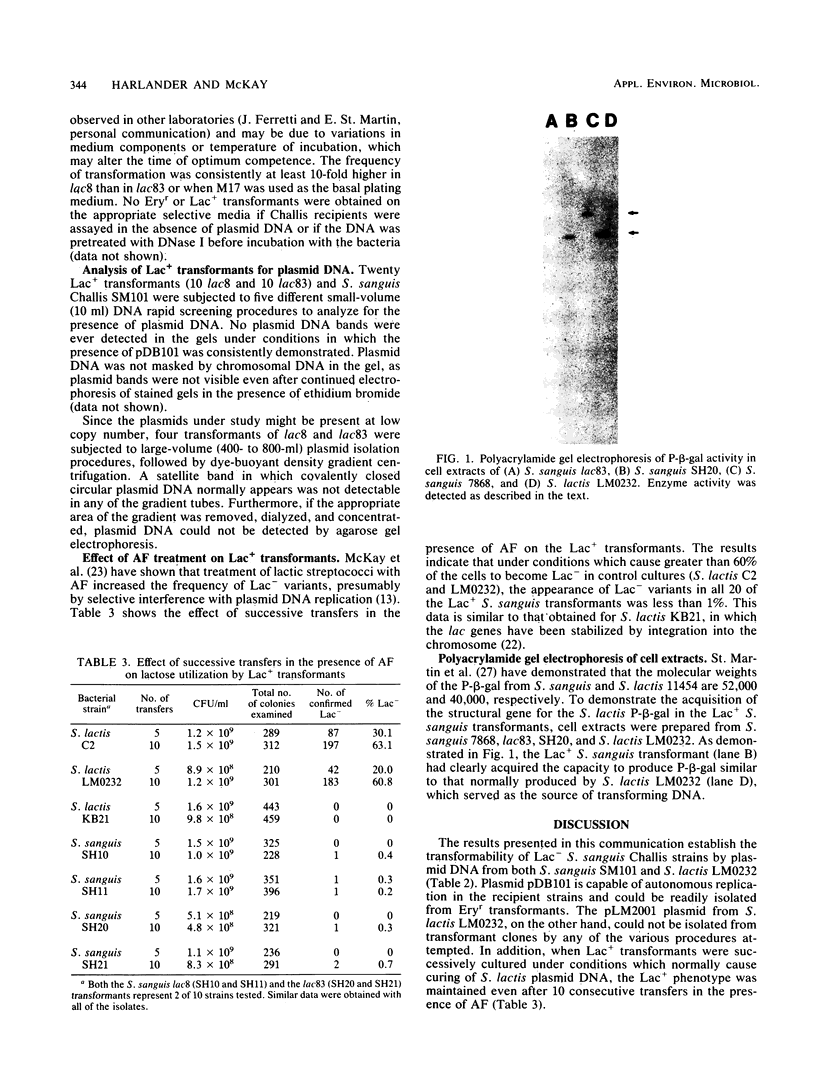
Abstract
Streptococcus lactis plasmid DNA, which is required for the fermentation of lactose (plasmid pLM2001), and a potential streptococcal cloning vector plasmid (pDB101) which confers resistance to erythromycin were evaluated by transformation into Streptococcus sanguis Challis. Plasmid pLM2001 transformed lactose-negative (Lac-) mutants of S. sanguis with high efficiency and was capable of conferring lactose-metabolizing ability to a mutant deficient in Enzyme IIlac, Factor IIIlac, and phospho-beta-galactosidase of the lactose phosphoenolpyruvate-phosphotransferase system. Plasmid pDB101 was capable of high-efficiency transformation of S. sanguis to antibiotic resistance, and the plasmid could be readily isolated from transformed strains. However, when 20 pLM2001 Lac+ transformants were analyzed by a variety of techniques for the presence of plasmids, none could be detected. In addition, attempts to cure the Lac+ transformants by treatment with acriflavin were unsuccessful. Polyacrylamide gel electrophoresis was used to demonstrate that the transformants had acquired a phospho-beta-galactosidase characteristic of that normally produced by S. lactis and not S. sanguis. It is proposed that the genes required for lactose fermentation may have become stabilized in the transformants due to their integration into the host chromosome. The efficient transformation into and expression of pLM2001 and pDB101 genes in S. sanguis provides a model system which could allow the development of a system for cloning genes from dairy starter cultures into S. sanguis to examine factors affecting their expression and regulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Plasmids, loss of lactose metabolism, and appearance of partial and full lactose-fermenting revertants in Streptococcus cremoris B1. J Bacteriol. 1977 Jan;129(1):367–377. doi: 10.1128/jb.129.1.367-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Ferretti J. J. Physical mapping of plasmid pDB101: a potential vector plasmid for molecular cloning in streptococci. Plasmid. 1980 Sep;4(2):130–138. doi: 10.1016/0147-619x(80)90002-5. [DOI] [PubMed] [Google Scholar]
- Behnke D. Plasmid transformation of Streptococcus sanguis (Challis) occurs by circular and linear molecules. Mol Gen Genet. 1981;182(3):490–497. doi: 10.1007/BF00293940. [DOI] [PubMed] [Google Scholar]
