Abstract
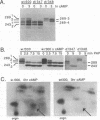
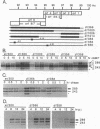
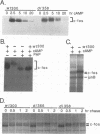
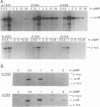
Adenovirus E1A protein and cyclic AMP cooperate to induce transcription factor AP-1 and viral gene expression in mouse S49 cells. We report that a protein encoded within the viral E4 gene region acts to counterbalance the induction of AP-1 DNA-binding activity by E1A and cyclic AMP. Studies with mutant adenoviruses demonstrated that in the absence of E4orf4 protein, AP-1 DNA-binding activity is induced to substantially higher levels than in wild-type virus-infected cells. The induction is the result of increased production of JunB and c-Fos proteins. Hyperphosphorylated forms of c-Fos and E1A proteins accumulate in the absence of functional E4orf4 protein. We propose that the E4orf4 protein acts to inhibit the activity of a cellular kinase that phosphorylates both the E1A and c-Fos proteins. Phosphorylation-dependent alterations in the activity of c-Fos, E1A, or some unidentified protein might, then, lead to decreased synthesis of AP-1 components. This E4 function likely plays an important role in natural infections, since a mutant virus unable to express the E4orf4 protein is considerably more cytotoxic than the wild-type virus.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991 Jul;11(7):3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate C., Luk D., Gagne E., Roeder R. G., Curran T. Fos and jun cooperate in transcriptional regulation via heterologous activation domains. Mol Cell Biol. 1990 Oct;10(10):5532–5535. doi: 10.1128/mcb.10.10.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S., Raychaudhuri P., Nevins J. R. Phosphorylation-dependent activation of the adenovirus-inducible E2F transcription factor in a cell-free system. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4352–4356. doi: 10.1073/pnas.86.12.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S., Weinmann R., Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991 Jun 14;65(6):1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- Barber J. R., Verma I. M. Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum. Mol Cell Biol. 1987 Jun;7(6):2201–2211. doi: 10.1128/mcb.7.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: characterization of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):611–620. doi: 10.1002/jcp.1040850313. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Bridge E., Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989 Feb;63(2):631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckbinder L., Miralles V. J., Reinberg D. TPA can overcome the requirement for EIa and together act synergistically in stimulating expression of the adenovirus EIII promoter. EMBO J. 1989 Dec 20;8(13):4239–4250. doi: 10.1002/j.1460-2075.1989.tb08609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., Kaelin W. G., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. doi: 10.1016/0092-8674(91)90559-h. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Ferreira P. C., Gentz R., Franza B. R., Jr, Curran T. The product of a fos-related gene, fra-1, binds cooperatively to the AP-1 site with Jun: transcription factor AP-1 is comprised of multiple protein complexes. Genes Dev. 1989 Feb;3(2):173–184. doi: 10.1101/gad.3.2.173. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Curran T., Van Beveren C., Verma I. M. Viral and cellular fos proteins are complexed with a 39,000-dalton cellular protein. Mol Cell Biol. 1985 Jan;5(1):167–172. doi: 10.1128/mcb.5.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont D. J., Tremblay M. L., Branton P. E. Phosphorylation at serine 89 induces a shift in gel mobility but has little effect on the function of adenovirus type 5 E1A proteins. J Virol. 1989 Feb;63(2):987–991. doi: 10.1128/jvi.63.2.987-991.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D. A., Hardy S., Shenk T. cAMP acts in synergy with E1A protein to activate transcription of the adenovirus early genes E4 and E1A. Genes Dev. 1988 Dec;2(12A):1517–1528. doi: 10.1101/gad.2.12a.1517. [DOI] [PubMed] [Google Scholar]
