Abstract
Previously, it was shown that cationic α-peptides derived from the human immunodeficiency virus TAT protein transduction domain blocked herpes simplex virus type 1 (HSV-1) entry. We now show that cationic oligomers of β-amino acids (“β-peptides”) inhibit HSV-1 infection. Among three cationic β-peptides tested, the most effective inhibition was observed for the one with a strong propensity to adopt a helical conformation in which cationic and hydrophobic residues are segregated from one another (“globally amphiphilic helix”). The antiviral effect was not cell type specific. Inhibition of virus infection by the β-peptides occurred at the postattachment penetration step, with a 50% effective concentration of 3 μM for the most-effective β-peptide. The β-peptides did not inactivate virions in solution, nor did they induce resistance to infection when cells were pretreated with the β-peptides. The β-peptides showed little if any toxicity toward Vero cells. These results raise the possibility that cationic β-peptides may be useful antiviral agents for HSV-1 and demonstrate the potential of β-peptides as novel antiviral drugs.
Herpes simplex virus type 1 (HSV-1) is a significant human pathogen causing mucocutaneous lesions primarily in the oral mucosa (cold sores), as well as other sites. More-severe diseases caused by HSV-1 infection include encephalitis, meningitis, and blinding keratitis (65), and HSV-1 is the leading cause of blindness due to infection in developed countries (5). Following an initial infection, HSV-1 establishes latent infection of neurons in sensory ganglia of the host (29), from where it periodically reactivates and causes recurrent lesions at the site of primary infection. To date, none of the currently approved antivirals can eliminate an established latent infection. Because of the difficulties dealing with latency, preventing HSV-1 from entering the cell is an attractive antiviral strategy.
HSV-1 entry is a complex process, involving multiple components on both the cell plasma membrane and the viral envelope. The initial interaction involves the binding of viral glycoprotein C (gC) or gB to cell surface heparan sulfate proteoglycan (58). Four viral glycoproteins, gB, gD, and the gH-gL heterodimer, are essential for the subsequent membrane fusion and entry steps (56, 57). Following attachment, gD interacts with any of three cellular receptors: herpes virus entry mediator, nectin-1, and 3-O-sulfated heparan sulfate, leading to a conformational change in gD (12, 13, 21, 22, 34, 39, 54, 62). This conformational change in gD is believed to trigger the formation of the fusion complex, which is thought to involve the sequential binding of gB to gD, followed by the binding of gH-gL to the gB-gD complex (10, 20, 23, 24, 36, 41, 50, 62).
Peptides that interrupt protein-protein interactions in viral infection have potential as antiviral drugs and may also be useful tools for elucidating the mechanisms underlying the entry process. For instance, the anti-human immunodeficiency virus (HIV) drug Fuzeon acts by blocking HIV entry (66). In another example, the enzymatic activity of HSV ribonucleotide reductase 1 (RR1)-RR2 complex can be inhibited by a peptide corresponding to the 9 carboxyl-terminal amino acids of the small-subunit RR2, which acts by disrupting the RR1-RR2 holoenzyme (14, 16, 17, 61). A peptidomimetic compound resembling the 6 carboxy-terminal amino acids of the RR2 subunit was shown to reduce the severity of HSV-1 keratitis and inhibit replication in vivo (6), supporting the potential use of peptides or peptidomimetic drugs for HSV-1 infection.
Previous work in our laboratory has shown that peptides containing cell-penetrating motifs are potent antivirals (7, 8). One of these cell-penetrating peptides, the cationic peptide GRKKRRQRRR derived from the HIV tat protein, was shown to inhibit HSV-1 infection at the entry step in cell culture with a 50% effective concentration (EC50) of 1 μM (7). Recently, we showed that the antiviral activity of TAT peptide could be improved by adding a single cysteine residue to the C terminus of the TAT peptide (TAT-C) (9). In addition to inhibiting entry, the added cysteine gave the peptide the capacity to inactivate virions in solution and induce a state of resistance to infection in cells pretreated with the peptides. The peptide composed entirely of d-amino acids (TAT-Cd) was equally as effective as the l-amino acid version, indicating that chirality is not critical (9). The TAT-C peptide has previously been shown to have low toxicity both in vitro and in vivo (1).
Antimicrobial peptides and proteins are often highly cationic and amphiphilic (33). Examples of these peptides include rabbit α-defensin NP-1, a synthetic θ-defensin (retrocyclin 2), magainins, lactoferricin, and dermaseptins. These peptides have been shown to block HSV infection at the attachment and/or entry step (2, 4, 18, 33, 55, 67). Lactoferricin binds to heparan sulfate, thereby blocking viral attachment (38). Retrocyclin 2 acts as a “mini-lectin” and interacts with the carbohydrate side chains on viral glycoproteins (28, 35, 67).
β-Peptides are oligomers of β-amino acids; these unnatural “foldamers” are capable of forming several distinct secondary structures, depending upon residue substitution patterns (11). In contrast to conventional α-peptides, β-peptides are resistant to proteolytic degradation (53). In addition, properly designed β-peptides are more conformationally stable in aqueous solution, especially at relatively short lengths, than are α-peptides (3, 48, 64). Therefore, β-peptides that mimic the structure and function of biologically active α-peptides can provide unique tools for elucidating structure-function relationships, and such β-peptides might ultimately be useful as therapeutic agents.
Helical β-peptides have been shown to mimic the antimicrobial activities of host-defense peptides (37, 43, 44, 49). This activity required the design of sequences that lead to global segregation of cationic and hydrophobic residues on opposite sides of the helix. More recently, β-peptides bearing guanidinium groups in their side chains have been shown to mimic the cell penetration behavior of arginine-rich α-peptides (45-47). These findings raise the possibility that β-peptides could be engineered to display antiviral activity against HSV-1. In the present study, we report that three cationic β-peptides, peptides 1 to 3 (Fig. 1), inhibit HSV-1 infection. The most effective β-peptide, peptide 1, blocked infection at the postattachment entry step in cell culture (EC50 = 3 μM) but did not inactivate virions in solution, induce cell resistance to infection, or inhibit viral attachment to the cell receptor. These data suggest the potential use of cationic β-peptides as antiviral agents.
