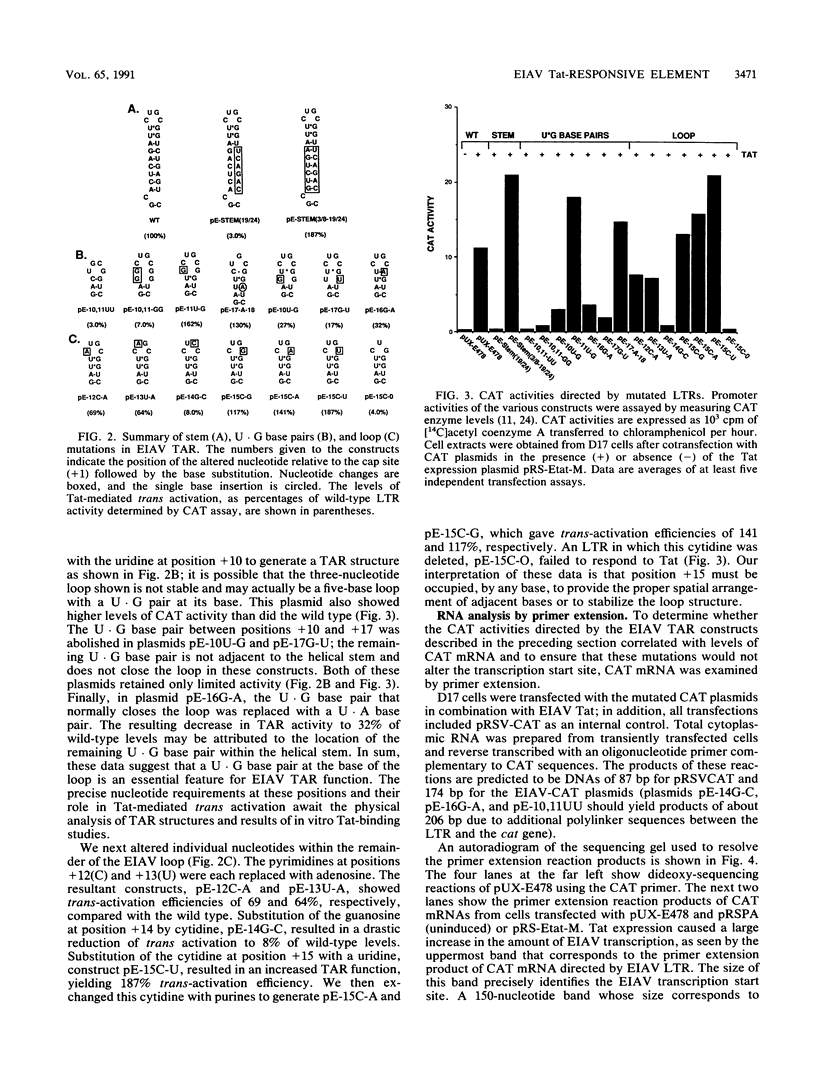
A hairpinlike structure is predicted to exist at the 5' end of equine infectious anemia virus (EIAV) RNA which is similar in many ways to the human immunodeficiency type 1 (HIV-1) Tat-responsive element (TAR). In EIAV, this structure has a shorter stem than in HIV-1 and lacks the uridine bulge. Primer extension analysis of EIAV RNA was used to identify the transcriptional start site in the viral long terminal repeat. Premature termination of primer elongation at the predicted double-stranded RNA region was frequently observed and suggests that the inferred hairpin structure exists under these conditions. We have functionally characterized EIAV TAR by site-directed mutagenesis and transient gene expression analysis. It is demonstrated here that the secondary structure of this element is essential for Tat action. Mutations that disrupted base pairing abolished TAR function, and compensatory mutations that restored the stem structure resulted in Tat activation. The TAR loop appears to be closed by two U.G base pairs that are likely to provide a unique structural motif recognized by the Tat protein. With one exception, substitutions of nucleotides within the EIAV loop sequence decreased TAR function. All nucleotide substitutions of the cytidine at position +14 increased EIAV Tat responsiveness; however, its deletion abolished trans activation. Our results lead us to propose that the EIAV and HIV-1 Tat systems employ closely related cis- and trans-acting components that probably act by the same mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Gallo R. C. Human immunodeficiency virus type 2 long terminal repeat: analysis of regulatory elements. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9753–9757. doi: 10.1073/pnas.85.24.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B., Jeang K. T. trans activation of human immunodeficiency virus type 1 is sequence specific for both the single-stranded bulge and loop of the trans-acting-responsive hairpin: a quantitative analysis. J Virol. 1989 Dec;63(12):5501–5504. doi: 10.1128/jvi.63.12.5501-5504.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B., Silverman R. H., Jeang K. T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989 Oct 20;59(2):273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Carroll R., Martarano L., Derse D. Identification of lentivirus tat functional domains through generation of equine infectious anemia virus/human immunodeficiency virus type 1 tat gene chimeras. J Virol. 1991 Jul;65(7):3460–3467. doi: 10.1128/jvi.65.7.3460-3467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Greene W. C. Functions of the auxiliary gene products of the human immunodeficiency virus type 1. Virology. 1990 Sep;178(1):1–5. doi: 10.1016/0042-6822(90)90373-y. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Ernberg I., Gait M. J., Green S. M., Heaphy S., Karn J., Lowe A. D., Singh M., Skinner M. A. HIV-1 tat protein stimulates transcription by binding to a U-rich bulge in the stem of the TAR RNA structure. EMBO J. 1990 Dec;9(12):4145–4153. doi: 10.1002/j.1460-2075.1990.tb07637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Ernberg I., Gait M. J., Green S. M., Heaphy S., Karn J., Lowe A. D., Singh M., Skinner M. A., Valerio R. Human immunodeficiency virus 1 tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6925–6929. doi: 10.1073/pnas.86.