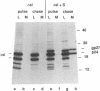
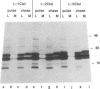
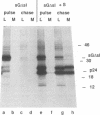
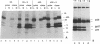Abstract
Cells infected with hepatitis B virus produce both virions and 20-nm subviral (surface antigen or HBsAg) particles; the latter are composed of viral envelope proteins and host-derived lipid. Although hepatitis B virus encodes three envelope proteins (L, M, and S), all of the information required to produce an HBsAg particle resides within the S protein. This polypeptide spans the bilayer at least twice and contains three hydrophobic regions, two of which are known to harbor topogenic signal sequences that direct this transmembrane orientation. We have examined the effects of mutations in these and other regions of the S protein on particle assembly and export. Lesions in the N terminal signal sequence (signal I) can still insert into the endoplasmic reticulum bilayer but do not participate in any of the subsequent steps in assembly. Deletion of the major internal signal (signal II) completely destabilizes the chain. Deletion of the C-terminal hydrophobic domain results in a stable, glycosylated, but nonsecreted chain. However, when coexpressed with wild-type S protein this mutant polypeptide can be incorporated into particles and secreted, indicating that the chain is still competent for some of the distal steps in particle assembly. The correct transmembrane disposition of the N terminus of the molecule is important for particle formation: addition of a heterologous (globin) domain to this region impairs secretion, but the defect can be corrected by provision of an N-terminal signal sequence that restores the proper topology of this region. The resulting chimeric chain is assembled into subviral particles that are secreted with normal efficiency.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruss V., Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpeyroux F., Chenciner N., Lim A., Lambert M., Malpièce Y., Streeck R. E. Insertions in the hepatitis B surface antigen. Effect on assembly and secretion of 22-nm particles from mammalian cells. J Mol Biol. 1987 May 20;195(2):343–350. doi: 10.1016/0022-2836(87)90655-3. [DOI] [PubMed] [Google Scholar]
- Delpeyroux F., Chenciner N., Lim A., Malpièce Y., Blondel B., Crainic R., van der Werf S., Streeck R. E. A poliovirus neutralization epitope expressed on hybrid hepatitis B surface antigen particles. Science. 1986 Jul 25;233(4762):472–475. doi: 10.1126/science.2425433. [DOI] [PubMed] [Google Scholar]
- Delpeyroux F., Peillon N., Blondel B., Crainic R., Streeck R. E. Presentation and immunogenicity of the hepatitis B surface antigen and a poliovirus neutralization antigen on mixed empty envelope particles. J Virol. 1988 May;62(5):1836–1839. doi: 10.1128/jvi.62.5.1836-1839.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., Lingappa V. R., Ganem D. Hepatitis B surface antigen: an unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol Cell Biol. 1986 May;6(5):1454–1463. doi: 10.1128/mcb.6.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., MacRae D. R., Lingappa V. R., Ganem D. Multiple topogenic sequences determine the transmembrane orientation of the hepatitis B surface antigen. Mol Cell Biol. 1987 Oct;7(10):3591–3601. doi: 10.1128/mcb.7.10.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Gavilanes F., Gonzalez-Ros J. M., Peterson D. L. Structure of hepatitis B surface antigen. Characterization of the lipid components and their association with the viral proteins. J Biol Chem. 1982 Jul 10;257(13):7770–7777. [PubMed] [Google Scholar]
- Gerber M. A., Hadziyannis S., Vissoulis C., Schaffner F., Paronetto F., Popper H. Electron microscopy and immunoelectronmicroscopy of cytoplasmic hepatitis B antigen in hepatocytes. Am J Pathol. 1974 Jun;75(3):489–502. [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuroki K., Floreani M., Mimms L. T., Ganem D. Epitope mapping of the PreS1 domain of the hepatitis B virus large surface protein. Virology. 1990 Jun;176(2):620–624. doi: 10.1016/0042-6822(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Kuroki K., Russnak R., Ganem D. Novel N-terminal amino acid sequence required for retention of a hepatitis B virus glycoprotein in the endoplasmic reticulum. Mol Cell Biol. 1989 Oct;9(10):4459–4466. doi: 10.1128/mcb.9.10.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub O., Rall L. B., Truett M., Shaul Y., Standring D. N., Valenzuela P., Rutter W. J. Synthesis of hepatitis B surface antigen in mammalian cells: expression of the entire gene and the coding region. J Virol. 1983 Oct;48(1):271–280. doi: 10.1128/jvi.48.1.271-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C., Yansura D., Levinson A. D. Direct expression of hepatitis B surface antigen in monkey cells from an SV40 vector. DNA. 1982;1(3):213–221. doi: 10.1089/dna.1.1982.1.213. [DOI] [PubMed] [Google Scholar]
- Michel M. L., Mancini M., Sobczak E., Favier V., Guetard D., Bahraoui E. M., Tiollais P. Induction of anti-human immunodeficiency virus (HIV) neutralizing antibodies in rabbits immunized with recombinant HIV--hepatitis B surface antigen particles. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7957–7961. doi: 10.1073/pnas.85.21.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer E. J., Nakamura G. R., Simonsen C. C., Levinson A. D., Brands R. Intracellular assembly and packaging of hepatitis B surface antigen particles occur in the endoplasmic reticulum. J Virol. 1986 Jun;58(3):884–892. doi: 10.1128/jvi.58.3.884-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer E. J., Nakamura G. R., Yaffe A. Intracellular transport and secretion of hepatitis B surface antigen in mammalian cells. J Virol. 1984 Aug;51(2):346–353. doi: 10.1128/jvi.51.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perara E., Rothman R. E., Lingappa V. R. Uncoupling translocation from translation: implications for transport of proteins across membranes. Science. 1986 Apr 18;232(4748):348–352. doi: 10.1126/science.3961485. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. A frameshift mutation in the pre-S region of the human hepatitis B virus genome allows production of surface antigen particles but eliminates binding to polymerized albumin. Proc Natl Acad Sci U S A. 1985 May;82(10):3440–3444. doi: 10.1073/pnas.82.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. L. Isolation and characterization of the major protein and glycoprotein of hepatitis B surface antigen. J Biol Chem. 1981 Jul 10;256(13):6975–6983. [PubMed] [Google Scholar]
- Peterson D. L. The structure of hepatitis B surface antigen and its antigenic sites. Bioessays. 1987 Jun;6(6):258–262. doi: 10.1002/bies.950060604. [DOI] [PubMed] [Google Scholar]
- Simon K., Lingappa V. R., Ganem D. Secreted hepatitis B surface antigen polypeptides are derived from a transmembrane precursor. J Cell Biol. 1988 Dec;107(6 Pt 1):2163–2168. doi: 10.1083/jcb.107.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Harley E. J., Zang E. A., Oleszko W. R., William D. C., Sadovsky R., Morrison J. M., Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980 Oct 9;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- Zerial M., Melancon P., Schneider C., Garoff H. The transmembrane segment of the human transferrin receptor functions as a signal peptide. EMBO J. 1986 Jul;5(7):1543–1550. doi: 10.1002/j.1460-2075.1986.tb04395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]