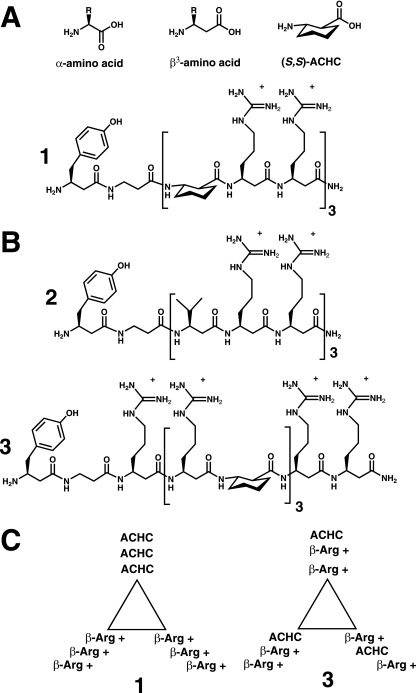
FIG. 1.
Structures of the β-peptides. (A) Generic structures of α- and β3-amino acids. The structure of ACHC is also shown. (B) Structures of β-peptides 1 to 3. (C) Schematic representation of the positions of the β-amino acid side chains of sequence-isomeric β-peptides 1 and 3 in the 14-helical conformation, viewed along the helical axis, showing the differential display of guanidinium residues.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney cells (Vero) and primary human corneal fibroblasts (keratocytes, HK320) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with a 10% serum (a 1:1 [vol/vol] mixture of fetal bovine serum and calf serum), 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate at 37°C in an atmosphere of 5% CO2. The isolation procedures for the primary keratocytes were described previously (42). Cells infected with virus were grown in DMEM supplemented with 2% serum. To prepare high-titer stocks of HSV-1 KOS virus, Vero cells were infected at a low multiplicity of infection (MOI = 0.01). When the cytopathic effect was 90 to 100%, the infected monolayers were harvested and subjected to three freeze-thaw cycles using a dry ice-ethanol bath, centrifuged at 1,000 × g for 5 min to remove debris, and stored at −80°C. Stocks of the HSV-1 KOS mutant hrR3, which expresses Escherichia coli β-galactosidase from the early ICP6 promoter (25), were purified on sucrose gradients as described previously (26). Viral titers were determined by plaque assay on Vero cells. To prepare Vero cells for all in vitro experiments described below, Vero cells were seeded in a 96-well plate (1 × 104 cells per well) and incubated for 4 days at 37°C. To prepare HK320 cells for a comprehensive assay, HK320 cells were seeded in a 96-well plate (5 × 104 cells per well) and incubated overnight at 37°C.
β-Peptide synthesis.
Synthesis of the 9-fluorenylmethoxy carbonyl (Fmoc) β-amino acids was described elsewhere (27). Briefly, Fmoc-protected β-amino acids were synthesized via Arndt-Eistert homologation (27) using the modified procedure described by Muller et al. (40). The synthesis of trans-2-(S,S)-aminocyclohexanecarboxylic acid (ACHC) was as described by Schinnerl et al. (51). The β-peptides were synthesized by manual Fmoc solid-phase synthesis on Novasyn TGR resin and purified by preparative reverse-phase high-pressure liquid chromatography. The desired β-amino acid composition was confirmed by matrix-assisted laser desorption ionization-time of flight analysis. β-Peptide concentrations were determined by calculating absorbance at 275 nm (46).
Comprehensive antiviral assay.
Both the hrR3 virus (1 × 106 PFU/ml) and Vero or HK320 cells were incubated with various concentrations of the β-peptides at 37°C for 1 h. The β-peptide-containing medium was removed from the cells, and the β-peptide-treated virus (MOI, 0.1) was added. After incubation at 37°C for 6 h, the cells were lysed with 0.5% NP-40 in phosphate-buffered saline (PBS) containing 2 mM MgCl2 and 5 mM chlorophenol red-β-galactopyranoside (Roche Diagnostics, Indianapolis, IN). The initial rate of β-d-galactosidase activity was measured with a 96-well plate reader (Biotek Instruments, Inc., Winooski, VT) at a wavelength of 570 nm. Background activity was measured in mock-infected cultures and was subtracted from the data. Each data point represents the mean, with standard deviation, of results for triplicate samples.
Inactivation of virions in solution.
Recombinant hrR3 virus (1 × 108 PFU/ml) was incubated with various concentrations of the β-peptides for 1 h at 37°C. Then, the sample was diluted 1,000-fold into DMEM supplemented with β-peptide-free serum, and the remaining infectious virus in Vero cell cultures was quantified by measuring the β-d-galactosidase activity as described above. The bKLA (biotinylated KLA) peptide (KLALKLALKALKAALKLA), which we previously showed was toxic to cells in culture and inactivates virus in solution (1, 8), was used as the positive control.
Assay to induce cell resistance.
Vero cells were incubated with various concentrations of the β-peptides for 1 h at 37°C. Following incubation, the β-peptides were removed and the cells were washed with DMEM supplemented with β-peptide-free serum. The β-peptide-treated cells were then infected with hrR3 virus (MOI, ∼0.1) at 37°C. After 1 h, the virus was replaced with serum-supplemented DMEM, the infected cells were incubated at 37°C for 6 h, and β-d-galactosidase activity was measured.
Entry assay.
Recombinant hrR3 virus was adsorbed for 1 h at 4°C to precooled Vero cells (MOI, ∼0.2). Various concentrations of β-peptides were then added, and the cultures were incubated for additional 15 min at 4°C. The cultures were then shifted to 37°C to initiate entry, and after 1 h, the wells were rinsed with PBS, treated for 1 min with low-pH citrate buffer (pH 3.0) to inactivate any remaining extracellular virus, and rinsed with PBS again. The cultures were then returned to serum-supplemented DMEM, incubated at 37°C, and assayed for β-d-galactosidase activity 6 h later, as described above.
Postentry assay.
