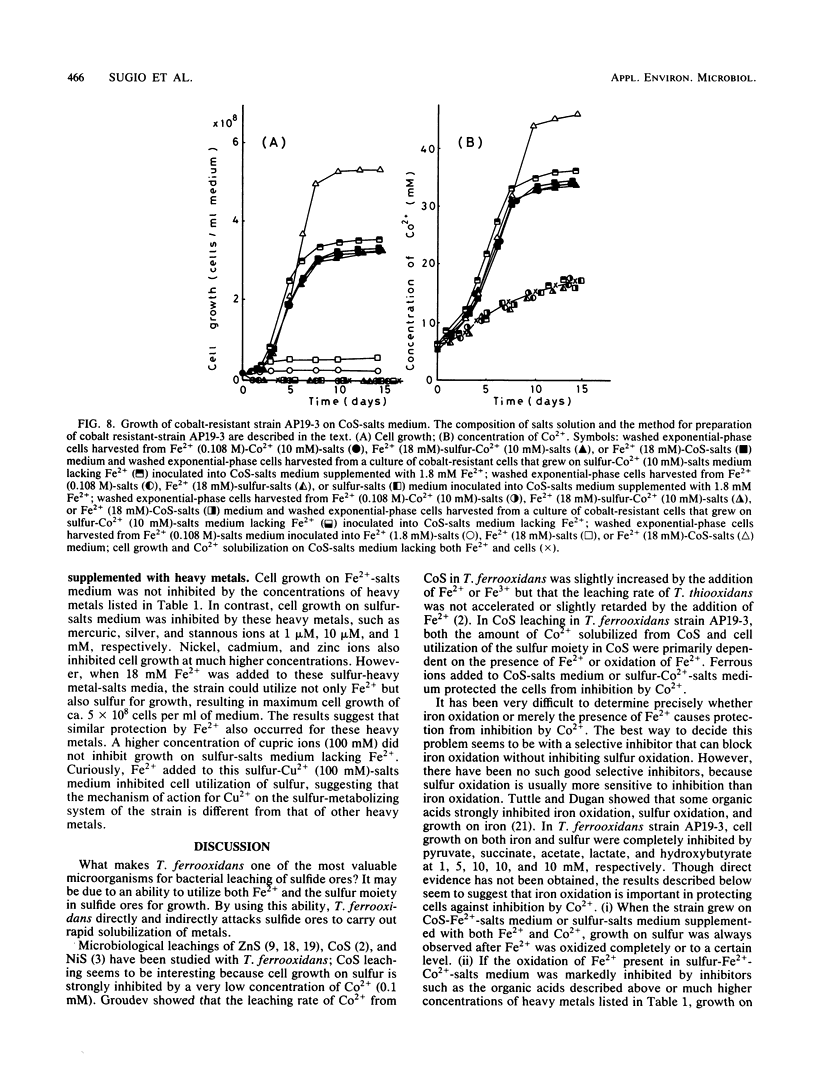
Abstract
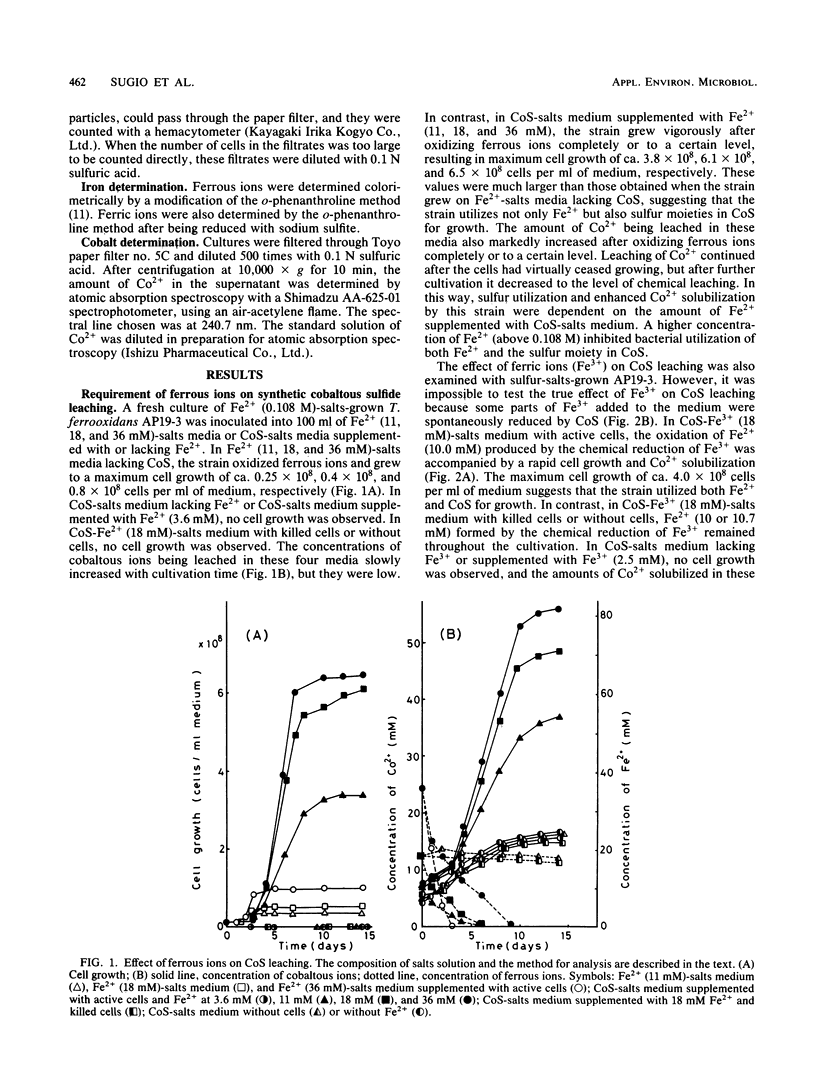
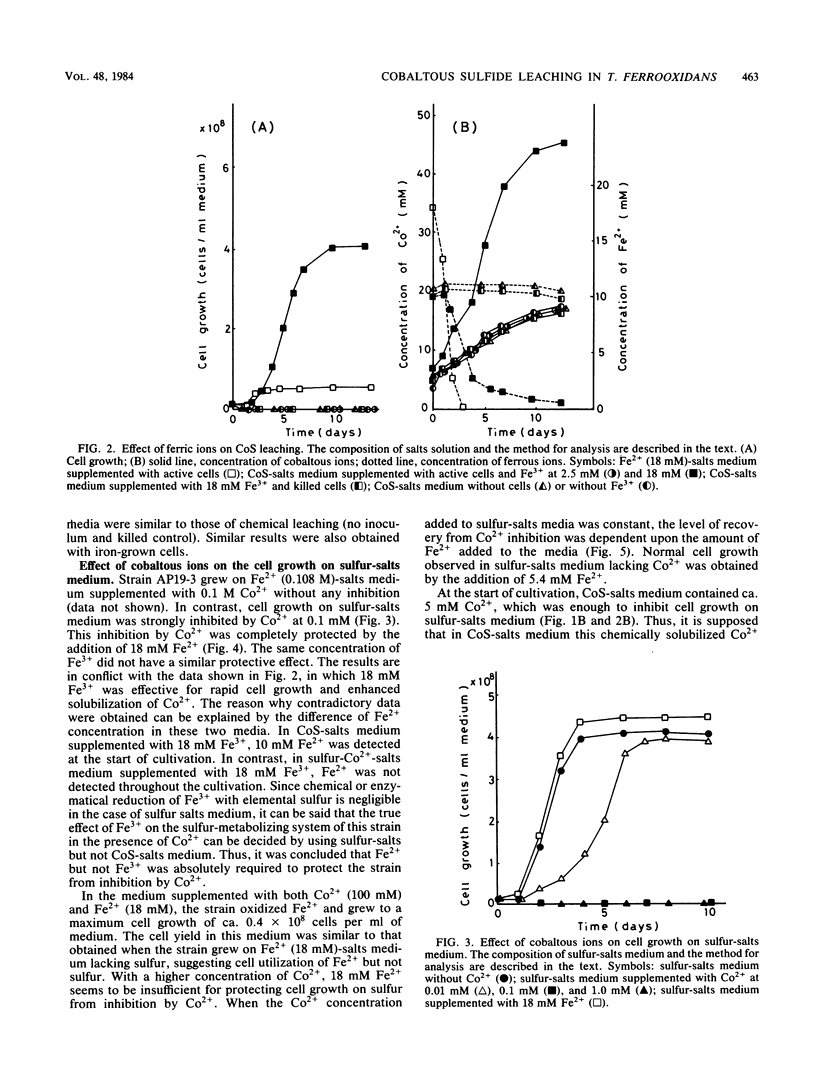
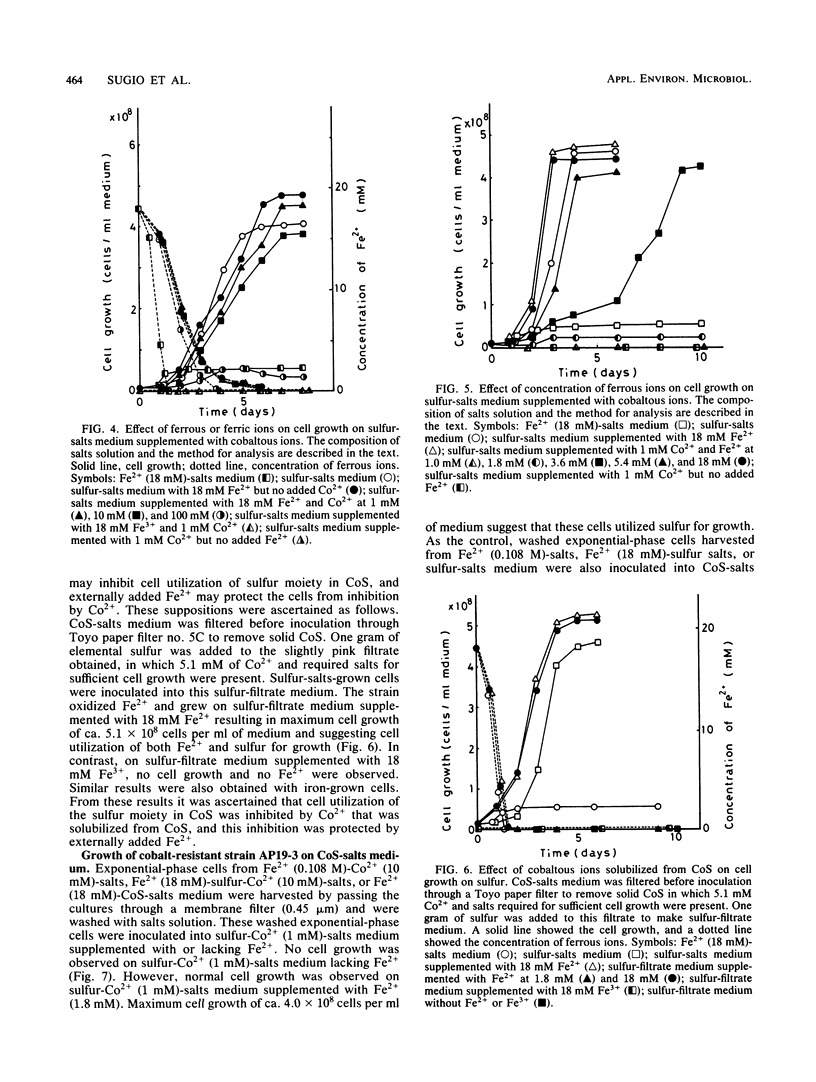
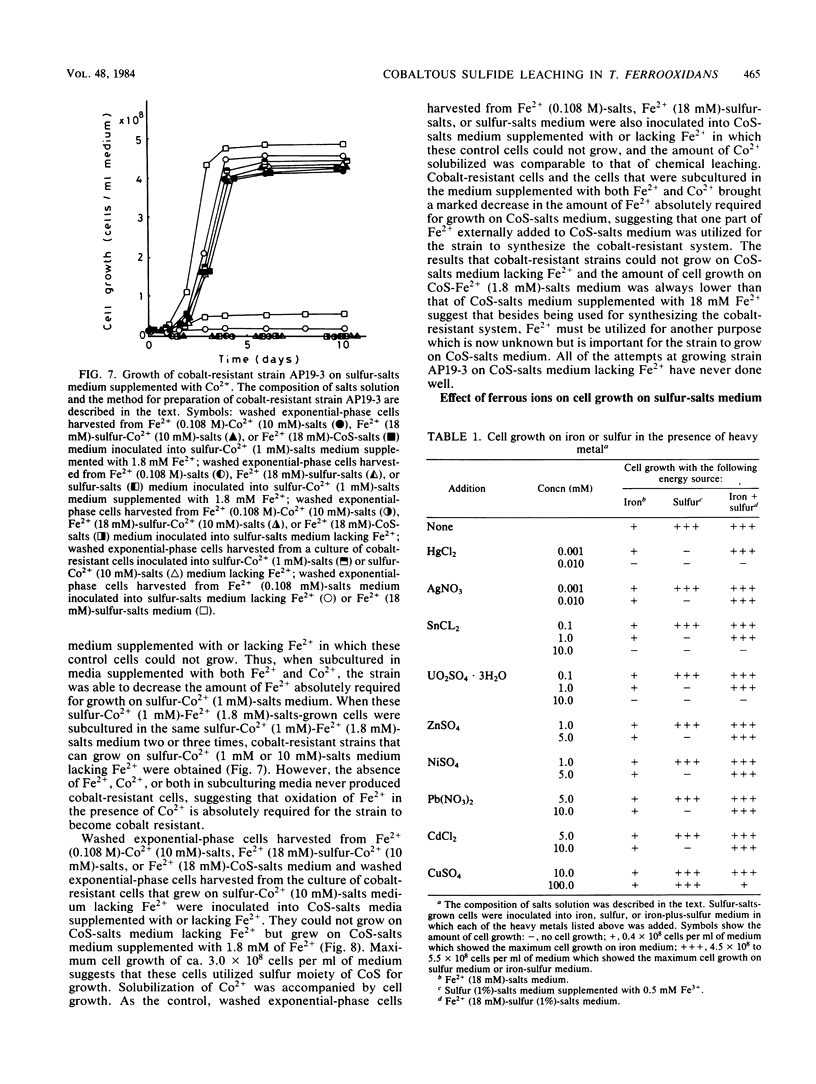
Microbiological leaching of synthetic cobaltous sulfide (CoS) was investigated with a pure strain of Thiobacillus ferroxidans. The strain could not grow on CoS-salts medium in the absence of ferrous ions (Fe2+). However, in CoS-salts medium supplemented with 18 mM Fe2+, the strain utilized both Fe2+ and the sulfur moiety in CoS for growth, resulting in an enhanced solubilization of Co2+. Cell growth on sulfur-salts medium was strongly inhibited by Co2+, and this inhibition was completely