Abstract
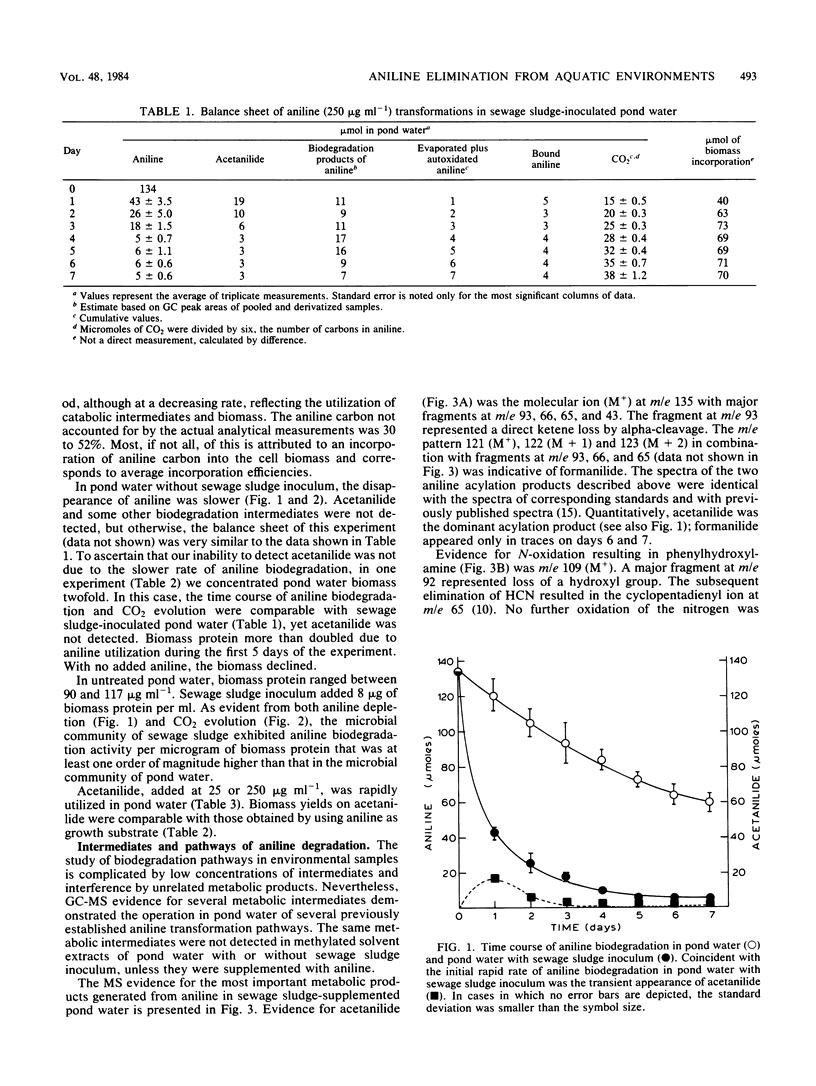
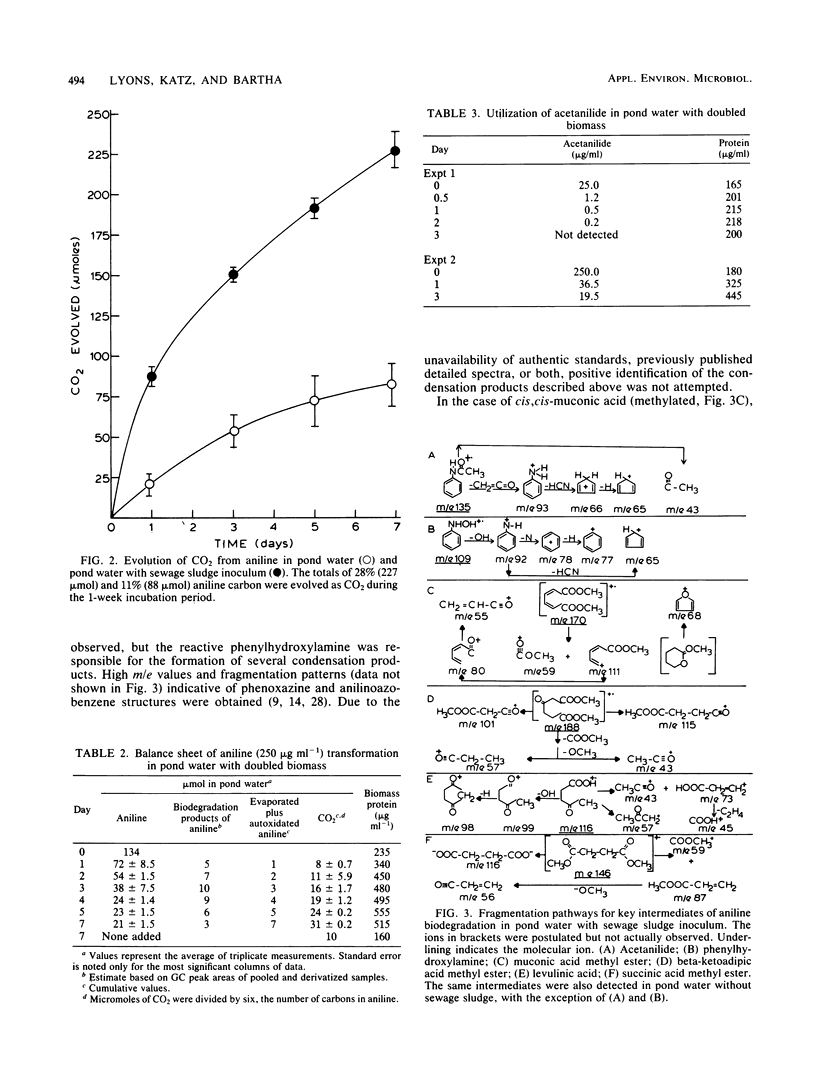
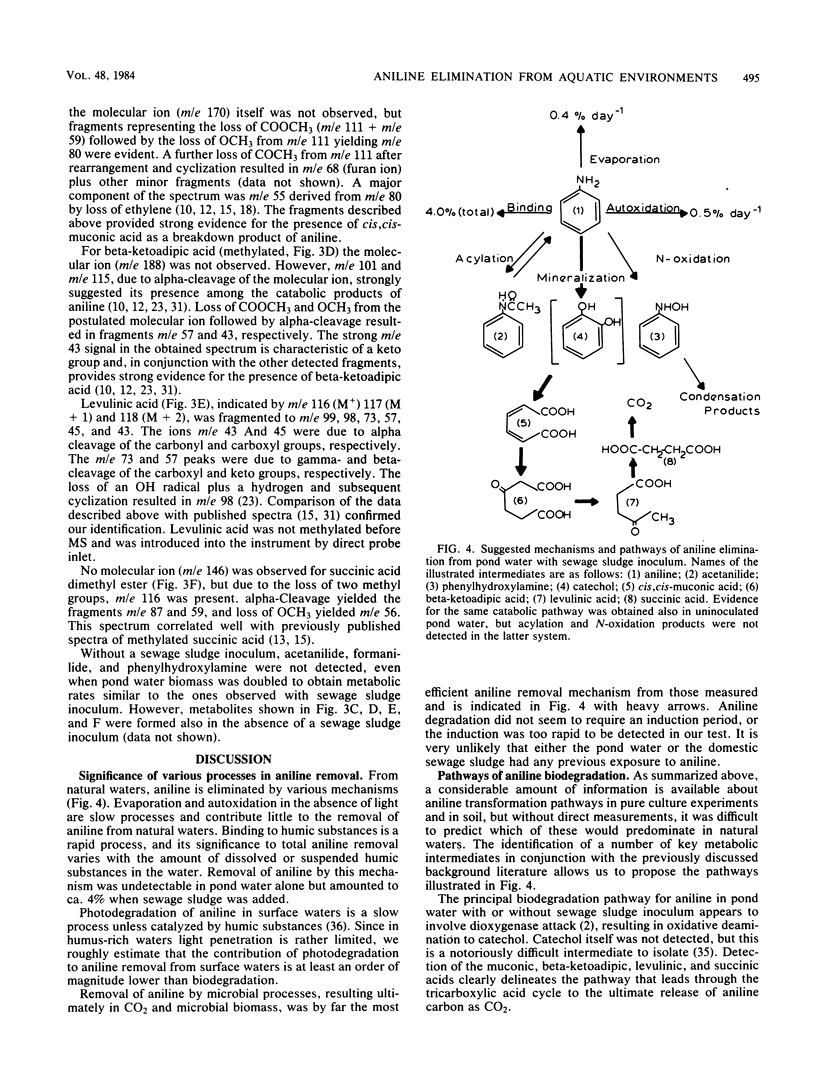
The fate of aniline, a representative of arylamine pollutants derived from the manufacture of dyes, coal liquefaction, and pesticide degradation, was comprehensively evaluated by use of unpolluted and polluted pond water as model environments. Evaporation plus autoxidation proved to be minor elimination mechanisms, removing ca. 1% of the added aniline per day. Instantaneous binding to humic components of a 0.1% sewage sludge inoculum removed 4%. Biodegradation of aniline in pond water was accelerated by the sewage sludge inoculum. A substantial portion of the degraded aniline carbon was mineralized to CO2 within a 1-week period, and microbial biomass was formed as a result of aniline utilization. Biodegradation was clearly the most significant removal mechanism of polluting aniline from pond water. A gas chromatographic-mass spectrometric analysis of biodegradation intermediates revealed that the major pathway of aniline biodegradation in pond water involved oxidative deamination to catechol, which was further metabolized through cis,cis-muconic, beta-ketoadipic, levulinic, and succinic acid intermediates to CO2. Minor biodegradation pathways involved reversible acylation to acetanilide and formanilide, whereas N-oxidation resulted in small amounts of oligomeric condensation products.