Vero cells were infected with hrR3 virus (MOI, ∼0.05) at 37°C. After 1 h, the wells were rinsed with PBS, treated for 1 min with low-pH citrate buffer (pH 3.0) to inactivate any remaining extracellular virus, and rinsed with PBS again. The cultures were then incubated at 37°C in serum-supplemented DMEM in the presence and absence of various concentrations of β-peptides and assayed for β-d-galactosidase activity 6 h later.
Attachment assay (cellular enzyme-linked immunosorbent assay).
Vero cells were precooled at 4°C for 30 min. Recombinant virus (5 × 107 PFU/ml) was mixed with various concentrations of β-peptides, and then the virus-β-peptide suspensions were added to cells at 4°C for 1 h. The cultures were rinsed with serum-free, carbonate-free DMEM (S−) once and fixed with 4% paraformaldehyde in S− for 1 h at room temperature. The cultures were washed three times with S− and blocked with 3% bovine serum albumin (BSA) in S− at room temperature. After the cultures were blocked for 1 h, polyclonal rabbit anti-HSV type 1 antibody (1:100 dilution in 0.05% BSA in S−; Dako North America, Inc., Carpinteria, CA) was added. After incubating for 1 h at room temperature, the cultures were rinsed three times for 5 min each with S−. The secondary antibody, goat anti-rabbit immunoglobulin G (IgG) conjugated with alkaline phosphatase (1:2,000 dilution in 0.05% BSA in S−; Sigma-Aldrich, St. Louis, MO) was then added. After 1 h at room temperature, the cultures were rinsed three times with S− for 5 min each, and the amount of adsorbed virus was measured by reading initial rates of change at 405 nm with a 96-well plate reader (Biotek Instruments, Inc., Winooski, VT) after substrate (p-nitrophenylphosphate; catalog no. P4744; Sigma-Aldrich, St. Louis, MO) addition.
Dye reduction assay.
β-Peptide cytotoxicity was determined using a commercially available assay (Celltiter 96 AQqueous One Solution cell proliferation assay reagent [Promega, Madison, WI]) as described previously (1). Briefly, Vero cells (1.5 × 104 cells/well) were seeded in a 96-well plate and incubated for 24 h at 37°C. Following incubation, 20 μl of medium containing the desired concentration (twofold dilution) of β-peptides was added to the cells. Control cells received medium only. The bKLA peptide (see above) was used as a positive control for toxicity. After incubating the cells in the presence of β-peptide overnight at 37°C, 20 μl of the 96 AQqueous One Solution cell proliferation assay reagent was added to each well. The plate was then incubated for 2 h at 37°C, and the absorbance at 490 nm was determined using a 96-well plate reader (Biotek Instruments, Inc., Winooski, VT).
RESULTS
β-Peptide design.
Figure 1 shows the three β-peptides used in this study; each molecule contains six β3-homoarginine (β3hArg) residues. These β-peptides were designed to adopt a 14-helix conformation, i.e., a conformation defined by 14-membered-ring hydrogen bonds between backbone amide groups [N-H(i)—O = C(i + 2)] (11). The 14-helix contains approximately three residues per helical turn. β-Peptides 1 and 2 contain repeating triads (X-β3hArg-β3hArg), where X is ACHC residues for β-peptide 1 and β3-homovaline residues for β-peptide 2. β-Peptide 3 is a sequence isomer of β-peptide 1. The ACHC residues in peptides 1 and 3 constrain the β-peptide backbone in a way that strongly promotes 14-helix formation; both peptide 1 and peptide 3 display 14-helical folding in aqueous solution, but peptide 2, lacking ACHC residues, does not (46). In the 14-helical conformation of peptide 1, the cationic guanidinium side chains (β3hArg) are clustered along one side (globally amphiphilic helix), as illustrated in the “helical wheel” diagram in Fig. 1C. In sequence isomer 3, the guanidinium side chains are dispersed around the entire periphery of the helix. Previously, we showed that variations in the geometry of the guanidinium group display affect the ability of the β-peptide to enter cells (46): β-peptide 1, which forms the globally amphiphilic helix, enters HeLa cells efficiently, but sequence isomer 3 does not. The behavior of peptide 2 showed that the stability of the helical conformation is important for cell entry. β-Peptide 2 can form a globally amphiphilic 14-helix, but this molecule, lacking ACHC residues, does not adopt the 14-helical conformation in aqueous solution, and peptide 2 does not enter HeLa cells efficiently (46).
β-Peptides are not toxic to Vero cells.
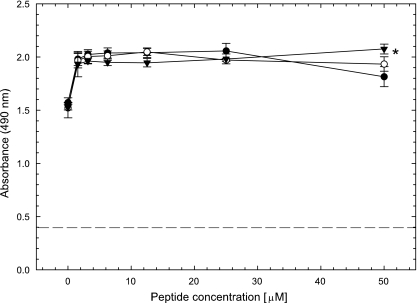
Prior to assessing the antiviral activities of the β-peptides, we asked whether the β-peptides were cytotoxic. Vero cells were incubated with various concentrations of the β-peptides for 24 h at 37°C, and cell viability was determined using a dye reduction assay. The bKLA peptide (5 μM) was used as a positive control, as this 18-mer α-peptide, which forms a globally amphiphilic α-helix, was previously shown to be toxic to Vero cells (1). Indeed, this α-peptide significantly reduced cell viability (Fig. 2). In contrast, none of the three β-peptides appeared to be toxic, as cell viability was not reduced at the maximum concentration tested (EC50 > 50 μM). It is possible that the β-peptides induce cell proliferation, since the number of viable cells increased about 33% in the presence of β-peptides compared to that in the no-peptide control, and this increase was significantly different (t test, P < 0.05).
FIG. 2.
β-Peptides are not cytotoxic to Vero cells. Confluent Vero cells in a 96-well plate were treated with various concentrations of the β-peptides overnight. The next day, 20 μl of Celltiter 96 AQqueous One Solution cell proliferation assay reagent (Promega, Madison, WI) was added to each well, and 2 h later, the absorbance at 490 nm was read in a 96-well plate reader. Each point and error bar represent the mean ± standard deviation of results for triplicate samples. The β-peptide concentration denoted 0 is the no-peptide control. The dashed line indicates the toxicity of a positive-control bKLA peptide (5 μM). The asterisk denotes that the peptides resulted in increased cell proliferation over that of the no-peptide control (P < 0.05). •, peptide 1; ○, peptide 2; ▾, peptide 3.
