Abstract
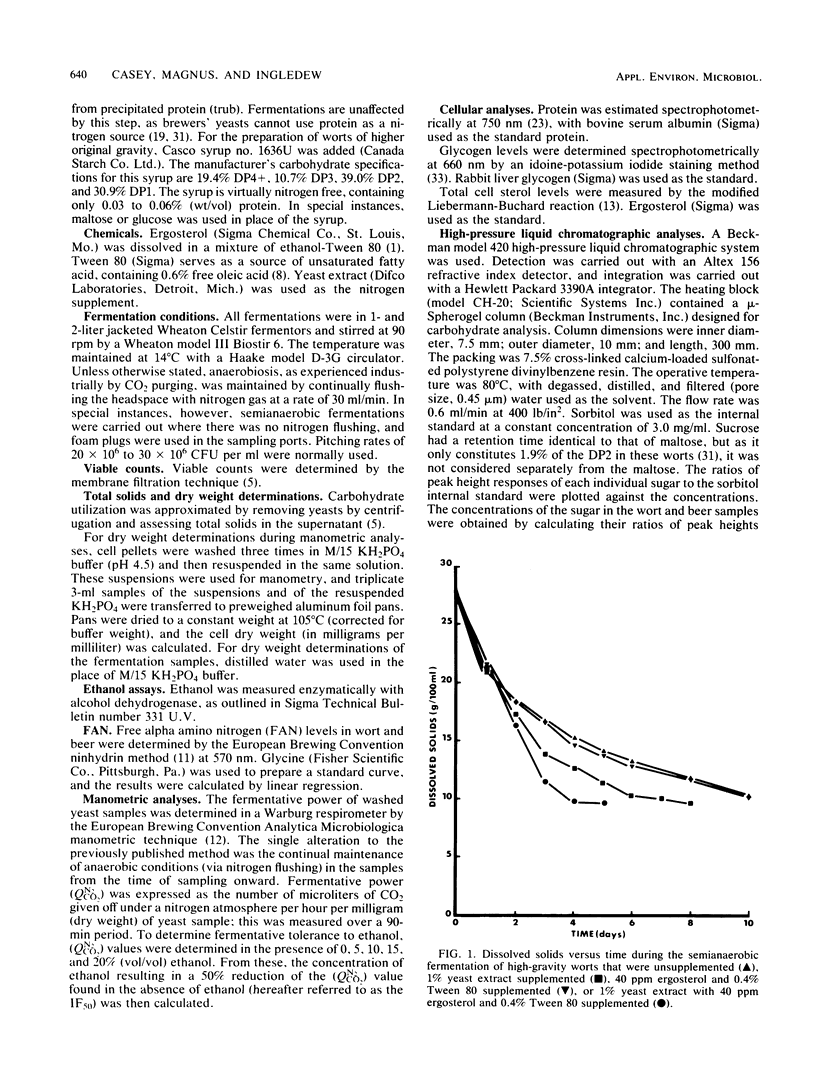
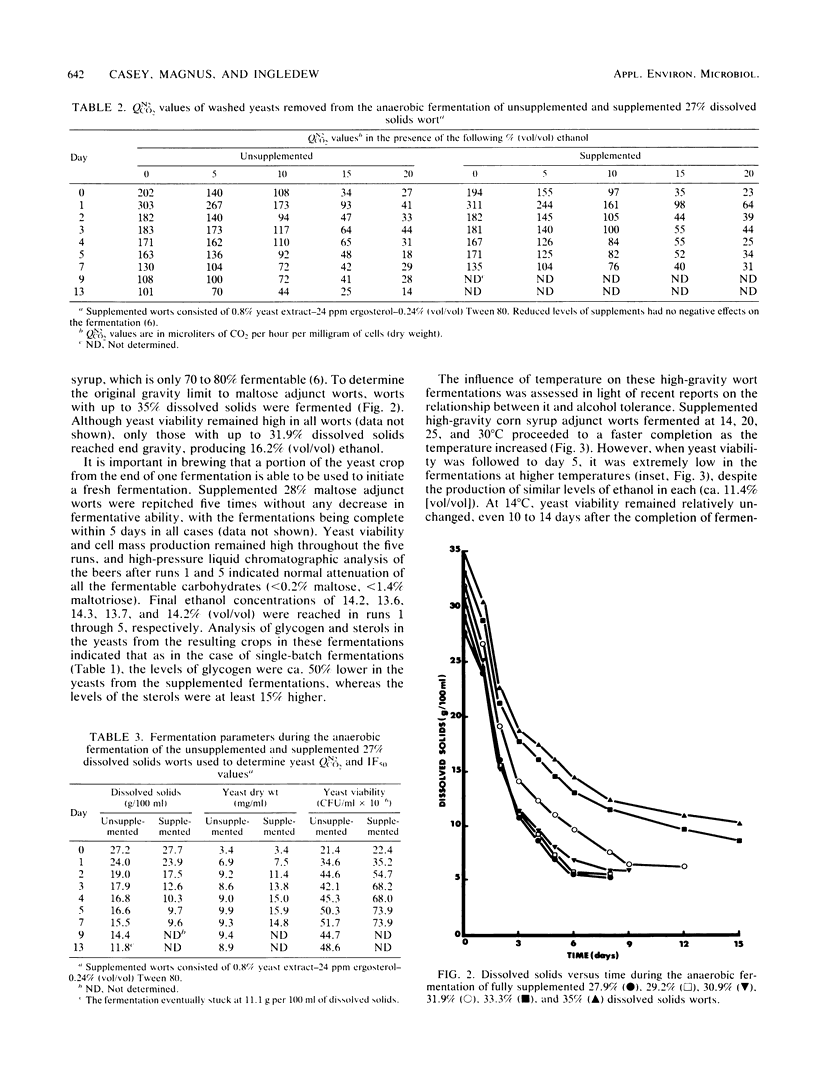
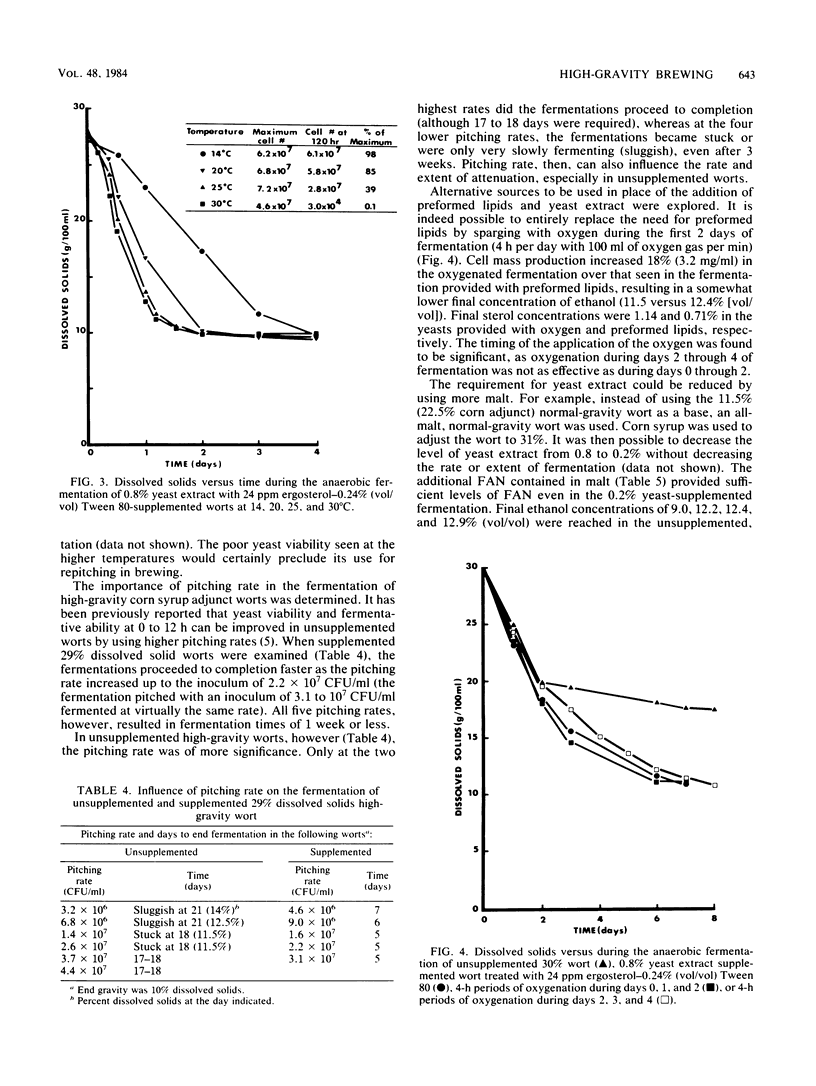
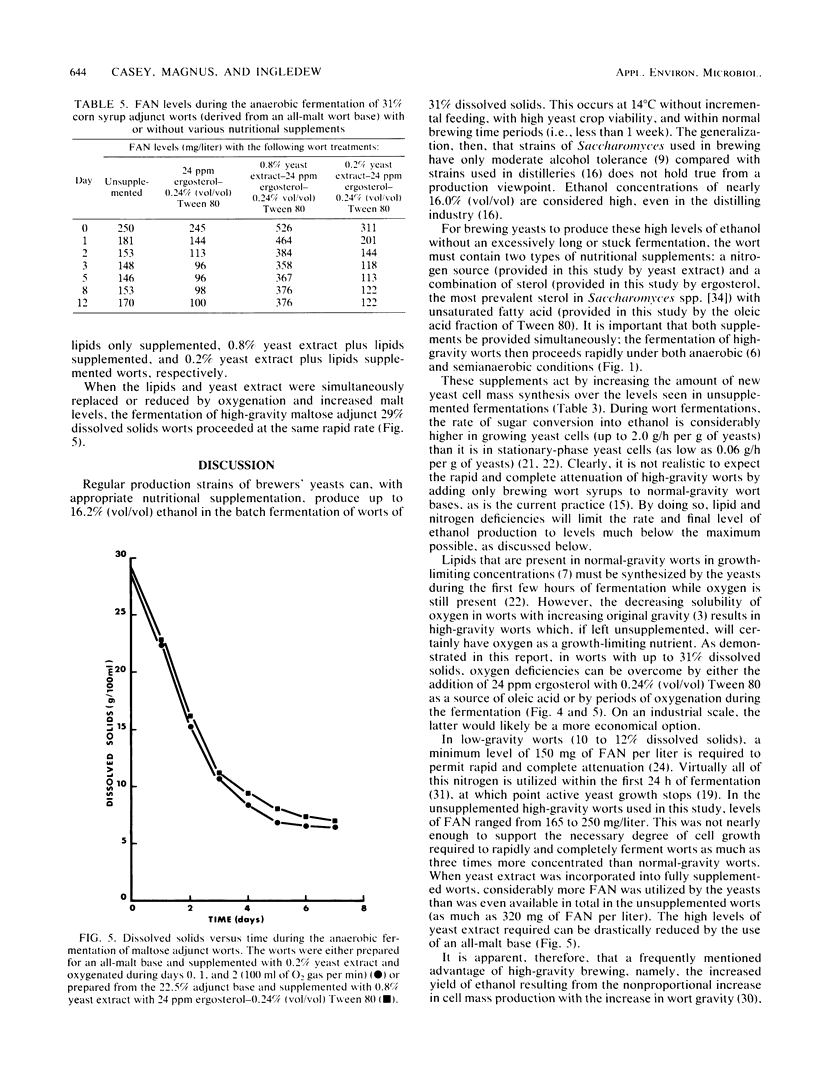
A number of economic and product quality advantages exist in brewing when high-gravity worts of 16 to 18% dissolved solids are fermented. Above this level, production problems such as slow or stuck fermentations and poor yeast viability occur. Ethanol toxicity has been cited as the main cause, as brewers' yeasts are reported to tolerate only 7 to 9% (vol/vol) ethanol. The inhibitory effect of high osmotic pressure has also been implicated. In this report, it is demonstrated that the factor limiting the production of high levels of ethanol by brewing yeasts is actually a nutritional deficiency. When a nitrogen source, ergosterol, and oleic acid are added to worts up to 31% dissolved solids, it is possible to produce beers up to 