Free radicals derived primarily from molecular oxygen have been implicated in a variety of human disorders including atherosclerosis, cancer, neurodegenerative diseases, and aging (1). Damage to tissue biomolecules, including lipids, proteins, and DNA, by free radicals is postulated to contribute importantly to the pathophysiology of oxidative stress. Lipids are readily attacked by free radicals resulting in the formation of a number of peroxidation products. One class of oxidation products formed in abundance in vitro and in vivo is the isoprostanes (IsoPs),2 which were discovered by our laboratory in 1990. IsoPs are a series of prostaglandin (PG)-like compounds produced by the free radical-catalyzed peroxidation of arachidonic acid independent of the cyclooxygenase (2). Over the past 20 years, we and others have carried out a large number of studies defining the basic chemistry and biochemistry involved in the formation and metabolism of the IsoPs. In addition, we have shown that levels of IsoPs are increased in a number of human diseases, and it is currently recognized that measurement of these molecules is the most accurate analytical method to assess oxidative injury in vivo. Further, a number of IsoPs have been found to possess potent biological activity and thus are likely also mediators of oxidant injury (3). In recent years, additional related compounds, derived from various polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) (4) and docosahexaenoic acid (DHA) (5), have been discovered to be formed as products of the IsoP pathway. It is the purpose herein to summarize our current knowledge regarding the IsoPs including the chemistry and biochemistry of their formation, the utility of measuring these compounds as markers of in vivo oxidant stress, and their pharmacological properties.
Mechanism of IsoP Formation
The first class of IsoPs discovered were the F2-IsoPs, so named because they contain F-type prostane rings analogous to PGF2α. A mechanism to explain the formation of the F2-IsoPs from arachidonic acid is outlined in Fig. 1 and is based on that proposed by Pryor et al. (6) for the generation of bicycloendoperoxide intermediates. Following abstraction of a bisallylic hydrogen atom and the addition of a molecule of oxygen to arachidonic acid to form a peroxyl radical, the radical undergoes 5-exo cyclization, and a second molecule of oxygen adds to the backbone of the compound to form PGG2-like compounds. These unstable bicycloendoperoxide intermediates are then reduced to the F2-IsoPs. Based on this mechanism of formation, four F2-IsoP regioisomers are generated (7). Compounds are denoted as 5-, 12-, 8-, or 15-series regioisomers depending on the carbon atom to which the side chain hydroxyl is attached (8). An alternative nomenclature system for the IsoPs has been proposed by FitzGerald and colleagues (9) in which the abbreviation iP is used for isoprostane and the regioisomers are denoted as III–VI based upon the number of carbons between the omega carbon and the first double bond.
FIGURE 1.
Mechanism of formation of the F2-IsoPs from the free radical-catalyzed peroxidation of arachidonate. Four regioisomers are generated, each consisting of 8 racemic diastereomers. For simplicity, stereochemistry is not indicated.
Although the initial abstraction of any the bisallylic hydrogen atoms of arachidonic acid is equally likely, the different IsoP regioisomers are not formed in equal amounts. When arachidonic acid is oxidized either in vitro or in vivo, the 5- and 15-series regioisomers are formed in significantly greater amounts than the 8- and 12-series regioisomers. One explanation for this difference has been recently elucidated by Yin et al. (10), who demonstrated that the arachidonyl hydroperoxides that give rise to the 8- and 12-series regioisomers readily undergo further oxidation to yield a newly discovered class of compounds that contains both bicycloendoperoxide and cyclic peroxide moieties; these compounds are termed dioxolane-IsoPs, and have been reported to be formed in vivo. 5- and 15-series regioisomers cannot undergo this further oxidation and can thus accumulate at higher concentrations in tissues and fluids.
An important structural distinction between IsoPs and cyclooxygenase-derived PGs is that the former contain side chains that are predominantly oriented cis to the prostane ring whereas the latter possess exclusively trans side chains (2). In this regard, however, we have recently reported that PGs can be formed via the IsoP pathway because smaller amounts of endoperoxides containing trans side chains are generated in vitro and in vivo by this mechanism (11). In this case, PGs derived via the IsoP pathway can be distinguished from those formed by cyclooxygenase because the former are generated as a racemic mixture whereas the latter are enantiomerically pure. A second important difference between IsoPs and PGs is that IsoPs are formed primarily in situ esterified to phospholipids and are subsequently released by a phospholipase(s) (3), whereas PGs are generated only from free arachidonic acid.