β-Peptides block HSV-1 infection.
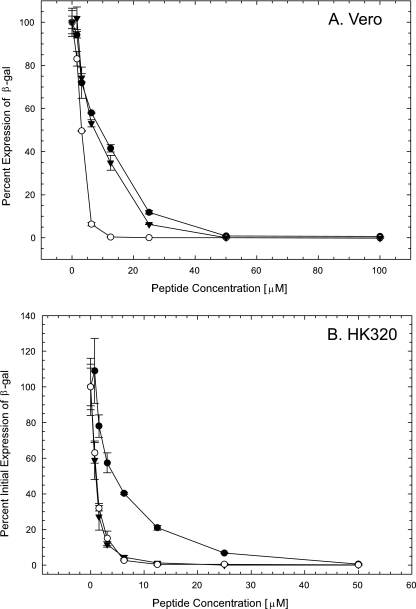
To determine whether the β-peptides were active against HSV-1 infection, a comprehensive assay was initially conducted. In this assay format, the β-peptide was present before, during, and after infection. To measure infectivity, we took advantage of the HSV-1 KOS mutant hrR3, which expresses a reporter β-galactosidase under the early ICP6 promoter. The expression of β-galactosidase protein indicates that the virus has entered the cell and released its genome to the nucleus, where transcription is activated. As shown in Fig. 3A, all three β-peptides inhibited viral infection at low micromolar concentrations. The EC50 for β-peptides 1, 2, and 3 were 3, 10, and 7 μM, respectively.
FIG. 3.
β-Peptides inhibit HSV-1 infection in a comprehensive assay. (A) Vero cells. (B) HK320 cells. Confluent cells in a 96-well plate and recombinant hrR3 KOS virus were incubated with various concentrations of the β-peptides for 1 h at 37°C prior to infection. Treated cells were then infected with treated viruses and incubated at 37°C for 6 h. Infected cells were lysed, chlorophenol red-β-galactopyranoside was added, and the kinetics of β-galactosidase (β-gal) activity, as a measure of infectivity, was then determined. The activity observed in cells that had not been exposed to a β-peptide was set to 100% infectivity. Each data point and error bar represent the mean ± standard deviation of results for triplicate samples. ○, peptide 1; •, peptide 2; ▾, peptide 3.
To demonstrate that the antiviral effect of the β-peptides on HSV-1 infection was not specific to a continuous cell line, the comprehensive assay was conducted using primary human keratocytes (HK320 cells). As shown in Fig. 3B, all three β-peptides effectively inhibited viral infection in a dose-dependent manner. The EC50 values for β-peptides 1, 2, and 3 were 1, 5, and 1 μM, respectively, in HK320 cells. These results suggest that the antiviral effects of the β-peptides are not dependent on cell type.
β-Peptides do not inactivate virions in solution.
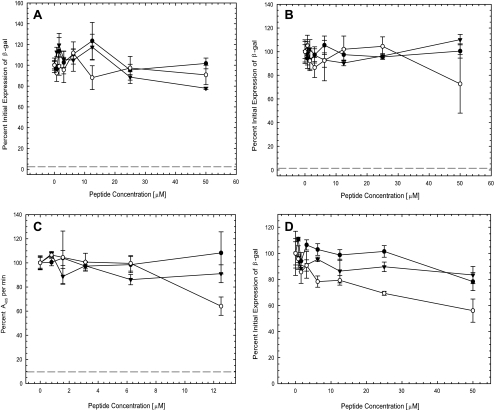
Having shown that the β-peptides inhibited HSV-1 infection, we sought to identify the step in infection that was blocked by the β-peptides. To determine if the β-peptides were virucidal, hrR3 virus was incubated with various concentrations of the β-peptides for 1 h at 37°C. After 1 h, the virus-β-peptide mixtures were diluted 1,000-fold, in order to reduce the β-peptide concentration to below the inhibitory concentration, and titers were determined on Vero cells. The controls included mock-infected cells, viruses only, and viruses that were treated with a 30 μM concentration of the bKLA peptide, which inactivates HSV-1 at this concentration (7). As shown in Fig. 4A, none of the β-peptides inhibited virus infection at concentrations as high as 50 μM, whereas infection was almost completely inhibited when viruses were treated with the bKLA peptide. Therefore, the inhibition of virus infection by β-peptides 1, 2, and 3 was not due to the irreversible inactivation of virions in solution.
FIG. 4.
Antiviral properties of β-peptides. To determine the step in infection blocked by the β-peptides, the time of introduction of β-peptides in four assays was varied as described in Materials and Methods. (A) Inactivation of virions in solution. Virus was incubated with various concentrations of the β-peptides at 37°C for 1 h. The β-peptide-virus mixtures were then diluted 1,000-fold, and the titer of infectious virus was determined. The bKLA peptide (30 μM) was included as a positive control for virus inactivation in solution (dashed line). Infectivity was determined after 6 h at 37°C by measuring β-galactosidase (β-gal) activity. (B) Induction of cellular resistance to infection. Vero cells were incubated with various concentrations of the β-peptides at 37°C for 1 h. β-Peptides were removed, and the cells were rinsed and infected with virus for 1 h at 37°C. The TAT-Cd peptide (100 μM) was included as a positive control for induction of resistance to infection (dashed line). Infectivity was determined after 6 h at 37°C by measuring β-galactosidase activity. (C) Viral attachment. Vero cells were cooled at 4°C for 30 min. The cultures were then incubated with virus in the presence of various concentrations of the β-peptides at 4°C for 1 h. The cultures were fixed with 4% paraformaldehyde and blocked with 3% BSA in medium for 1 h at room temperature. Primary and secondary antibodies were polyclonal rabbit anti-HSV-1 antibody (Dako North America, Inc.) and goat anti-rabbit IgG conjugated with alkaline phosphatase (Sigma-Aldrich), respectively. The kinetics of absorbance at 405 nm was recorded as a measure of viral attachment. Heparin (5 μM) was included as a positive control for inhibition of viral attachment (dashed line). (D) Postentry inhibition. Vero cells were infected with virus for 1 h at 37°C. The cultures were then rinsed, treated with low-pH citrate buffer (pH 3.0) to inactivate extracellular virus, rinsed again, and refed with medium containing various concentrations of the β-peptides. Infectivity was determined after 6 h at 37°C by measuring β-galactosidase activity. The negative control was a sample without β-peptide (set as 100%) in all assays. Each data point and error bar represent the mean ± standard deviation of results for triplicate samples. ○, peptide 1; •, peptide 2; ▾, peptide 3.