protected by Fe2+. Cobalt-resistant cells, obtained by subculturing the strain in medium supplemented with both Fe2+ and Co2+, brought a marked decrease in the amount of Fe2+ absolutely required for cell growth on CoS-salts medium. As one mechanism of protection by Fe2+, it is proposed that the strain utilizes one part of Fe2+ externally added to CoS-salts medium to synthesize the cobalt-resistant system. Since a similar protective effect by Fe2+ was also observed for cell inhibition by stannous, nickel, zinc, silver, and mercuric ions, a new role of Fe2+ in bacterial leaching in T. ferrooxidans is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hoffman L. E., Hendrix J. L. Inhibition of Thiobacillus ferrooxidans by soluble silver. Biotechnol Bioeng. 1976 Aug;18(8):1161–1165. doi: 10.1002/bit.260180811. [DOI] [PubMed] [Google Scholar]
- Olson G. J., Porter F. D., Rubinstein J., Silver S. Mercuric reductase enzyme from a mercury-volatilizing strain of Thiobacillus ferrooxidans. J Bacteriol. 1982 Sep;151(3):1230–1236. doi: 10.1128/jb.151.3.1230-1236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZZELL W. E., TRUSELL P. C. ISOLATION AND PROPERTIES OF AN IRON-OXIDIZING THIOBACILLUS. J Bacteriol. 1963 Mar;85:595–603. doi: 10.1128/jb.85.3.595-603.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. P. Mechanism of bacterial pyrite oxidation. J Bacteriol. 1967 Oct;94(4):1046–1051. doi: 10.1128/jb.94.4.1046-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torma A. E., Walden C. C., Branion R. M. Microbiological leaching of a zinc sulfide concentrate. Biotechnol Bioeng. 1970 Jul;12(4):501–517. doi: 10.1002/bit.260120403. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Niemelä S. I., Gyllenberg H. G. Tolerance of Thiobacillus ferrooxidans to some metals. Antonie Van Leeuwenhoek. 1971;37(4):489–496. doi: 10.1007/BF02218519. [DOI] [PubMed] [Google Scholar]
- Tuttle J. H., Dugan P. R. Inhibition of growth, iron, and sulfur oxidation in Thiobacillus ferrooxidans by simple organic compounds. Can J Microbiol. 1976 May;22(5):719–730. doi: 10.1139/m76-105. [DOI] [PubMed] [Google Scholar]