- Engel D. A., Muller U., Gedrich R. W., Eubanks J. S., Shenk T. Induction of c-fos mRNA and AP-1 DNA-binding activity by cAMP in cooperation with either the adenovirus 243- or the adenovirus 289-amino acid E1A protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3957–3961. doi: 10.1073/pnas.88.9.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer G. A., Katoh Y., Roberts R. J. Characterization of the major mRNAs from adenovirus 2 early region 4 by cDNA cloning and sequencing. Nucleic Acids Res. 1984 Apr 25;12(8):3503–3519. doi: 10.1093/nar/12.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., Lee J. H., Scheppler J. A., Herrmann C., Harlow E., Deuschle U., Beach D., Franza B. R., Jr Cell cycle regulation of histone H1 kinase activity associated with the adenoviral protein E1A. Science. 1991 Sep 13;253(5025):1271–1275. doi: 10.1126/science.1653969. [DOI] [PubMed] [Google Scholar]
- Gius D., Cao X. M., Rauscher F. J., 3rd, Cohen D. R., Curran T., Sukhatme V. P. Transcriptional activation and repression by Fos are independent functions: the C terminus represses immediate-early gene expression via CArG elements. Mol Cell Biol. 1990 Aug;10(8):4243–4255. doi: 10.1128/mcb.10.8.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Allegretto E. A., Karin M., Green M. R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988 Oct;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Shenk T. Adenoviral control regions activated by E1A and the cAMP response element bind to the same factor. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4171–4175. doi: 10.1073/pnas.85.12.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. Sequence-independent autoregulation of the adenovirus type 5 E1A transcription unit. Mol Cell Biol. 1985 Nov;5(11):3214–3221. doi: 10.1128/mcb.5.11.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Kovelman R., Roeder R. G. Activation of transcription factor IIIC by the adenovirus E1A protein. Cell. 1988 Jun 17;53(6):907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Horikoshi N., Maguire K., Kralli A., Maldonado E., Reinberg D., Weinmann R. Direct interaction between adenovirus E1A protein and the TATA box binding transcription factor IID. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5124–5128. doi: 10.1073/pnas.88.12.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. T., Schneider R. J. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap-binding protein. Cell. 1991 Apr 19;65(2):271–280. doi: 10.1016/0092-8674(91)90161-q. [DOI] [PubMed] [Google Scholar]
- Hurst H. C., Jones N. C. Identification of factors that interact with the E1A-inducible adenovirus E3 promoter. Genes Dev. 1987 Dec;1(10):1132–1146. doi: 10.1101/gad.1.10.1132. [DOI] [PubMed] [Google Scholar]
- Hérissé J., Rigolet M., de Dinechin S. D., Galibert F. Nucleotide sequence of adenovirus 2 DNA fragment encoding for the carboxylic region of the fiber protein and the entire E4 region. Nucleic Acids Res. 1981 Aug 25;9(16):4023–4042. doi: 10.1093/nar/9.16.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger T., Shenk T. A protein kinase is present in a complex with adenovirus E1A proteins. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11143–11147. doi: 10.1073/pnas.88.24.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf U., Büscher M., Schönthal A., Rahmsdorf H. J., Herrlich P. Autoregulation of fos: the dyad symmetry element as the major target of repression. EMBO J. 1989 Sep;8(9):2559–2566. doi: 10.1002/j.1460-2075.1989.tb08394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Hai T. Y., SivaRaman L., Thimmappaya B., Hurst H. C., Jones N. C., Green M. R. A cellular protein, activating transcription factor, activates transcription of multiple E1A-inducible adenovirus early promoters. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8355–8359. doi: 10.1073/pnas.84.23.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. S., Kao C. C., Bryant G. O., Liu X., Berk A. J. Adenovirus E1A activation domain binds the basic repeat in the TATA box transcription factor. Cell. 1991 Oct 18;67(2):365–376. doi: 10.1016/0092-8674(91)90188-5. [DOI] [PubMed] [Google Scholar]