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn P. L., Derse D. cis- and trans-acting regulation of gene expression of equine infectious anemia virus. J Virol. 1988 Sep;62(9):3522–3526. doi: 10.1128/jvi.62.9.3522-3526.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn P., DaSilva L., Martarano L., Derse D. Equine infectious anemia virus tat: insights into the structure, function, and evolution of lentivirus trans-activator proteins. J Virol. 1990 Apr;64(4):1616–1624. doi: 10.1128/jvi.64.4.1616-1624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo S., Kubota S., Siomi H., Adachi A., Oroszlan S., Maki M., Hatanaka M. A region of basic amino-acid cluster in HIV-1 Tat protein is essential for trans-acting activity and nucleolar localization. Virus Genes. 1989 Nov;3(2):99–110. doi: 10.1007/BF00125123. [DOI] [PubMed] [Google Scholar]
- Feng S., Holland E. C. HIV-1 tat trans-activation requires the loop sequence within tar. Nature. 1988 Jul 14;334(6178):165–167. doi: 10.1038/334165a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. A., Harrich D., Soultanakis E., Wu F., Mitsuyasu R., Gaynor R. B. Human immunodeficiency virus type 1 LTR TATA and TAR region sequences required for transcriptional regulation. EMBO J. 1989 Mar;8(3):765–778. doi: 10.1002/j.1460-2075.1989.tb03437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatignol A., Kumar A., Rabson A., Jeang K. T. Identification of cellular proteins that bind to the human immunodeficiency virus type 1 trans-activation-responsive TAR element RNA. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7828–7832. doi: 10.1073/pnas.86.20.7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R., Soultanakis E., Kuwabara M., Garcia J., Sigman D. S. Specific binding of a HeLa cell nuclear protein to RNA sequences in the human immunodeficiency virus transactivating region. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4858–4862. doi: 10.1073/pnas.86.13.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Cullen B. R. Mutational analysis of the trans-activation-responsive region of the human immunodeficiency virus type I long terminal repeat. J Virol. 1988 Mar;62(3):673–679. doi: 10.1128/jvi.62.3.673-679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A., Smith D. H., Jakobovits E. B., Capon D. J. A discrete element 3' of human immunodeficiency virus 1 (HIV-1) and HIV-2 mRNA initiation sites mediates transcriptional activation by an HIV trans activator. Mol Cell Biol. 1988 Jun;8(6):2555–2561. doi: 10.1128/mcb.8.6.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Kono Y. Serial passages of equine infectious anemia virus in horse leukocyte culture. Natl Inst Anim Health Q (Tokyo) 1967 Spring;7(1):1–7. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Marciniak R. A., Garcia-Blanco M. A., Sharp P. A. Identification and characterization of a HeLa nuclear protein that specifically binds to the trans-activation-response (TAR) element of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 May;87(9):3624–3628. doi: 10.1073/pnas.87.9.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain W. H., Chen Y. M., Foss K., Schneider J. Association of transfer RNA acceptor identity with a helical irregularity. Science. 1988 Dec 23;242(4886):1681–1684. doi: 10.1126/science.2462282. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Wyatt J. R., Tinoco I., Jr Solution conformation of an RNA hairpin loop. Biochemistry. 1990 May 1;29(17):4215–4226. doi: 10.1021/bi00469a026. [DOI] [PubMed] [Google Scholar]