16.2% (vol/vol) ethanol. Yeast viability remains high, and the yeasts can be repitched at least five times. Supplementation does not increase the fermentative tolerance of the yeasts to ethanol but increases the length and level of new yeast cell mass synthesis over that seen in unsupplemented wort (and therefore the period of more rapid wort attenuation). Glycogen, protein, and sterol levels in yeasts were examined, as was the importance of pitching rate, temperature, and degree of anaerobiosis. The ethanol tolerance of brewers' yeast is suggested to be no different than that of sake or distillers' yeast.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Gray W. D. Studies on the Alcohol Tolerance of Yeasts. J Bacteriol. 1941 Nov;42(5):561–574. doi: 10.1128/jb.42.5.561-574.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagodawithana T. W., Castellano C., Steinkraus K. H. Effect of dissolved oxygen, temperature, initial cell count, and sugar concentration on the viability of Saccharomyces cerevisiae in rapid fermentations. Appl Microbiol. 1974 Sep;28(3):383–391. doi: 10.1128/am.28.3.383-391.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro J. M., Finck J. D. Evolution de l'activite 'hexokinase' de Saccharomyces uvarum fermentant le saccharose. Cell Mol Biol. 1982;28(1):85–89. [PubMed] [Google Scholar]
- Patel G. B., Ingledew W. M. Trends in wort carbohydrate utilization. Appl Microbiol. 1973 Sep;26(3):349–353. doi: 10.1128/am.26.3.349-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray J. B., Schibeci A., Kidby D. K. Lipids of yeasts. Bacteriol Rev. 1975 Sep;39(3):197–231. doi: 10.1128/br.39.3.197-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uden N., da Cruz Duarte H. Effects of ethanol on the temperature profile of Saccharomyces cerevisiae. Z Allg Mikrobiol. 1981;21(10):743–750. [PubMed] [Google Scholar]