Pretreatment of cells with β-peptides does not inhibit infection.
Pretreatment with certain cationic peptides, such as TAT-Cd, can render cells resistant to infection with HSV-1 (9). To determine whether pretreatment with the β-peptides induced resistance to infection, cells were incubated with various concentrations of the β-peptides at 37°C for 1 h prior to virus infection. The cultures were then rinsed with β-peptide-free serum-supplement DMEM and infected. Mock-infected cells, infected cells that were not treated with any peptide, and cells treated with 100 μM TAT-Cd served as controls. As shown in Fig. 4B, cells exposed to TAT-Cd were resistant to infection, whereas none of the β-peptides induced resistance to infection up to a concentration of 50 μM. These results suggest that the inhibitory activity of the β-peptides was not due to an effect of the β-peptides on cells.
Inhibition of viral attachment to the cell surface is not a major activity of the antiviral β-peptides.
To determine whether the antiviral effect was due to the inhibition of viral attachment to the cell surface, a cellular enzyme-linked immunosorbent binding assay was performed. Viral attachment in the absence of β-peptides was set at 100%. Heparin (5 μM) served as a positive control and blocked virus binding to the cell surface by 90% (Fig. 4C). As shown in Fig. 4C, increasing concentrations of β-peptides 2 and 3 had no effect on attachment. However, β-peptide 1 reduced viral attachment to 65% at the highest concentration tested (12.5 μM), and this difference was significant (t test, P < 0.05) compared to values for the control or β-peptide 2 and 3 groups. Compared to the results from the other assays, these results suggest that the inhibition of virus attachment is not the major source of the antiviral effect exerted by the β-peptides.
Postentry effect of β-peptides.
To test whether the β-peptides had any effect on infection after virus had entered the cells, the β-peptides were added to cells that had been exposed to virus for an hour at 37°C. Figure 4D shows that both peptides 2 and 3 had little if any inhibitory effects when they were added after viral entry at the highest concentrations tested (50 μM). β-Peptide 1 had some inhibitory effect, yielding 45% inhibition at a concentration of 50 μM, and this difference with β-peptide 1 was significant (t test, P < 0.05) compared to the effects of β-peptides 2 and 3 and the control. These results suggest that antiviral activities employed by all three β-peptides are not primarily due to the effect of peptides after viral entry into the cell.
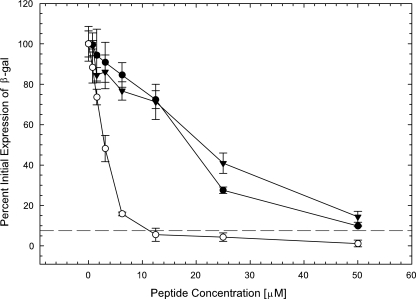
β-Peptides inhibit viral infection at the postattachment entry step.
To determine whether the β-peptides inhibited viral infection at a step between attachment and entry, virus was allowed to attach to cells at 4°C for 1 h. The cultures were then exposed to various concentrations of the β-peptides, and after an additional 15 min at 4°C, the cultures were shifted to 37°C to initiate virus entry. At 1 h following the temperature shift, any virus remaining outside the cells was inactivated by washing the cultures with low-pH citrate buffer, and the cultures were rinsed and returned to DMEM supplemented with β-peptide-free serum. The controls included mock-infected cells, infected cells that were not treated with any peptide, and cells that were treated with 10 μM TAT-Cd, a peptide that had previously been shown to inhibit virus entry (7). As shown in Fig. 5, all three β-peptides exhibited a dose-dependent postattachment inhibitory effect. The most effective β-peptide was 1, with an EC50 of 3 μM. This was significantly different from the EC50 values for β-peptides 2 and 3 (EC50s, ∼19 μM and 22 μM, respectively; P < 0.05). These results show that β-peptide 1 is a particularly effective entry inhibitor.
FIG. 5.
β-Peptides inhibit HSV-1 entry. Recombinant hrR3 virus was adsorbed to precooled confluent Vero cells in a 96-well plate at 4°C for 1 h. Unbound virus was removed by rinsing with medium, and various concentrations of the β-peptides were added to the cultures. After incubation at 4°C for 15 min, the cultures were shifted to 37°C for 1 h. The cultures were then rinsed, treated with low-pH citrate buffer (pH 3.0) to inactivate extracellular virus, rinsed again, and refed. After 6 h at 37°C, β-galactosidase (β-gal) activity was measured. The controls were samples without β-peptide (set as 100%) and samples in the presence of a 10 μM concentration of the TAT-Cd peptide (dashed line). Each data point and error bar represent the mean ± standard deviation of results for triplicate samples. ○, peptide 1; •, peptide 2; ▾, peptide 3.
DISCUSSION
β-Peptides that mimic the structures of naturally active peptides are gaining interest because they have two key advantages over natural α-peptides: resistance to proteolytic degradation (52) and an ability to form stable secondary structures at short lengths (11, 15). Many naturally occurring antimicrobial host defense peptides, such as magainins (69) and cecropins (59), are cationic and form globally amphiphilic α-helices (68). We previously reported that cationic β-peptides that form a globally amphiphilic helix display antimicrobial activity and that the amphiphilic helix is necessary for activity (44, 49). Several studies have demonstrated that cationic antimicrobial peptides have anti-HSV-1 activity and that the antiviral activity requires a globally amphiphilic helical fold (30-33). Therefore, we expected a cationic β-peptide that adopts a globally amphiphilic helix to be active against HSV-1. Our data show for the first time that amphiphilic cationic β-peptides are indeed effective against HSV-1.