- Rappaport J., Lee S. J., Khalili K., Wong-Staal F. The acidic amino-terminal region of the HIV-1 Tat protein constitutes an essential activating domain. New Biol. 1989 Oct;1(1):101–110. [PubMed] [Google Scholar]
- Rosen C. A. Regulation of HIV gene expression by RNA-protein interactions. Trends Genet. 1991 Jan;7(1):9–14. doi: 10.1016/0168-9525(91)90015-i. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Roy S., Delling U., Chen C. H., Rosen C. A., Sonenberg N. A bulge structure in HIV-1 TAR RNA is required for Tat binding and Tat-mediated trans-activation. Genes Dev. 1990 Aug;4(8):1365–1373. doi: 10.1101/gad.4.8.1365. [DOI] [PubMed] [Google Scholar]
- Roy S., Parkin N. T., Rosen C., Itovitch J., Sonenberg N. Structural requirements for trans activation of human immunodeficiency virus type 1 long terminal repeat-directed gene expression by tat: importance of base pairing, loop sequence, and bulges in the tat-responsive sequence. J Virol. 1990 Mar;64(3):1402–1406. doi: 10.1128/jvi.64.3.1402-1406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K., Olsen K., Stiegler G., Payne S. L., Montelaro R. C., Issel C. J. Lentivirus genomic organization: the complete nucleotide sequence of the env gene region of equine infectious anemia virus. Virology. 1986 Dec;155(2):309–321. doi: 10.1016/0042-6822(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Selby M. J., Bain E. S., Luciw P. A., Peterlin B. M. Structure, sequence, and position of the stem-loop in tar determine transcriptional elongation by tat through the HIV-1 long terminal repeat. Genes Dev. 1989 Apr;3(4):547–558. doi: 10.1101/gad.3.4.547. [DOI] [PubMed] [Google Scholar]
- Selby M. J., Peterlin B. M. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990 Aug 24;62(4):769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- Siomi H., Shida H., Maki M., Hatanaka M. Effects of a highly basic region of human immunodeficiency virus Tat protein on nucleolar localization. J Virol. 1990 Apr;64(4):1803–1807. doi: 10.1128/jvi.64.4.1803-1807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Derse D., Rice N. R. Cloning and characterization of cDNAs encoding equine infectious anemia virus tat and putative Rev proteins. J Virol. 1990 Aug;64(8):3716–3725. doi: 10.1128/jvi.64.8.3716-3725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Venkatesh L., Srinivasan A., Chinnadurai G. Functional substitution of the basic domain of the HIV-1 trans-activator, Tat, with the basic domain of the functionally heterologous Rev. Virology. 1990 May;176(1):178–183. doi: 10.1016/0042-6822(90)90242-j. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Weeks K. M., Ampe C., Schultz S. C., Steitz T. A., Crothers D. M. Fragments of the HIV-1 Tat protein specifically bind TAR RNA. Science. 1990 Sep 14;249(4974):1281–1285. doi: 10.1126/science.2205002. [DOI] [PubMed] [Google Scholar]