Quantification of F2-IsoPs
A number of methods have been developed to quantify the IsoPs. Our laboratory uses a gas chromatographic/negative ion chemical ionization mass spectrometric (GC/NICI-MS) approach employing stable isotope dilution (12). For quantification purposes, we measure the F2-IsoP, 15-F2t-IsoP, and other F2-IsoPs that co-elute with this compound. Several internal standards are available from commercial sources to quantify the IsoPs. The advantages of MS over other approaches include its high sensitivity and specificity, which yield quantitative results in the low picogram range. Its drawbacks are that it is labor intensive and requires considerable expenditures on equipment.
Several alternative GC/MS assays have been developed by different investigators including FitzGerald and colleagues (13) that quantify other IsoP regioisomers. In addition, a number of liquid chromatographic (LC)/MS methods for F2-IsoPs have been developed (14–16). One advantage of LC/MS methods is that the sample preparation for analysis is simpler than that for GC/MS because no derivatization of the molecule is required.
Alternative methods have also been developed to quantify IsoPs using immunological approaches (17). Antibodies have been generated against 15-F2t-IsoP, and several immunoassay kits are commercially available. A potential drawback of these methods is that limited information is currently available regarding their precision and accuracy. In addition, little data exist comparing IsoP levels determined by immunoassay to MS. Despite potential limitations, immunoassays have expanded IsoP research because of their low cost and relative ease of use (12).
F2-IsoPs as Index of Oxidative Stress in Vivo
A true utility of the F2-IsoPs is in the quantification of lipid peroxidation and thus oxidant stress status in vivo (3, 12). In this regard, measurement of the F2-IsoPs has revolutionized our ability to quantify oxidative injury in vivo. F2-IsoPs are stable, robust molecules and are detectable in all human tissues and biological fluids analyzed, including plasma, urine, bronchoalveolar lavage fluid, cerebrospinal fluid, and bile, as well as various tissues. The quantification of F2-IsoPs in urine and plasma, however, is most convenient and least invasive. Further, based on available data, quantification of these compounds in either plasma or urine is representative of their endogenous production and thus gives a highly precise and accurate index of in vivo oxidant stress.
Normal levels of F2-IsoPs in healthy humans have been defined (3, 12). Defining these levels is particularly important in that it allows for an assessment of the effects of diseases on endogenous oxidant tone and allows for the determination of the extent to which various therapeutic interventions affect levels of oxidant stress. A number of methods exist to quantify free radicals and their oxidation products although many of these techniques suffer from a lack of sensitivity and specificity, especially when used to assess oxidant stress status in vivo. In a recent multi-investigator study, termed the Biomarkers of Oxidative Stress (BOSS) Study, sponsored by the National Institutes of Health, it was found that the most accurate method to assess in vivo oxidant stress status is the quantification of plasma or urinary IsoPs, and thus, currently, quantification of these compounds provides the “gold standard” to assess oxidative injury in vivo (18).
Elevations of IsoPs in human body fluids and tissues have been found in a diverse array of human disorders, including atherosclerosis (19, 20), diabetes (21), obesity (22), cigarette smoking (23), neurodegenerative diseases (24), and many others. Further, treatments for some of these conditions, including antioxidant supplementation, antidiabetic treatments, cessation of smoking, and even weight loss, have been shown to decrease production of F2-IsoPs (25, 26). As an example, we recently defined, for the first time, the clinical pharmacology of vitamin E as an antioxidant (26). We determined that doses of α-tocopherol of 1600 IU/day or greater are required to statistically affect plasma F2-IsoP levels. Interestingly, unlike vitamin E, vitamin C supplementation does not alter IsoP levels in humans (27).
Biological Activities of F2-IsoPs
In addition to being robust markers of in vivo oxidant stress, F2-IsoPs and other classes of IsoPs can exert potent biological activity and potentially mediate some of the adverse effects of oxidant injury. As mentioned previously, IsoPs are initially formed in vivo esterified to glycerophospholipids. Molecular modeling of IsoP-containing phospholipids reveals them to be remarkably distorted molecules (28). Thus, the formation of these abnormal phospholipids would be expected to exert profound effects on membrane fluidity and integrity, well known sequelae of oxidant injury.
