Abstract
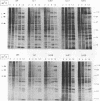
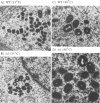
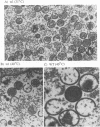
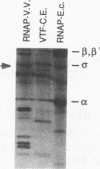
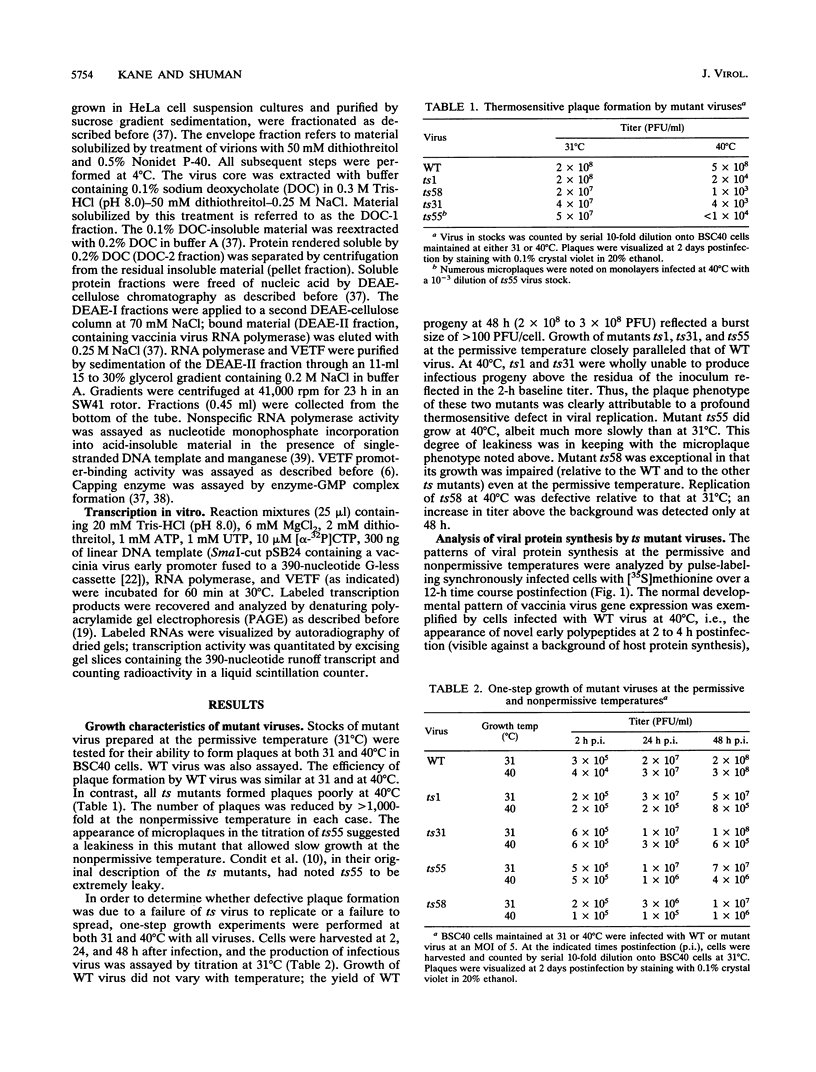
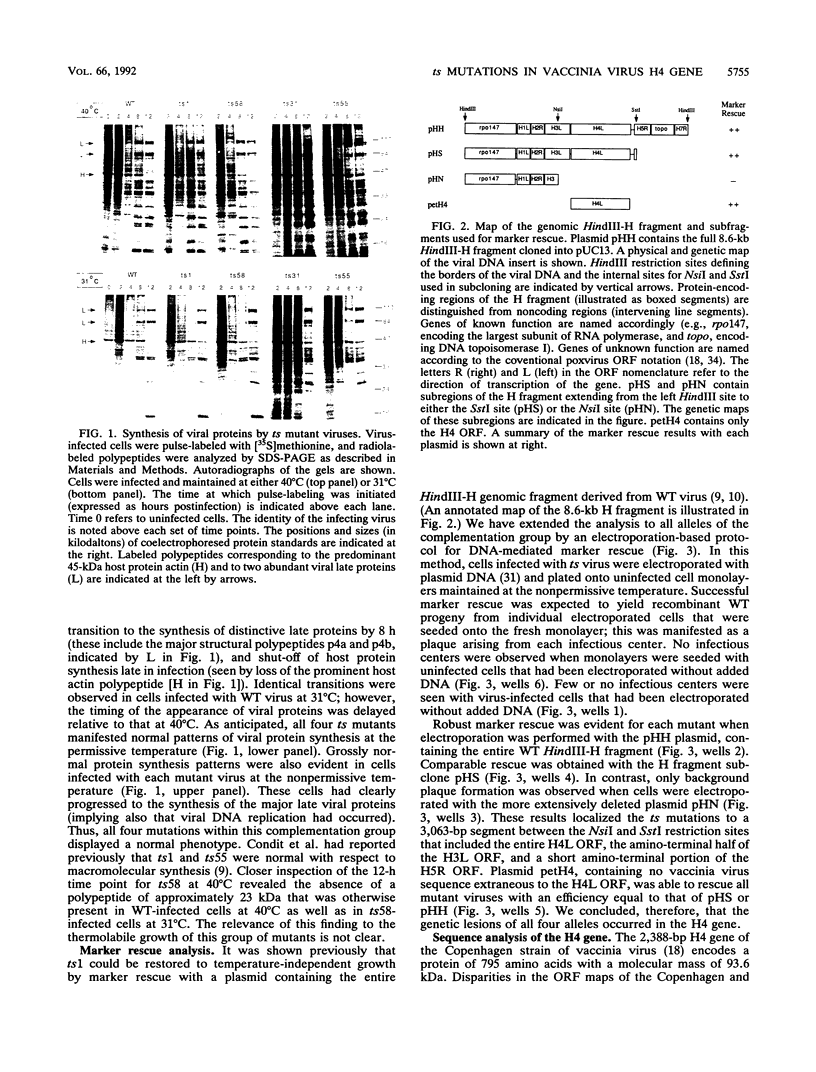
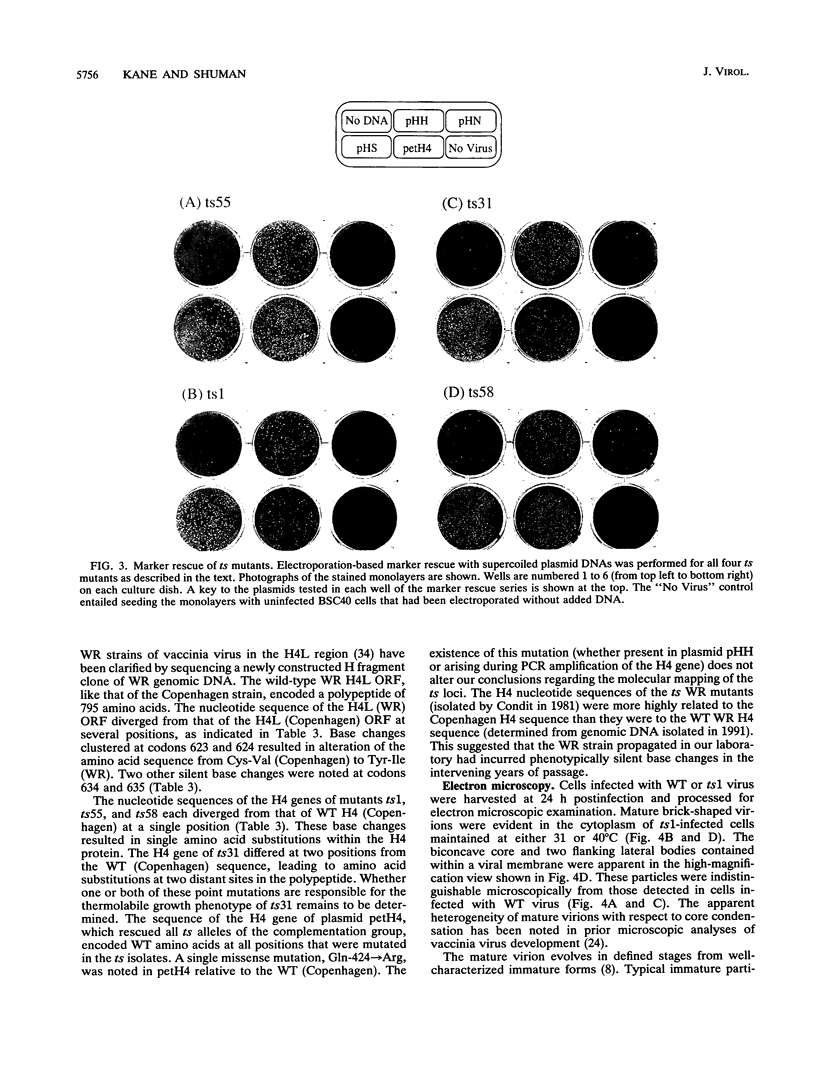
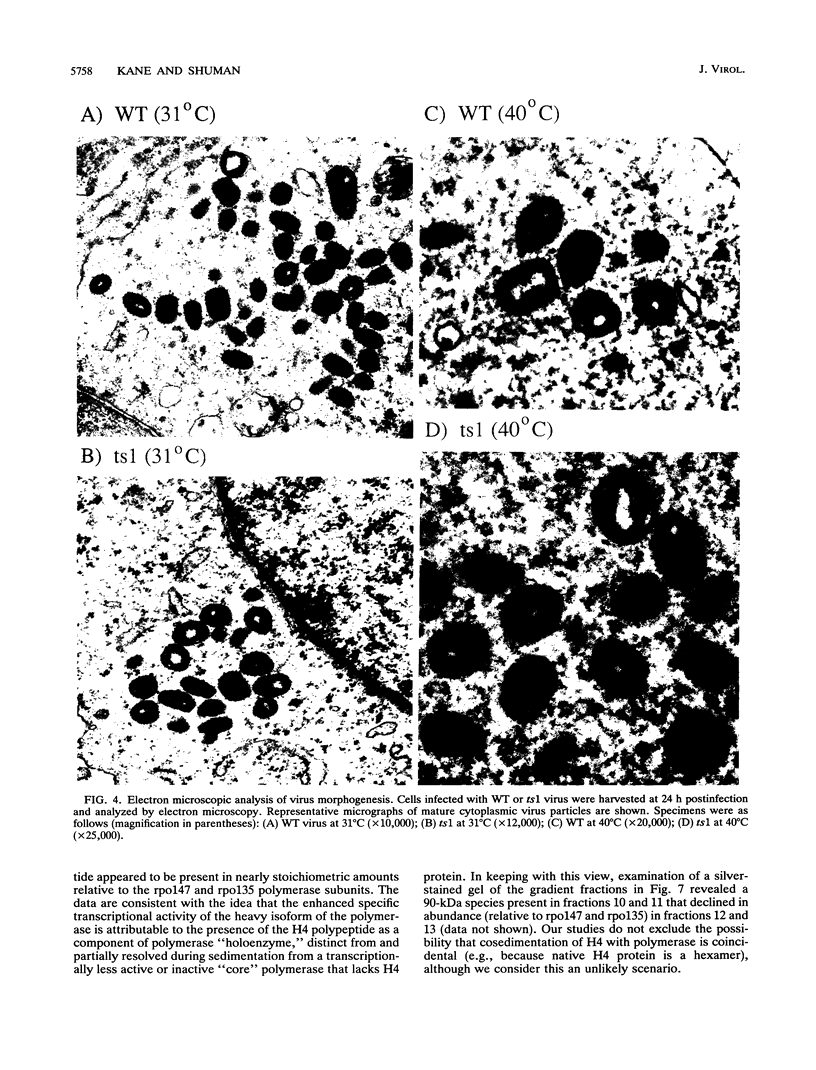
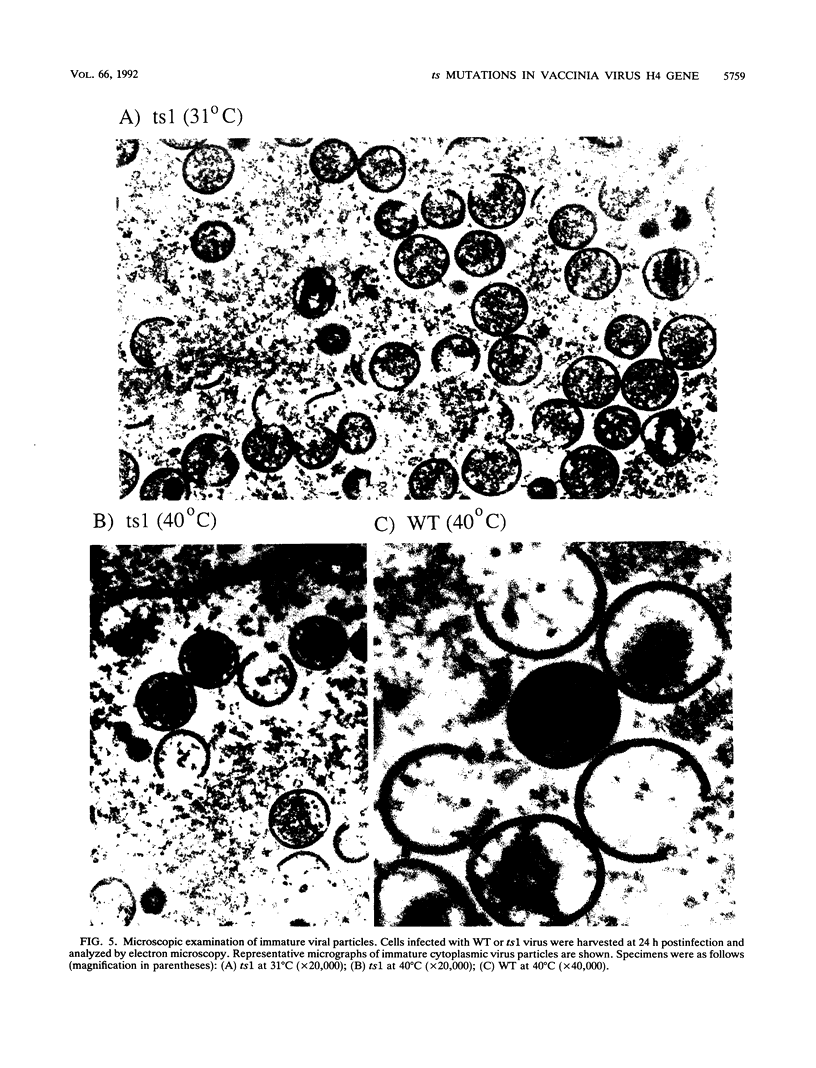
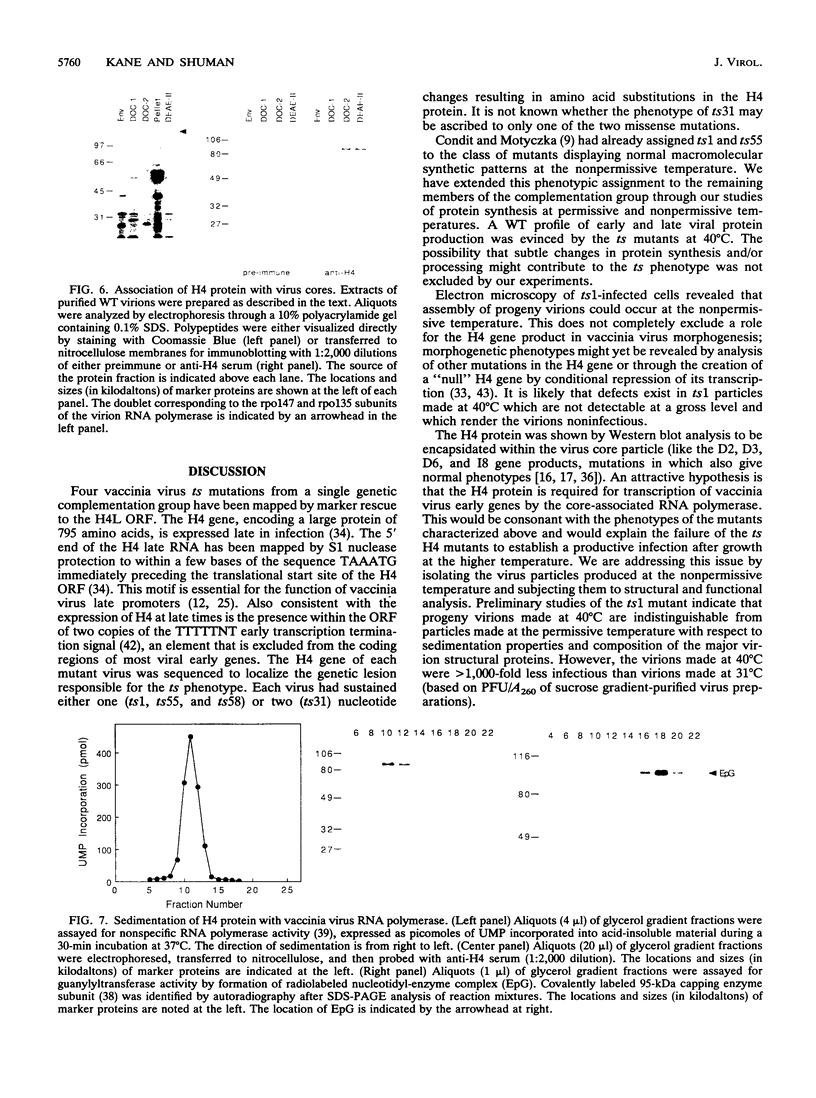
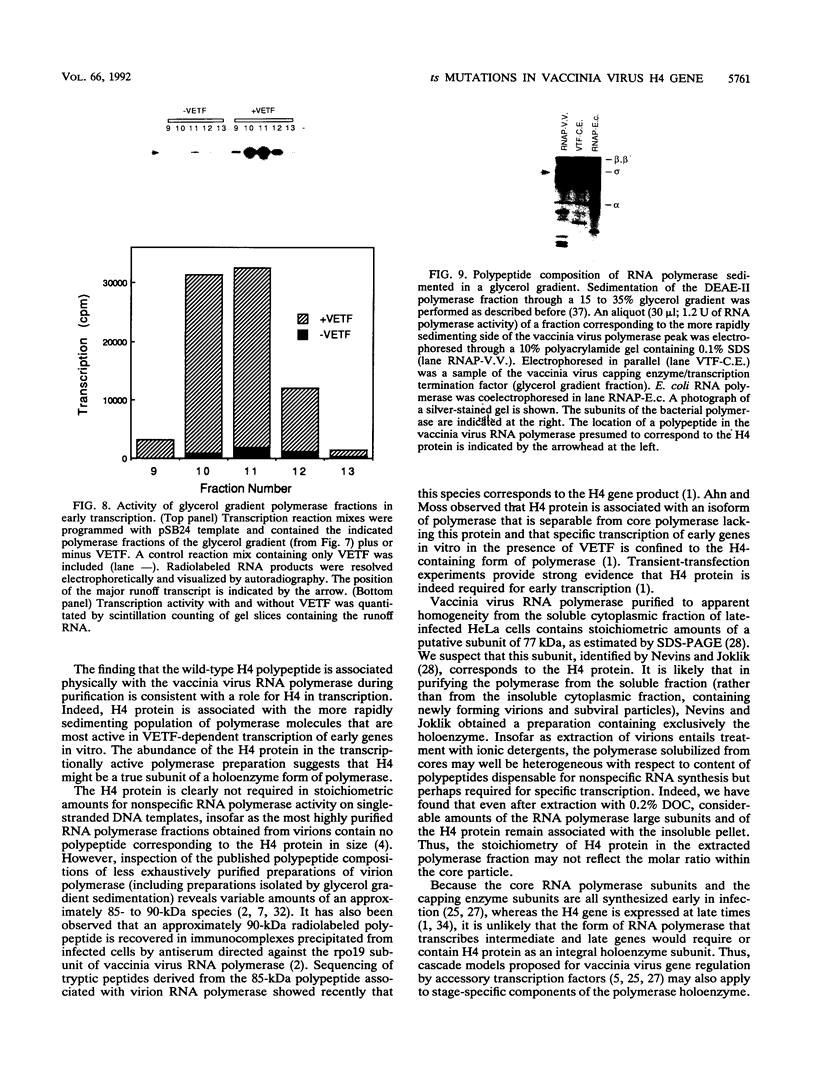
Four previously isolated temperature-sensitive (ts) mutants of vaccinia virus WR (ts1, ts31, ts55, and ts58) comprising a single complementation group (R. C. Condit, A. Motyczka, and G. Spizz, Virology 128:429-443, 1983) have been mapped by marker rescue to the H4L open reading frame located within the genomic HindIII-H DNA fragment. The H4 gene is predicted to encode a 93.6-kDa polypeptide expressed at late times during infection. Nucleotide sequence alterations responsible for thermolabile growth lead to amino acid substitutions in the H4 gene product. All four ts alleles display "normal" patterns of early and late viral protein synthesis at the nonpermissive temperature (40 degrees C). Mature virion particles, microscopically indistinguishable from wild-type