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1980 Nov;18(5):753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Duncan C. H., Wilson G. A., Young F. E. Mechanism of integrating foreign DNA during transformation of Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3664–3668. doi: 10.1073/pnas.75.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaustad P. Genetic transformation in Streptococcus sanguis. Competence factor and competence factor inactivator. Acta Pathol Microbiol Scand B. 1981 Apr;89(2):67–73. doi: 10.1111/j.1699-0463.1981.tb00155_89b.x. [DOI] [PubMed] [Google Scholar]
- Gibson E. M., Chace N. M., London S. B., London J. Transfer of plasmid-mediated antibiotic resistance from streptococci to lactobacilli. J Bacteriol. 1979 Jan;137(1):614–619. doi: 10.1128/jb.137.1.614-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Larsen L. D., McKay L. L. Plasmid Profiles of Lactose-Negative and Proteinase-Deficient Mutants of Streptococcus lactis C10, ML(3), and M18. Appl Environ Microbiol. 1979 Jun;37(6):1193–1195. doi: 10.1128/aem.37.6.1193-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc D. J., Lee L. N. Rapid screening procedure for detection of plasmids in streptococci. J Bacteriol. 1979 Dec;140(3):1112–1115. doi: 10.1128/jb.140.3.1112-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G. Isolation of a protein-containing cell surface component from Streptococcus sanguis which affects its adherence to saliva-coated hydroxyapatite. Infect Immun. 1981 Nov;34(2):428–434. doi: 10.1128/iai.34.2.428-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Stabilization of Lactose Metabolism in Streptococcus lactis C2. Appl Environ Microbiol. 1978 Aug;36(2):360–367. doi: 10.1128/aem.36.2.360-367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Slade H. D. Effect of filtrates from transformable and nontransformable streptococci on the transformation of streptococci. J Bacteriol. 1966 Jun;91(6):2216–2222. doi: 10.1128/jb.91.6.2216-2222.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Snook R. J., McKay L. L. Conjugal Transfer of Lactose-Fermenting Ability Among Streptococcus cremoris and Streptococcus lactis Strains. Appl Environ Microbiol. 1981 Nov;42(5):904–911. doi: 10.1128/aem.42.5.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. M., McKay L. L. Recombinant plasmid associated cell aggregation and high-frequency conjugation of Streptococcus lactis ML3. J Bacteriol. 1981 Jun;146(3):937–944. doi: 10.1128/jb.146.3.937-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger C. L., Beaman A. J., Lee L. N. Tween 80 effect on glucosyltransferase synthesis by Streptococcus salivarius. J Bacteriol. 1978 Jan;133(1):231–239. doi: 10.1128/jb.133.1.231-239.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