Despite their initial formation esterified in lipids, studies exploring the bioactivity of IsoPs have been performed using unesterified compounds as free acids. One F2-IsoP that is produced abundantly in vivo and has been extensively tested for biological activity is 15-F2t-IsoP (8-iso-PGF2α), which differs from cyclooxygenase-derived PGF2α only in the inversion of the upper side chain stereochemistry (29). This IsoP has been found to be a potent vasoconstrictor in a variety of vascular beds, including the kidney, lung, heart, brain, and others (30). In addition, 15-F2t-IsoP induces endothelin release and proliferation of vascular smooth muscle cells. There is also additional evidence that this molecule can increase resistance to aspirin inhibition of platelet aggregation in platelets as well as inhibit platelet aggregation in human whole blood. These vasoactive and platelet effects of 15-F2t-IsoP have been shown to result from interaction with the thromboxane receptor, a G-protein-coupled transmembrane eicosanoid receptor, based on the finding that these effects could be abrogated by thromboxane receptor antagonists (31).
The testing of other F2-IsoPs for biological activity has been limited. It has been shown, however, that 15-F2c-IsoP (12-iso-PGF2α) activates the PGF2α receptor (32).
Formation of IsoPs with Alternative Ring Structures
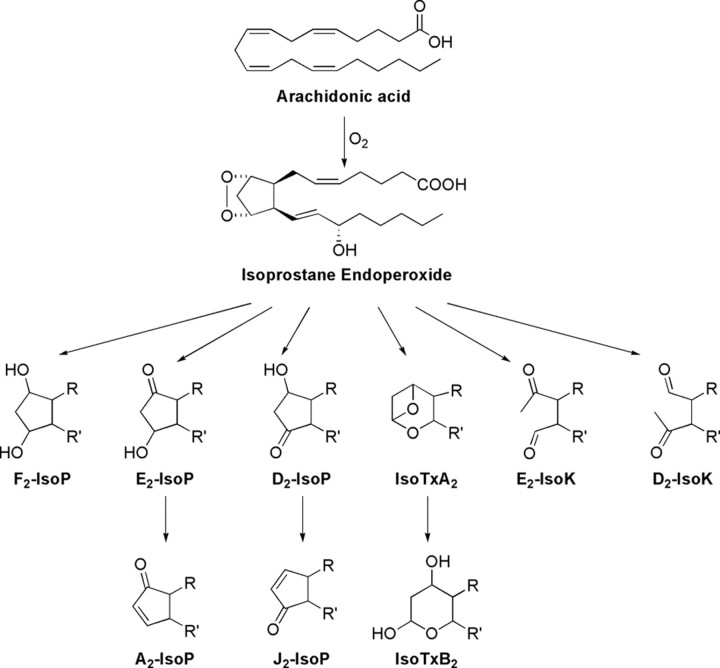
Since the initial discovery of the F2-IsoPs, our laboratory has shown that the IsoP pathway provides a mechanism for the generation of other classes of IsoPs from arachidonic acid, which differ with regard to the functional groups on the prostane ring. In addition to undergoing reduction to yield F2-IsoPs, the arachidonyl endoperoxide intermediate can undergo isomerization to yield E- and D-ring IsoPs (Fig. 2), which are isomeric to PGE2 and PGD2, respectively (3). E2/D2-IsoPs are formed competitively with F2-IsoPs, and studies have demonstrated that the depletion of cellular reducing agents, such as GSH or α-tocopherol, favors the formation of E2/D2-IsoPs over that of reduced F2-IsoPs (33).
FIGURE 2.
Structures of other classes of IsoPs and related compounds formed via IsoP endoperoxide intermediates.
The biological properties of E-ring IsoPs have been examined to a limited extent. Interestingly, the E-ring analogue of 15-F2t-IsoP, 15-E2t-IsoP, has been shown to have biological properties very similar to 15-F2t-IsoP and is a ligand for the thromboxane receptor (30). In addition, others have noted that 15-E2t-IsoP is a ligand for various PGE2 (EP) receptors.
E2/D2-IsoPs are not terminal products of the IsoP pathway. These compounds readily dehydrate in vivo to yield A2/J2-IsoPs, which are also known as cyclopentenone IsoPs because they contain an α,β-unsaturated cyclopentenone ring structure (Fig. 2) (34). A2/J2-IsoPs are highly reactive electrophiles that readily form Michael adducts with cellular thiols, including those found on cysteine residues in proteins and GSH (35). These cyclopentenone IsoPs are rapidly metabolized in vivo by GSH transferase enzymes to water-soluble modified GSH conjugates (36). A major urinary cyclopentenone IsoP metabolite in rats, a 15-A2t-IsoP mercapturic acid sulfoxide conjugate, was recently identified.