The finding that peptide 1 was the most effective against HSV-1 among the three β-peptides that we evaluated suggests that 14-helical propensity and the geometry of guanidinium group display influences antiviral activity; however, neither a strong helical propensity nor a global segregation of guanidinium groups is absolutely required for activity. β-Peptides 1 and 2 were designed to have similar guanidinium group arrangements in a 14-helical conformation, but peptide 2 has a substantially lower folding propensity than peptide 1 (46). The modestly improved activity of peptide 1 relative to that of peptide 2 may arise because peptide 1 is preorganized in the necessary conformation prior to encountering its target. The modestly improved activity of peptide 1 relative to the activity of sequence isomer 3 suggests that a spatial clustering of guanidinium groups is favorable for antiviral activity. Perhaps this clustering facilitates the interaction of the oligo-cation with negatively charged sialic acid units on the virus. Recently, we have shown that sialic acid on HSV-1 envelope glycoproteins is required for efficient infection (60), raising the possibility that the binding of β-peptide 1 to sialic acid could inhibit the function of viral entry proteins.
Several pieces of evidence indicate that the cationic β-peptides examined in this study inhibit HSV-1 infection at the entry step following viral attachment to the target cell surface. The fact that the EC50 values observed for β-peptide 1 in the comprehensive assay and the entry assay were identical (3 μM) suggests that inhibition of entry accounted for most of the antiviral activity of peptide 1. The observation that pretreatment of cells with β-peptides does not confer resistance to infection suggests that any interactions of the β-peptide with cell surface molecules do not interfere with viral attachment and subsequent entry. It is also important to note that the β-peptides themselves did not interfere with β-galactosidase activity because β-galactosidase activity was not inhibited when cells were pretreated with the β-peptides, and these peptides can be taken up by cells (46). Our results showed that the β-peptides were not inhibitory when preincubated with virus prior to infection, which suggests that any interaction between the peptides and virus is reversible.
Although the data strongly support the conclusion that the principal effect of β-peptide 1 was to inhibit viral entry, we found that β-peptide 1 reduced β-galactosidase activity by about 45% when it was added postinfection but that β-peptides 2 and 3 had no effect. This is consistent with previous data showing that β-peptide 1 but not β-peptides 2 and 3 could enter cells (46). At the present time, the reason for this effect is not clear. It is not due to the inhibition of β-galactosidase activity by β-peptide 1, as the β-galactosidase activity was not inhibited when cells were pretreated with the β-peptide 1 (Fig. 4B). We used a low MOI in these studies, raising the possibility that the effect could be due to the inhibition of the reinfection of new cells. However, the infection was allowed to proceed for only 6 h, which is not sufficient for the initiation of a second round of infection. Further studies will be needed to determine if β-peptide 1 has antiviral effects inside the cell.
Preliminary studies with cationic TAT peptides indicate that they bind to sialic acids on HSV-1 glycoproteins, and when considered with our recent finding that virion sialic acids are required for infection (60), this observation raises the possibility that the cationic β-peptides inhibit HSV-1 entry through an interaction with sialic acid on one or more envelope glycoproteins. If so, then it might be expected that binding to virion sialic acid would inactivate the virus. However, previous work in one of our laboratories showed that the TAT peptide itself (GRKKRRQRRR) inhibited entry but was not virucidal and did not induce cellular resistance to infection (9). Virucidal and cell resistance activities of the TAT peptide required the addition of a C-terminal cysteine (TAT-C). Thus, it is not surprising that the β-peptides functioned only as entry inhibitors. Further studies will be needed to identify the target on the virus for the β-peptides.
The initial attachment of HSV-1 to cells occurs via binding to cell surface heparan sulfate (58). Cationic TAT peptides have been shown to bind heparan sulfate, and this is required for the cell penetration activity of TAT peptides (63, 70). The cationic β-peptides are also likely to bind to heparan sulfate, raising the question of why the cationic β-peptides do not block attachment. In our studies with TAT peptides, we found that attachment was not inhibited to a significant extent, suggesting that TAT peptides and HSV-1 recognize different structural features in heparan sulfate (9). Thus, it is possible that the cationic β-peptides act similarly to TAT and therefore do not inhibit attachment of the virus to cells.
One of our laboratories recently reported that specific β-peptides can block the infection of cells in culture by human cytomegalovirus (19). In that case, however, activity was sensitive to the β-peptide sequence, which contrasts with the observations reported here for β-peptides 1 to 3. We believe that the mode of anti-human cytomegalovirus action involves the disruption of a specific protein-protein interaction involved in the viral entry process.
In summary, we report for the first time that amphiphilic, cationic β-peptides inhibit HSV-1 infection at low micromolar concentrations in cell culture. The inhibition by these β-peptides was not due to the inactivation of virions in solution, induction of cell resistance to infection, blocking of viral attachment, or toxicity to the cells themselves. Rather, the β-peptides appear to inhibit HSV-1 infection at a step between attachment and entry. These β-peptides may be useful as tools for extending our understanding of the processes of viral entry into cells and offer a new, potentially exciting, direction in antiviral drug development.
Acknowledgments
We thank Sharon Altmann, Hermann Bultmann, Suping Cai, Gilbert Jose, Aaron Kolb, and Jeremy Teuton for helpful comments on the manuscript; Elizabeth Froelich for administrative assistance; and Justin Murray for technical assistance.
This work was supported by grants AI52089, EY07336, and EY016665 to C.R.B.; by grant GM56414 to S.H.G.; and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, University of Wisconsin. E.P.E. was supported in part by an NDSEG Fellowship (DoD) and training grant T32GM008505.