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachofer R., Lingens F., Schäfer W. Conversion of aniline into pyrocatechol by a Nocardia sp.; incorporation of oxygen-18. FEBS Lett. 1975 Feb 1;50(2):288–290. doi: 10.1016/0014-5793(75)80510-2. [DOI] [PubMed] [Google Scholar]
- Bordeleau L. M., Rosen J. D., Bartha R. Herbicide-derived chloroazobenzene residues: pathway of formation. J Agric Food Chem. 1972 May-Jun;20(3):573–578. doi: 10.1021/jf60181a001. [DOI] [PubMed] [Google Scholar]
- Duxbury J. M., Tiedje J. M., Alexander M., Dawson J. E. 2,4-D metabolism: enzymatic conversion of chloromaleylacetic acid to succinic acid. J Agric Food Chem. 1970 Mar-Apr;18(2):199–201. doi: 10.1021/jf60168a029. [DOI] [PubMed] [Google Scholar]
- Gledhill W. E. Screening test for assessment of ultimate biodegradability: linear alkylbenzene sulfonates. Appl Microbiol. 1975 Dec;30(6):922–929. doi: 10.1128/am.30.6.922-929.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. D., Plimmer J. R., Klingebiel U. I. Microbial oxidation of 4-chloroaniline. J Agric Food Chem. 1973 Jan-Feb;21(1):127–132. doi: 10.1021/jf60185a028. [DOI] [PubMed] [Google Scholar]