The chemical reactivity of cyclopentenone IsoPs suggested that these compounds might be biologically active. The synthesis of two cyclopentenone IsoPs regioisomers, 15-A2-IsoPs and 15-J2-IsoPs, has allowed us to examine their bioactivity. Studies employing primary cortical neuronal cultures demonstrated that both 15-A2-IsoP and 15-J2-IsoP potently induce neuronal apoptosis and exacerbate neurodegeneration caused by other insults at concentrations as low as 100 nm (37). Cyclopentenone IsoPs also exert biological effects in non-neural tissue. They potently suppress lipopolysaccharide-induced inflammatory signaling via inhibition of the NF-κB pathway (38).
Thromboxane-like molecules have also been reported to be generated via the IsoP pathway although compounds with structures similar to prostacyclin have not been reported (Fig. 2). Further, Roberts and co-workers (39) have reported that a series of compounds termed isoketals (IsoKs or isolevuglandins) can be generated via the IsoP pathway and result from opening of the cyclopentane ring (Fig. 2). These compounds are also highly reactive and readily adduct lysine residues on proteins resulting in covalent modification and protein dysfunction and cross-linking.
Formation of IsoPs from Other Polyunsaturated Fatty Acids
Arachidonic acid is not the only polyunsaturated fatty acid that can be oxidized to generate IsoPs. F-ring IsoPs have been shown to be generated from the peroxidation of linolenic acid (C18:3, ω-3, F1-IsoPs), EPA (C20:5, ω-3, F3-IsoPs), and DHA (C22:6, ω-3, F4-neuroprostanes) (Fig. 3, A and B) (4, 5). Similar to the distribution of F2-IsoP regioisomers, certain F3-IsoP and F4-neuroprostane regioisomers are generated in greater amounts than others (40). In addition to F-ring compounds, E- and D-ring as well as A- and J-ring or cyclopentenone, IsoP-like compounds are generated from the oxidation of DHA and presumably EPA.
FIGURE 3.
A, structures of theω-3 polyunsaturated fatty acids, EPA and DHA, in comparison with the ω-6 polyunsaturated fatty acid arachidonic acid. B, pathway for the formation of an F3-IsoP from EPA.
Our interest in examining the formation of IsoP-like compounds from EPA and DHA stems from emerging evidence that has implicated increased dietary intake of fish oil, which contains large amounts of EPA and DHA, as being beneficial in the prevention and treatment of a number of diseases, including atherosclerotic cardiovascular disease and sudden death, neurodegeneration, and various inflammatory disorders among others (41). Further, recent data have suggested that the anti-inflammatory effects and other biologically relevant properties of ω-3 fatty acids are due, in part, to the generation of various bioactive oxidation products (42, 43). We thus hypothesized that EPA- and DHA-derived IsoPs could contribute to the beneficial biological effects of fish oil supplementation. Indeed, one report states that the EPA-derived IsoP, 15-F3t-IsoP, possesses activity that is different from 15-F2t-IsoP in that it does not affect human platelet shape change or aggregation (44). The lack of activity of 15-F3t-IsoP is consistent with observations regarding EPA-derived PGs in that many of these latter compounds exert either weaker agonist or no effects in comparison with arachidonic acid-derived PGs (45).
Interestingly, our laboratory has recently shown that the levels of IsoPs generated from the oxidation of EPA significantly exceed those of F2-IsoPs generated from arachidonic acid, perhaps because EPA contains more double bonds and is therefore more easily oxidizable (4). Additionally, in vivo in mice, levels of F3-IsoPs in tissues such as heart were virtually undetectable at base line, but supplementation of animals with EPA markedly increased quantities up to 27.4 ± 5.6 ng/g of heart tissue. Of particular note, we found that EPA supplementation also markedly reduced levels of arachidonate-derived F2-IsoP mouse heart tissues by over 60% (p < 0.05). These observations are quite significant because F2-IsoPs are generally considered to be pro-inflammatory molecules associated with the pathophysiological sequelae of oxidant stress. It is thus intriguing to propose that part of the mechanism by which EPA prevents certain diseases is its ability to decrease F2-IsoP generation. In addition, it suggests that supplementation with fish oil may be of benefit to populations associated with increased levels of F2-IsoPs.