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Akkarawongsa, R., A. E. Cullinan, A. Zinkel, J. Clarin, and C. R. Brandt. 2006. Corneal toxicity of cell-penetrating peptides that inhibit herpes simplex virus entry. J. Ocul. Pharmacol. Ther. 22:279-289. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, J., H. Jenssen, K. Sandvik, and T. J. Gutteberg. 2004. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 74:262-271. [DOI] [PubMed] [Google Scholar]
- 3.Appella, D. H., J. J. Barchi, S. R. Durell, and S. H. Gellman. 1999. Formation of short, stable helices in aqueous solution by beta-amino acid hexamer. J. Am. Chem. Soc. 121:2309-2310. [Google Scholar]
- 4.Belaid, A., M. Aouni, R. Khelifa, A. Trabelsi, M. Jemmali, and K. Hani. 2002. In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J. Med. Virol. 66:229-234. [DOI] [PubMed] [Google Scholar]
- 5.Binder, P. S. 1977. Herpes simplex keratitis. Surv. Ophthalmol. 21:313-330. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, C. R., B. Spencer, P. Imesch, M. Garneau, and R. Deziel. 1996. Evaluation of a peptidomimetic ribonucleotide reductase inhibitor with a murine model of herpes simplex virus type 1 ocular disease. Antimicrob. Agents Chemother. 40:1078-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bultmann, H., and C. R. Brandt. 2002. Peptides containing membrane-transiting motifs inhibit virus entry. J. Biol. Chem. 277:36018-36023. [DOI] [PubMed] [Google Scholar]
- 8.Bultmann, H., J. S. Busse, and C. R. Brandt. 2001. Modified FGF4 signal peptide inhibits entry of herpes simplex virus type 1. J. Virol. 75:2634-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bultmann, H., J. Teuton, and C. R. Brandt. 2007. Addition of a C-terminal cysteine improves the anti-herpes simplex virus activity of a peptide containing the human immunodeficiency virus type 1 TAT protein transduction domain. Antimicrob. Agents Chemother. 51:1596-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, R. P., S. H. Gellman, and W. F. DeGrado. 2001. β-Peptides: from structure to function. Chem. Rev. 101:3219-3232. [DOI] [PubMed] [Google Scholar]
- 12.Cocchi, F., D. Fusco, L. Menotti, T. Gianni, R. J. Eisenberg, G. H. Cohen, and G. Campadelli-Fiume. 2004. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 101:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, E. A., P. Gandreau, P. Brazeau, and Y. Langelier. 1986. Specific inhibition of herpesvirus ribonucleotide reductase by a nonapeptide derived from the carboxy terminus of subunit 2. Nature 321:441-443. [DOI] [PubMed] [Google Scholar]
- 15.DeGrado, W. F., J. P. Schneider, and Y. Hamuro. 1999. The twists and turns of beta-peptides. J. Pept. Res. 54:206-217. [DOI] [PubMed] [Google Scholar]
- 16.Digard, P., K. P. Williams, P. Hensley, I. S. Brooks, C. E. Dahl, and D. M. Coen. 1995. Specific inhibition of herpes simplex virus DNA polymerase by helical peptides corresponding to the subunit interface. Proc. Natl. Acad. Sci. USA 92:1456-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutia, B. M., M. C. Frame, J. H. Subak-Sharpe, W. N. Clark, and H. S. Marsden. 1986. Specific inhibition of herpesvirus ribonucleotide reductase by synthetic peptides. Nature 321:439-441. [DOI] [PubMed] [Google Scholar]
- 18.Egal, M., M. Conrad, D. L. MacDonald, W. L. Maloy, M. Motley, and C. A. Genco. 1999. Antiviral effects of synthetic membrane-active peptides on herpes simplex virus, type 1. Int. J. Antimicrob. Agents 13:57-60. [DOI] [PubMed] [Google Scholar]
- 19.English, E. P., R. S. Chumanov, S. H. Gellman, and T. Compton. 2006. Rational development of beta-peptide inhibitors of human cytomegalovirus entry. J. Biol. Chem. 281:2661-2667. [DOI] [PubMed] [Google Scholar]
- 20.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fusco, D., C. Forghieri, and G. Campadelli-Fiume. 2005. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc. Natl. Acad. Sci. USA 102:9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 23.Gianni, T., G. Campadelli-Fiume, and L. Menotti. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 78:12268-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianni, T., C. Forghieri, and G. Campadelli-Fiume. 2006. The herpesvirus glycoproteins B and H·L are sequentially recruited to the receptor-bound gD to effect membrane fusion at virus entry. Proc. Natl. Acad. Sci. USA 103:14572-14577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Goldstein, D. J., and S. K. Weller. 1988. Herpes simplex virus type-1 ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 62:196-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grau, D. R., R. J. Visalli, and C. R. Brandt. 1989. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Investig. Ophthalmol. Vis. Sci. 30:2474-2480. [PubMed] [Google Scholar]
- 27.Guichard, G., S. Abele, and D. Seebach. 1998. Preparation of N-Fmoc-protected β2- and β3-amino acids and their use as building blocks for the solid-phase synthesis of β-peptides. Helv. Chim. Acta 81:187-206. [Google Scholar]
- 28.Hazrati, E., B. Galen, W. Lu, W. Wang, Y. Ouyang, M. J. Keller, R. I. Lehrer, and B. C. Herold. 2006. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 177:8658-8666. [DOI] [PubMed] [Google Scholar]
- 29.Hill, J. M., B. M. Gebhardt, R. Wen, A. M. Bouterie, H. W. Thompson, R. J. O'Callaghan, W. P. Halford, and H. E. Kaufman. 1996. Quantitation of herpes simplex virus type 1 DNA and latency-associated transcripts in rabbit trigeminal ganglia demonstrates a stable reservoir of viral nucleic acids during latency. J. Virol. 70:3137-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenssen, H. 2005. Anti herpes simplex virus activity of lactoferrin/lactoferricin—an example of antiviral activity of antimicrobial protein/peptide. Cell. Mol. Life Sci. 62:3002-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenssen, H., J. Andersen, D. Mantzilas, and T. J. Gutteberg. 2004. A wide range of medium-sized, highly cationic, a-helical peptides show antiviral activity against herpes simplex virus. Antivir. Res. 64:119-126. [DOI] [PubMed] [Google Scholar]