virions, are produced in the cytoplasm of cells infected with ts1 at 40 degrees C. Western immunoblot analysis localizes the H4 protein to the virion core. After solubilization from cores, the H4 protein is associated during purification with transcriptionally active vaccinia virus DNA-dependent RNA polymerase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Moss B. RNA polymerase-associated transcription specificity factor encoded by vaccinia virus. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3536–3540. doi: 10.1073/pnas.89.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. Y., Rosel J., Cole N. B., Moss B. Identification and expression of rpo19, a vaccinia virus gene encoding a 19-kilodalton DNA-dependent RNA polymerase subunit. J Virol. 1992 Feb;66(2):971–982. doi: 10.1128/jvi.66.2.971-982.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldick C. J., Jr, Moss B. Resistance of vaccinia virus to rifampicin conferred by a single nucleotide substitution near the predicted NH2 terminus of a gene encoding an Mr 62,000 polypeptide. Virology. 1987 Jan;156(1):138–145. doi: 10.1016/0042-6822(87)90444-2. [DOI] [PubMed] [Google Scholar]
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Broyles S. S., Fesler B. S. Vaccinia virus gene encoding a component of the viral early transcription factor. J Virol. 1990 Apr;64(4):1523–1529. doi: 10.1128/jvi.64.4.1523-1529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Li J., Moss B. Promoter DNA contacts made by the vaccinia virus early transcription factor. J Biol Chem. 1991 Aug 15;266(23):15539–15544. [PubMed] [Google Scholar]
- Broyles S. S., Pennington M. J. Vaccinia virus gene encoding a 30-kilodalton subunit of the viral DNA-dependent RNA polymerase. J Virol. 1990 Nov;64(11):5376–5382. doi: 10.1128/jvi.64.11.5376-5382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Palumbo G. J. Poxvirus pathogenesis. Microbiol Rev. 1991 Mar;55(1):80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Drillien R., Spehner D., Kirn A. Complementation and genetic linkage between vaccinia virus temperature-sensitive mutants. Virology. 1982 Jun;119(2):372–381. doi: 10.1016/0042-6822(82)90096-4. [DOI] [PubMed] [Google Scholar]
- Dyster L. M., Niles E. G. Genetic and biochemical characterization of vaccinia virus genes D2L and D3R which encode virion structural proteins. Virology. 1991 Jun;182(2):455–467. doi: 10.1016/0042-6822(91)90586-z. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J. Isolation and genetic characterization of temperature-sensitive mutants of vaccinia virus WR. J Virol. 1982 Sep;43(3):778–790. doi: 10.1128/jvi.43.3.778-790.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi Z., Condit R. C. Genetic and molecular biological characterization of a vaccinia virus temperature-sensitive complementation group affecting a virion component. Virology. 1991 Mar;181(1):258–272. doi: 10.1016/0042-6822(91)90491-s. [DOI] [PubMed] [Google Scholar]
- Fathi Z., Condit R. C. Phenotypic characterization of a vaccinia virus temperature-sensitive complementation group affecting a virion component. Virology. 1991 Mar;181(1):273–276. doi: 10.1016/0042-6822(91)90492-t. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Hagler J., Shuman S. A freeze-frame view of eukaryotic transcription during elongation and capping of nascent mRNA. Science. 1992 Feb 21;255(5047):983–986. doi: 10.1126/science.1546295. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Künzi M. S., Traktman P. Genetic evidence for involvement of vaccinia virus DNA-dependent ATPase I in intermediate and late gene expression. J Virol. 1989 Sep;63(9):3999–4010. doi: 10.1128/jvi.63.9.3999-4010.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Hagler J., Shuman S. Discrete functional stages of vaccinia virus early transcription during a single round of RNA synthesis in vitro. J Biol Chem. 1991 Jul 15;266(20):13303–13310. [PubMed] [Google Scholar]
- Miner J. N., Hruby D. E. Rifampicin prevents virosome localization of L65, an essential vaccinia virus polypeptide. Virology. 1989 May;170(1):227–237. doi: 10.1016/0042-6822(89)90370-x. [DOI] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Moss B., Ahn B. Y., Amegadzie B., Gershon P. D., Keck J. G. Cytoplasmic transcription system encoded by vaccinia virus. J Biol Chem. 1991 Jan 25;266(3):1355–1358. [PubMed] [Google Scholar]
- Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and properties of the vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1977 Oct 10;252(19):6930–6938. [PubMed] [Google Scholar]
- Niles E. G., Condit R. C., Caro P., Davidson K., Matusick L., Seto J. Nucleotide sequence and genetic map of the 16-kb vaccinia virus HindIII D fragment. Virology. 1986 Aug;153(1):96–112. doi: 10.1016/0042-6822(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Perkus M. E., Goebel S. J., Davis S. W., Johnson G. P., Norton E. K., Paoletti E. Deletion of 55 open reading frames from the termini of vaccinia virus. Virology. 1991 Jan;180(1):406–410. doi: 10.1016/0042-6822(91)90047-f. [DOI] [PubMed] [Google Scholar]