In related studies, we have also determined that cyclopentenone IsoPs derived from EPA possess potent bioactivity in that they readily form Michael adducts with proteins and alter protein structure and function. In this regard, we have reported that J3-IsoPs are produced in large amounts in tissues of mice supplemented with EPA and that these compounds are capable of readily modulating the nuclear translocation of the transcription factor Nrf2, a major regulator of the antioxidant response in cells (46). This likely occurs via the ability of J3-IsoPs to modify critical cysteine residues in the cytosolic Nrf2·Keap1 complex that subsequently destabilizes the complex.
Summary and Thoughts for the Future
The discovery of the IsoPs as products of non-enzymatic lipid peroxidation has been a major breakthrough in the field of lipid oxidation and free radical chemistry. Previous research elucidated basic mechanisms of lipid peroxidation leading to the formation of compounds with cyclopentane ring structures. The discovery that identical compounds, subsequently termed IsoPs, were formed in vivo from the peroxidation of arachidonate allowed for the translation of fundamental chemical principles to human biology. The quantification of IsoPs has opened up new areas of investigation regarding the role of free radicals in human physiology and pathophysiology and is the most useful tool currently available to explore the role of lipid peroxidation in the pathogenesis of human disease. Our understanding of the IsoP pathway continues to expand, providing new insights into the nature of lipid peroxidation in vivo and revealing new molecules that exert potent biological actions and might serve as unique indices of disease. Basic research into the biochemistry and pharmacology of the IsoPs, coupled with clinical studies employing these molecules as biomarkers, should continue to provide important insights into the role of oxidant stress in human physiology and pathophysiology.
This work was supported, in whole or in part, by National Institutes of Health Grants GM15431, DK48831, ES13125, and ES00267. This is the fifth article of seven in the Oxidized Lipids Minireview Series. This minireview will be reprinted in the 2008 Minireview Compendium, which will be available in January, 2009.
Footnotes
The abbreviations used are: IsoP, isoprostane; PG, prostaglandin; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; GC, gas chromatography; MS, mass spectrometry.
References
- 1.Halliwell, B., and Gutteridge, J. M. (1990) Methods Enzymol. 186 1–85 [DOI] [PubMed] [Google Scholar]
- 2.Morrow, J. D., Hill, K. E., Burk, R. F., Nammour, T. M., Badr, K. F., and Roberts, L. J., II (1990) Proc. Natl. Acad. Sci. U. S. A. 87 9383–9387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Famm, S. S., and Morrow, J. D. (2003) Curr. Med. Chem. 10 1723–1740 [DOI] [PubMed] [Google Scholar]
- 4.Gao, L., Yin, H., Milne, G. L., Porter, N. A., and Morrow, J. D. (2006) J. Biol. Chem. 281 14092–14099 [DOI] [PubMed] [Google Scholar]
- 5.Roberts, L. J., II, Montine, T. J., Markesbery, W. R., Tapper, A. R., Hardy, P., Chemtob, S., Dettbarn, W. D., and Morrow, J. D. (1998) J. Biol. Chem. 273 13605–13612 [DOI] [PubMed] [Google Scholar]
- 6.Pryor, W. A., Stanley, J. P., and Blair, E. (1976) Lipids 11 370–379 [DOI] [PubMed] [Google Scholar]
- 7.Morrow, J. D., Harris, T. M., and Roberts, L. J., II (1990) Anal. Biochem. 184 1–10 [DOI] [PubMed] [Google Scholar]