- Leza M. A., Hearing P. Cellular transcription factor binds to adenovirus early region promoters and to a cyclic AMP response element. J Virol. 1988 Aug;62(8):3003–3013. doi: 10.1128/jvi.62.8.3003-3013.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leza M. A., Hearing P. Independent cyclic AMP and E1A induction of adenovirus early region 4 expression. J Virol. 1989 Jul;63(7):3057–3064. doi: 10.1128/jvi.63.7.3057-3064.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Interaction of a common cellular transcription factor, ATF, with regulatory elements in both E1a- and cyclic AMP-inducible promoters. Proc Natl Acad Sci U S A. 1988 May;85(10):3396–3400. doi: 10.1073/pnas.85.10.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Green M. R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell. 1990 Jun 29;61(7):1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Lowag C., Neuberg M., Müller R. trans-repression of the mouse c-fos promoter: a novel mechanism of Fos-mediated trans-regulation. Cell. 1989 Dec 22;59(6):999–1007. doi: 10.1016/0092-8674(89)90756-3. [DOI] [PubMed] [Google Scholar]
- Maguire K., Shi X. P., Horikoshi N., Rappaport J., Rosenberg M., Weinmann R. Interactions between adenovirus E1A and members of the AP-1 family of cellular transcription factors. Oncogene. 1991 Aug;6(8):1417–1422. [PubMed] [Google Scholar]
- Marton M. J., Baim S. B., Ornelles D. A., Shenk T. The adenovirus E4 17-kilodalton protein complexes with the cellular transcription factor E2F, altering its DNA-binding properties and stimulating E1A-independent accumulation of E2 mRNA. J Virol. 1990 May;64(5):2345–2359. doi: 10.1128/jvi.64.5.2345-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Hashimoto S., Wold W. S., Symington J., Rankin A., Green M. Identification of adenovirus 2 early region 4 polypeptides by in vitro translation and tryptic peptide map analysis. J Virol. 1982 Jan;41(1):334–339. doi: 10.1128/jvi.41.1.334-339.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Muchardt C., Li C., Kornuc M., Gaynor R. CREB regulation of cellular cyclic AMP-responsive and adenovirus early promoters. J Virol. 1990 Sep;64(9):4296–4305. doi: 10.1128/jvi.64.9.4296-4305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Müller D., Kurz C., Renz M. Different types of modification in c-fos and its associated protein p39: modulation of DNA binding by phosphorylation. Oncogene Res. 1987;2(1):19–32. [PubMed] [Google Scholar]
- Müller U., Roberts M. P., Engel D. A., Doerfler W., Shenk T. Induction of transcription factor AP-1 by adenovirus E1A protein and cAMP. Genes Dev. 1989 Dec;3(12A):1991–2002. doi: 10.1101/gad.3.12a.1991. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Offringa R., Gebel S., van Dam H., Timmers M., Smits A., Zwart R., Stein B., Bos J. L., van der Eb A., Herrlich P. A novel function of the transforming domain of E1a: repression of AP-1 activity. Cell. 1990 Aug 10;62(3):527–538. doi: 10.1016/0092-8674(90)90017-9. [DOI] [PubMed] [Google Scholar]
- Ofir R., Dwarki V. J., Rashid D., Verma I. M. Phosphorylation of the C terminus of Fos protein is required for transcriptional transrepression of the c-fos promoter. Nature. 1990 Nov 1;348(6296):80–82. doi: 10.1038/348080a0. [DOI] [PubMed] [Google Scholar]
- Pei R., Berk A. J. Multiple transcription factor binding sites mediate adenovirus E1A transactivation. J Virol. 1989 Aug;63(8):3499–3506. doi: 10.1128/jvi.63.8.3499-3506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Bagchi S., Nevins J. R. DNA-binding activity of the adenovirus-induced E4F transcription factor is regulated by phosphorylation. Genes Dev. 1989 May;3(5):620–627. doi: 10.1101/gad.3.5.620. [DOI] [PubMed] [Google Scholar]