- 32.Jenssen, H., J. H. Andersen, L. Uhlin-Hansen, T. J. Gutteberg, and O. Rekdal. 2004. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antivir. Res. 61:101-109. [DOI] [PubMed] [Google Scholar]
- 33.Jenssen, H., P. Hamill, and R. E. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leikina, E., H. Delanoe-Ayari, K. Melikov, M. S. Cho, A. Chen, A. J. Waring, W. Wang, Y. Xie, J. A. Loo, R. I. Lehrer, and L. V. Chernomordik. 2005. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat. Immunol. 6:995-1001. [DOI] [PubMed] [Google Scholar]
- 36.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, D., and W. F. DeGrado. 2001. De novo design, synthesis, and characterization of antimicrobial beta-peptides. J. Am. Chem. Soc. 123:7553-7559. [DOI] [PubMed] [Google Scholar]
- 38.Marchetti, M., E. Trybala, F. Superti, M. Johansson, and T. Bergstrom. 2004. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology 318:405-413. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 40.Muller, A., C. Vogt, and N. Sewald. 1998. Synthesis of Fmoc-β-homoamino acids by ultrasound-promoted Wolff-rearrangement. Synthesis 6:837-841. [Google Scholar]
- 41.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters, D. M., N. Dowd, C. Brandt, and T. Compton. 1996. Human papilloma virus E6/E7 genes can expand the lifespan of human corneal fibroblasts. In Vitro Cell. Dev. Biol. Anim. 32:279-284. [DOI] [PubMed] [Google Scholar]
- 43.Porter, E. A., X. Wang, H. S. Lee, B. Weisblum, and S. H. Gellman. 2000. Non-haemolytic beta-amino-acid oligomers. Nature 404:565. [DOI] [PubMed] [Google Scholar]
- 44.Porter, E. A., B. Weisblum, and S. H. Gellman. 2002. Mimicry of host-defense peptides by unnatural oligomers: antimicrobial beta-peptides. J. Am. Chem. Soc. 124:7324-7330. [DOI] [PubMed] [Google Scholar]
- 45.Potocky, T. B., A. K. Menon, and S. H. Gellman. 2003. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J. Biol. Chem. 278:50188-50194. [DOI] [PubMed] [Google Scholar]
- 46.Potocky, T. B., A. K. Menon, and S. H. Gellman. 2005. Effects of conformational stability and geometry of guanidinium display on cell entry by beta-peptides. J. Am. Chem. Soc. 127:3686-3687. [DOI] [PubMed] [Google Scholar]
- 47.Potocky, T. B., J. Silvius, A. K. Menon, and S. H. Gellman. 2007. HeLa cell entry by guanidinium-rich beta-peptides: importance of specific cation-cell surface interactions. Chembiochem 8:917-926. [DOI] [PubMed] [Google Scholar]
- 48.Raguse, T. L., J. R. Lai, and S. H. Gellman. 2003. Environment-independent 14-helix formation in short beta-peptides: striking a balance between shape control and functional diversity. J. Am. Chem. Soc. 125:5592-5593. [DOI] [PubMed] [Google Scholar]
- 49.Raguse, T. L., E. A. Porter, B. Weisblum, and S. H. Gellman. 2002. Structure-activity studies of 14-helical antimicrobial beta-peptides: probing the relationship between conformational stability and antimicrobial potency. J. Am. Chem. Soc. 124:12774-12785. [DOI] [PubMed] [Google Scholar]
- 50.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schinnerl, M., J. K. Murray, J. M. Langenhan, and S. H. Gellman. 2003. Asymmetric synthesis of a new helix-forming β-amino acid: trans-4-aminopiperidine-3-carboxylic acid. Eur. J. Org. Chem. 8:721-726. [Google Scholar]
- 52.Schreiber, J. V., J. Frackenpohl, F. Moser, T. Fleischmann, H. P. Kohler, and D. Seebach. 2002. On the biodegradation of beta-peptides. Chembiochem 3:424-432. [DOI] [PubMed] [Google Scholar]
- 53.Seebach, D., S. Abele, J. V. Schreiber, B. Martinoni, A. K. Nussbaum, H. Schild, H. Schulz, H. Hennecke, R. Woessner, and F. Bitsch. 1998. Biological and pharmacokinetic studies with beta-peptides. Chimica 52:734-739. [Google Scholar]
- 54.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 55.Sinha, S., N. Cheshenko, R. I. Lehrer, and B. C. Herold. 2003. NP-1, a rabbit alpha-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. Antimicrob. Agents Chemother. 47:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 57.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spear, P. G., M. T. Shieh, B. C. Herold, D. WuDunn, and T. I. Koshy. 1992. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv. Exp. Med. Biol. 313:341-353. [DOI] [PubMed] [Google Scholar]
- 59.Steiner, H., D. Hultmark, A. Engstrom, H. Bennich, and H. G. Boman. 1981. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292:246-248.7019715 [Google Scholar]
- 60.Teuton, J. R., and C. R. Brandt. 2007. Sialic acid on herpes simplex virus type 1 envelope glycoproteins is required for efficient infection of cells. J. Virol. 81:3731-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trybala, E., T. Bergstrom, B. Svennerholm, S. Jeansson, J. C. Glorioso, and S. Olofsson. 1994. Localization of a functional site on herpes simplex virus type 1 glycoprotein C involved in binding to cell surface heparan sulphate. J. Gen. Virol. 75:743-752. [DOI] [PubMed] [Google Scholar]
- 62.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type I are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyagi, M., M. Rusnati, M. Presta, and M. Giacca. 2001. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276:3254-3261. [DOI] [PubMed] [Google Scholar]
- 64.Wang, X., J. F. Espinosa, and S. H. Gellman. 2000. 12-Helix formation in aqueous solution with short beta-peptides containing pyrrolidine-based residues. J. Am. Chem. Soc. 122:4821-4822. [Google Scholar]
- 65.Whitley, R. J. 1996. Herpes simplex viruses, p. 2297-2342. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven, Philadelphia, PA. [Google Scholar]
- 66.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yasin, B., W. Wang, M. Pang, N. Cheshenko, T. Hong, A. J. Waring, B. C. Herold, E. A. Wagar, and R. I. Lehrer. 2004. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78:5147-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 69.Zasloff, M. 1987. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 84:5449-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ziegler, A., and J. Seelig. 2004. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters. Biophys. J. 86:252-263. [DOI] [PMC free article] [PubMed] [Google Scholar]