- 8.Taber, D. F., Morrow, J. D., and Roberts, L. J., II (1997) Prostaglandins 53 63–67 [DOI] [PubMed] [Google Scholar]
- 9.Rokach, J., Khanapure, S. P., Hwang, S. W., Adiyaman, M., Lawson, J. A., and FitzGerald, G. A. (1997) Prostaglandins 54 853–873 [DOI] [PubMed] [Google Scholar]
- 10.Yin, H., Morrow, J. D., and Porter, N. A. (2004) J. Biol. Chem. 279 3766–3776 [DOI] [PubMed] [Google Scholar]
- 11.Yin, H., Gao, L., Tai, H. H., Murphey, L. J., Porter, N. A., and Morrow, J. D. (2007) J. Biol. Chem. 282 329–336 [DOI] [PubMed] [Google Scholar]
- 12.Milne, G. L., Yin, H., Brooks, J. D., Sanchez, S., Roberts, L. J., II and Morrow, J. D. (2007) Methods Enzymol. 433 113–126 [DOI] [PubMed] [Google Scholar]
- 13.Pratico, D., Barry, O. P., Lawson, J. A., Adiyaman, M., Hwang, S. W., Khanapure, S. P., Iuliano, L., Rokach, J., and FitzGerald, G. A. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 3449–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang, Y., Wei, P., Duke, R. W., Reaven, P. D., Harman, S. M., Cutler, R. G., and Heward, C. B. (2003) Free Radic. Biol. Med. 34 409–418 [DOI] [PubMed] [Google Scholar]
- 15.Bohnstedt, K. C., Karlberg, B., Wahlund, L. O., Jonhagen, M. E., Basun, H., and Schmidt, S. (2003) J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 796 11–19 [DOI] [PubMed] [Google Scholar]
- 16.Taylor, A. W., Bruno, R. S., Frei, B., and Traber, M. G. (2006) Anal. Biochem. 350 41–51 [DOI] [PubMed] [Google Scholar]
- 17.Basu, S. (1998) Prostaglandins Leukotrienes Essent. Fatty Acids 58 319–325 [DOI] [PubMed] [Google Scholar]
- 18.Kadiiska, M. B., Gladen, B. C., Baird, D. D., Germolec, D., Graham, L. B., Parker, C. E., Nyska, A., Wachsman, J. T., Ames, B. N., Basu, S., Brot, N., Fitzgerald, G. A., Floyd, R. A., George, M., Heinecke, J. W., Hatch, G. E., Hensley, K., Lawson, J. A., Marnett, L. J., Morrow, J. D., Murray, D. M., Plastaras, J., Roberts, L. J., II, Rokach, J., Shigenaga, M. K., Sohal, R. S., Sun, J., Tice, R. R., Van Thiel, D. H., Wellner, D., Walter, P. B., Tomer, K. B., Mason, R. P., and Barrett, J. C. (2005) Free Radic. Biol. Med. 38 698–710 [DOI] [PubMed] [Google Scholar]
- 19.Morrow, J. D. (2005) Arterioscler. Thromb. Vasc. Biol. 25 279–286 [DOI] [PubMed] [Google Scholar]
- 20.Pratico, D., Tangirala, R. K., Rader, D. J., Rokach, J., and FitzGerald, G. A. (1998) Nat. Med. 4 1189–1192 [DOI] [PubMed] [Google Scholar]
- 21.Davi, G., Chiarelli, F., Santilli, F., Pomilio, M., Vigneri, S., Falco, A., Basili, S., Ciabattoni, G., and Patrono, C. (2003) Circulation 107 3199–3203 [DOI] [PubMed] [Google Scholar]
- 22.Keaney, J. F., Jr., Larson, M. G., Vasan, R. S., Wilson, P. W., Lipinska, I., Corey, D., Massaro, J. M., Sutherland, P., Vita, J. A., and Benjamin, E. J. (2003) Arterioscler. Thromb. Vasc. Biol. 23 434–439 [DOI] [PubMed] [Google Scholar]
- 23.Morrow, J. D., Frei, B., Longmire, A. W., Gaziano, J. M., Lynch, S. M., Shyr, Y., Strauss, W. E., Oates, J. A., and Roberts, L. J., II (1995) N. Engl. J. Med. 332 1198–1203 [DOI] [PubMed] [Google Scholar]
- 24.Montine, K. S., Quinn, J. F., Zhang, J., Fessel, J. P., Roberts, L. J., II, Morrow, J. D., and Montine, T. J. (2004) Chem. Phys. Lipids 128 117–124 [DOI] [PubMed] [Google Scholar]
- 25.Davi, G., Ciabattoni, G., Consoli, A., Mezzetti, A., Falco, A., Santarone, S., Pennese, E., Vitacolonna, E., Bucciarelli, T., Costantini, F., Capani, F., and Patrono, C. (1999) Circulation 99 224–229 [DOI] [PubMed] [Google Scholar]