- Rice S. A., Klessig D. F. Isolation and analysis of adenovirus type 5 mutants containing deletions in the gene encoding the DNA-binding protein. J Virol. 1985 Dec;56(3):767–778. doi: 10.1128/jvi.56.3.767-778.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. D., Slavicek J. M., Schneider J. F., Jones N. C. Heterogeneity of adenovirus type 5 E1A proteins: multiple serine phosphorylations induce slow-migrating electrophoretic variants but do not affect E1A-induced transcriptional activation or transformation. J Virol. 1988 Jun;62(6):1948–1955. doi: 10.1128/jvi.62.6.1948-1955.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Sassone-Corsi P. Cyclic AMP induction of early adenovirus promoters involves sequences required for E1A trans-activation. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7192–7196. doi: 10.1073/pnas.85.19.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Ciesiolka T., Gruss P. A nexus between Oct-4 and E1A: implications for gene regulation in embryonic stem cells. Cell. 1991 Jul 26;66(2):291–304. doi: 10.1016/0092-8674(91)90619-a. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Schütte J., Viallet J., Nau M., Segal S., Fedorko J., Minna J. jun-B inhibits and c-fos stimulates the transforming and trans-activating activities of c-jun. Cell. 1989 Dec 22;59(6):987–997. doi: 10.1016/0092-8674(89)90755-1. [DOI] [PubMed] [Google Scholar]
- Shenk T., Flint J. Transcriptional and transforming activities of the adenovirus E1A proteins. Adv Cancer Res. 1991;57:47–85. doi: 10.1016/s0065-230x(08)60995-1. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Tremblay M. L., Dumont D. J., Branton P. E. Analysis of phosphorylation sites in the exon 1 region of E1A proteins of human adenovirus type 5. Virology. 1989 Apr;169(2):397–407. doi: 10.1016/0042-6822(89)90165-7. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Tsukamoto A. S., Ponticelli A., Berk A. J., Gaynor R. B. Genetic mapping of a major site of phosphorylation in adenovirus type 2 E1A proteins. J Virol. 1986 Jul;59(1):14–22. doi: 10.1128/jvi.59.1.14-22.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen A., Gilardi P., Näslund A., LeMoullec J. M., Pettersson U., Perricaudet M. mRNAs from human adenovirus 2 early region 4. J Virol. 1984 Sep;51(3):822–831. doi: 10.1128/jvi.51.3.822-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. The oncogene jun and nuclear signalling. Trends Biochem Sci. 1989 May;14(5):172–175. doi: 10.1016/0968-0004(89)90268-5. [DOI] [PubMed] [Google Scholar]
- Wang H. G., Draetta G., Moran E. E1A induces phosphorylation of the retinoblastoma protein independently of direct physical association between the E1A and retinoblastoma products. Mol Cell Biol. 1991 Aug;11(8):4253–4265. doi: 10.1128/mcb.11.8.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. A cell line that supports the growth of a defective early region 4 deletion mutant of human adenovirus type 2. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5383–5386. doi: 10.1073/pnas.80.17.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Fos C-terminal mutations block down-regulation of c-fos transcription following serum stimulation. EMBO J. 1988 Dec 20;7(13):4193–4202. doi: 10.1002/j.1460-2075.1988.tb03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Shenk T. Dissection of overlapping functions within the adenovirus type 5 E1A gene. EMBO J. 1984 Aug;3(8):1907–1912. doi: 10.1002/j.1460-2075.1984.tb02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. P., Rowe D. T., Tremblay M. L., McDermott M., Branton P. E. Identification of human adenovirus early region 1 products by using antisera against synthetic peptides corresponding to the predicted carboxy termini. J Virol. 1983 Jun;46(3):1003–1013. doi: 10.1128/jvi.46.3.1003-1013.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R., Foulkes N., Mulder M., Kruijer W., Sassone-Corsi P. Positive regulation of jun/AP-1 by E1A. Mol Cell Biol. 1991 Jan;11(1):192–201. doi: 10.1128/mcb.11.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]