- Kearney P. C., Plimmer J. R. Metabolism of 3,4-dichloroaniline in soils. J Agric Food Chem. 1972 May-Jun;20(3):584–585. doi: 10.1021/jf60181a008. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Minard R. D., Russel S., Bollag J. M. Chemical transformation of 4-chloroaniline to a triazene in a bacterial culture medium. J Agric Food Chem. 1977 Jul-Aug;25(4):841–844. doi: 10.1021/jf60212a011. [DOI] [PubMed] [Google Scholar]
- Plimmer J. R., Kearney P. C., Chisaka H., Yount J. B., Klingebiel U. I. 1,3-bis(3,4-dichlorophenyl) triazene from propanil in soils. J Agric Food Chem. 1970 Sep-Oct;18(5):859–861. doi: 10.1021/jf60171a033. [DOI] [PubMed] [Google Scholar]
- Rosen J. D., Siewierski M., Winnett G. FMN-sensitized photolyses of chloroanilines. J Agric Food Chem. 1970 May-Jun;18(3):494–496. doi: 10.1021/jf60169a019. [DOI] [PubMed] [Google Scholar]
- Rubin I. B., Guerin M. R. Fractionation of synthetic crude oils from coal for biological testing. Environ Res. 1976 Dec;12(3):358–365. doi: 10.1016/0013-9351(76)90046-3. [DOI] [PubMed] [Google Scholar]
- Russel S., Bollag J. M. Formylation and acetylation of 4-chloroaniline by a Streptomyces sp. Acta Microbiol Pol. 1977;26(1):59–64. [PubMed] [Google Scholar]
- Tweedy B. G., Loeppky C., Ross J. A. Metobromuron: acetylation of the aniline moiety as a detoxification mechanism. Science. 1970 Apr 24;168(3930):482–483. doi: 10.1126/science.168.3930.482. [DOI] [PubMed] [Google Scholar]