- 26.Roberts, L. J., II, Oates, J. A., Linton, M. F., Fazio, S., Meador, B. P., Gross, M. D., Shyr, Y., and Morrow, J. D. (2007) Free Radic. Biol. Med. 43 1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine, M., Wang, Y., Padayatty, S. J., and Morrow, J. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 9842–9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow, J. D., Awad, J. A., Boss, H. J., Blair, I. A., and Roberts, L. J., II (1992) Proc. Natl. Acad. Sci. U. S. A. 89 10721–10725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow, J. D., Minton, T. A., Badr, K. F., and Roberts, L. J., II (1994) Biochim. Biophys. Acta 1210 244–248 [DOI] [PubMed] [Google Scholar]
- 30.Morrow, J. D. (2006) Curr. Pharm. Des. 12 895–902 [DOI] [PubMed] [Google Scholar]
- 31.Takahashi, K., Nammour, T. M., Fukunaga, M., Ebert, J., Morrow, J. D., Roberts, L. J., II, Hoover, R. L., and Badr, K. F. (1992) J. Clin. Invest. 90 136–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunapuli, P., Lawson, J. A., Rokach, J., and FitzGerald, G. A. (1997) J. Biol. Chem. 272 27147–27154 [DOI] [PubMed] [Google Scholar]
- 33.Montine, T. J., Montine, K. S., Reich, E. E., Terry, E. S., Porter, N. A., and Morrow, J. D. (2003) Biochem. Pharmacol. 65 611–617 [DOI] [PubMed] [Google Scholar]
- 34.Chen, Y., Morrow, J. D., and Roberts, L. J., II (1999) J. Biol. Chem. 274 10863–10868 [DOI] [PubMed] [Google Scholar]
- 35.Milne, G. L., Zanoni, G., Porta, A., Sasi, S., Vidari, G., Musiek, E. S., Freeman, M. L., and Morrow, J. D. (2004) Chem. Res. Toxicol. 17 17–25 [DOI] [PubMed] [Google Scholar]
- 36.Milne, G. L., Gao, L., Porta, A., Zanoni, G., Vidari, G., and Morrow, J. D. (2005) J. Biol. Chem. 280 25178–25184 [DOI] [PubMed] [Google Scholar]
- 37.Musiek, E. S., Breeding, R. S., Milne, G. L., Zanoni, G., Morrow, J. D., and McLaughlin, B. (2006) J. Neurochem. 97 1301–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musiek, E. S., Gao, L., Milne, G. L., Han, W., Everhart, M. B., Wang, D., Backlund, M. G., DuBois, R. N., Zanoni, G., Vidari, G., Blackwell, T. S., and Morrow, J. D. (2005) J. Biol. Chem. 280 35562–35570 [DOI] [PubMed] [Google Scholar]
- 39.Brame, C. J., Salomon, R. G., Morrow, J. D., and Roberts, L. J., II (1999) J. Biol. Chem. 274 13139–13146 [DOI] [PubMed] [Google Scholar]
- 40.Yin, H., Musiek, E. S., Gao, L., Porter, N. A., and Morrow, J. D. (2005) J. Biol. Chem. 280 26600–26611 [DOI] [PubMed] [Google Scholar]
- 41.Ruxton, C. H., Reed, S. C., Simpson, M. J., and Millington, K. J. (2004) J. Hum. Nutr. Diet 17 449–459 [DOI] [PubMed] [Google Scholar]
- 42.Serhan, C. N., Clish, C. B., Brannon, J., Colgan, S. P., Chiang, N., and Gronert, K. (2000) J. Exp. Med. 192 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sethi, S., Eastman, A. Y., and Eaton, J. W. (1996) J. Lab. Clin. Med. 128 27–38 [DOI] [PubMed] [Google Scholar]
- 44.Pratico, D., Smyth, E. M., Violi, F., and FitzGerald, G. A. (1996) J. Biol. Chem. 271 14916–14924 [DOI] [PubMed] [Google Scholar]
- 45.Wada, M., DeLong, C. J., Hong, Y. H., Rieke, C. J., Song, I., Sidhu, R. S., Yuan, C., Warnock, M., Schmaier, A. H., Yokoyama, C., Smyth, E. M., Wilson, S. J., FitzGerald, G. A., Garavito, R. M., Sui, D. X., Regan, J. W., and Smith, W. L. (2007) J. Biol. Chem. 282 22254–22266 [DOI] [PubMed] [Google Scholar]
- 46.Gao, L., Wang, J., Sekhar, K. R., Yin, H., Yared, N. F., Schneider, S. N., Sasi, S., Dalton, T. P., Anderson, M. E., Chan, J. Y., Morrow, J. D., and Freeman, M. L. (2007) J. Biol. Chem. 282 2529–2537 [DOI] [PubMed] [Google Scholar]