- Perkus M. E., Limbach K., Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989 Sep;63(9):3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick S. D., Broyles S. S. Vaccinia virus gene D7R encodes a 20,000-dalton subunit of the viral DNA-dependent RNA polymerase. Virology. 1990 Oct;178(2):603–605. doi: 10.1016/0042-6822(90)90362-u. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. F., Smith G. L. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 1990 Sep 25;18(18):5347–5351. doi: 10.1093/nar/18.18.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosel J. L., Earl P. L., Weir J. P., Moss B. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J Virol. 1986 Nov;60(2):436–449. doi: 10.1128/jvi.60.2.436-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Seto J., Celenza L. M., Condit R. C., Niles E. G. Genetic map of the vaccinia virus HindIII D Fragment. Virology. 1987 Sep;160(1):110–119. doi: 10.1016/0042-6822(87)90051-1. [DOI] [PubMed] [Google Scholar]
- Shuman S., Broyles S. S., Moss B. Purification and characterization of a transcription termination factor from vaccinia virions. J Biol Chem. 1987 Sep 5;262(25):12372–12380. [PubMed] [Google Scholar]
- Shuman S., Morham S. G. Domain structure of vaccinia virus mRNA capping enzyme. Activity of the Mr 95,000 subunit expressed in Escherichia coli. J Biol Chem. 1990 Jul 15;265(20):11967–11972. [PubMed] [Google Scholar]
- Spencer E., Shuman S., Hurwitz J. Purification and properties of vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1980 Jun 10;255(11):5388–5395. [PubMed] [Google Scholar]
- Tartaglia J., Paoletti E. Physical mapping and DNA sequence analysis of the rifampicin resistance locus in vaccinia virus. Virology. 1985 Dec;147(2):394–404. doi: 10.1016/0042-6822(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Condit R. C. Marker rescue mapping of vaccinia virus temperature-sensitive mutants using overlapping cosmid clones representing the entire virus genome. Virology. 1986 Apr 15;150(1):10–20. doi: 10.1016/0042-6822(86)90261-8. [DOI] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Moss B. Inducer-dependent conditional-lethal mutant animal viruses. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1511–1515. doi: 10.1073/pnas.88.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]